Abstract
AIM: To ascertain whether caecal pH is different in patients with irritable bowel syndrome (IBS), whose primary symptoms are bloating and distension, to healthy controls.
METHODS: Motility and pH data were reviewed from 16 patients with Rome III defined IBS and 16 healthy controls, who had undergone a wireless motility capsule (WMC) study using a standardized protocol. Motility measures were anchored around known anatomical landmarks as identified by compartmental pH changes. Sixty-minute epochs were used to quantify antral, duodenal, ileal, caecal and distal colonic contractility. The maximum and minimum pH was measured either side of the ileo-caecal junction.
RESULTS: No differences were seen in motility parameters, compartmental transit times or maximal ileal pH between the two groups. Caecal pH was significantly lower in patients compared to controls (5.12 ± 0.05 vs 6.16 ± 0.15, P < 0.0001). The ileal:caecal Δchange was greater in patients than controls (-2.63 ± 0.08 vs -1.42 ± 0.11, P < 0.0001). There was a significant correlation between caecal pH and right colonic contractility (r = 0.54, P = 0.002).
CONCLUSION: Patients with bloating and distension have a lower caecal pH compared to controls. The measurement of caecal pH using the WMC provides a quantifiable biomarker of fermentation potentially identifying those patients that may preferentially benefit from antibiotic or dietary interventions.
Keywords: Caecal pH, Caecoparesis, Bloating, Colonic microbiota, Fermentation
Core tip: Colonic bacterial fermentation has been implicated in the pathogenesis of irritable bowel syndrome. Hitherto, the measurement of fermentation in vivo in humans has been invasive and technically challenging. A major by product of colonic bacterial fermentation are short chain fatty acids. These short chain fatty acids act to reduce colonic pH. Herein, we demonstrate that the measurement of caecal ph using the wireless motility capsule provides a quantifiable biomarker of fermentation potentially identifying those patients with irritable bowel syndrome that may preferentially benefit from antibiotic or dietary interventions.
INTRODUCTION
Bloating and distension are both common and vexatious symptoms with community-based estimates of prevalence of 19% and 8.9% respectively[1]. Bloating is largely regarded as a subjective sensation of abdominal swelling, whereas distension refers to an observable increase in abdominal girth[2]. Bloating is associated with a reduction in quality of life, is a cause for healthcare seeking and represents a considerable challenge to manage effectively[3,4]. Bloating and distension are common complaints in patients with functional gastrointestinal disorders (FGID) such as irritable bowel syndrome (IBS) and functional dyspepsia[5-7].
The pathophysiological mechanisms that account for bloating and distension are poorly understood. They have been proposed to include disturbances in the handling of gas and its elimination from the gastrointestinal (GI) tract[8], psychological factors[9], carbohydrate malabsorption[10], musculoskeletal abnormalities[11], sensorimotor aberrancies[5], small intestinal bacterial overgrowth (SIBO)[12] and alterations within the GI microbiota[13].
The human microbiota is a complex symbiotic ecosystem residing largely in the GI tract. The composition and concentration of the microbiota varies along the length of the GI tract[14]. In humans, the colon receives digested material from the small bowel where it is mixed, stored and eventually excreted as faeces. The anaerobic breakdown of carbohydrates and protein by bacteria, largely occurring within the proximal colon, is through a process known as fermentation, the principal products of which are short chain fatty acids (SCFA)[15,16]. The direct in vivo measurement of SCFA concentrations in the human proximal colon is technically difficult and invasive[17,18]. Given that the degree of bacterial fermentation is directly proportional to the concentration of SCFA, the measurement of segmental intra-colonic pH is an inverse surrogate proxy of the degree of fermentation occurring within that territory[19]. It has been over 40 years since the stereotypical pH profile of the GI tract was first investigated using radio-telemetric techniques[20]. Upon entering the acidic environment of the stomach there is an immediate fall in pH, followed by a sharp rise on exiting the stomach, and a further fall in pH some hours later, a fall hypothesized to occur across the ileo-caecal junction (ICJ)[21]. Until recently, controversy remained as to the exact location of this fall in pH, as previous methods directed at validating position of the capsule within the GI tract were subject to limitations, particularly regarding accurate anatomical localization. These concerns were resolved in a study by Zarate et al[22], using a dual-scintigraphic technique of direct GI manometric measurements and pH evaluation using a wireless motility capsule (WMC), demonstrating that the drop in pH did indeed occur across the ICJ. The WMC is an ambulatory and relatively non-invasive diagnostic technique that continuously samples intraluminal pH, temperature and pressure as it traverses the GI tract. As changes in GI microbiota and fermentation have been linked to the development of bloating and distension, it is not known whether the measurement of caecal pH and the pH gradient across the ICJ, using WMC, offers a relatively non-invasive objective surrogate biomarker of this process. The WMC also allows examination of the hypothesis that these pH changes influence motility and transit parameters. In this retrospective study we aimed to address these knowledge gaps.
MATERIALS AND METHODS
Subjects
Sixteen consecutive outpatients, whose chief complaint and indication for the WMC investigation was bloating and distension were enrolled in the study between June 2011 and August 2012. Sixteen healthy age and sex-matched participants were recruited as controls. All participants provided written informed consent to undertake the investigations and the retrospective analysis of data was approved by the local institutional committee and East London and The City Research Ethics Committee (reference no. 07/H0703/77, permission granted March 2008).
Patients
All patients underwent a detailed clinical history and physical examination. Standard haematological, biochemical, immunological, upper and lower GI endoscopy with histology were performed in all patients prior to the WMC study by their referring gastroenterologist to rule out structural or biochemical causes for their symptoms. Patients did not undergo direct small bowel visualisation with enteroscopy or wireless capsule endoscopy. Bloating and/or distension were the chief presenting complaint in all patients. All patients fulfilled the Rome III criteria for IBS and had alternating bowel habit, characterized by variable stool consistency and frequency[23,24].
Healthy controls
All healthy subjects had a normal bowel habit, defined as between three bowel movements a per day and one bowel movement every three days, with no symptoms of suggestive of SIBO or a rectal evacuatory disorder. No subject had any GI symptoms or history of metabolic, neurogenic, or endocrine disorder known to influence GI motor activity. In addition, no subject had undergone GI surgery other than appendicectomy and none were taking either laxatives or medications known to influence GI motility or pH.
Exclusion criteria
The presence of a positive pregnancy test, “red flag”/alarm symptoms (such as weight loss, anaemia or rectal bleeding), a positive microbiological, immunological or histological investigation suggesting another cause for symptoms, recent antibiotic use in the preceding 4 wk, recent probiotic use in the last 2 wk, concurrent use of promotile or acid-suppressing medications, history of a systemic disorder with known GI manifestations (such as diabetes mellitus, connective tissue disorders etc.) and previous GI tract surgery were treated as criteria for exclusion. Specific contraindications to WMC were dysphagia, recent abdominal surgery, Crohn’s disease and diverticulitis.
Wireless motility capsule study
All subjects were kept nil-by mouth except for small amounts of water from 9 pm on the before the study. Prior to ingestion of the WMC, all subjects were given a test meal (SmartBar, SmartPill Corporation, Buffalo, United States), a cereal bar of known calorific and nutritional content (260 kcal, 2% fat, 1 g fibre). The WMC was then swallowed with 60 mL of water. Once the communication was established between the WMC and data receiver, and the capsule was confirmed to be in the stomach (pH < 4), the patient was instructed in receiver care and allowed to leave the unit. No further meals or drinks were allowed for 6-h post capsule ingestion. After this, patients were allowed to eat and drink normally. After each bowel movement, the patient was instructed to wait for 1-min prior to flushing the toilet. Once they had flushed the toilet, they checked the data receiver to see if the signal connection had been lost and this confirmed exit of the capsule. The patient would then call the department, be instructed in how to turn the receiver off on return the box for download of the data. Continuous pH and pressure data were obtained by using the WMC system (SmartPill, Given Imaging Ltd, Yoqneam, Israel). The WMC contains sensors for pH, temperature and pressure and which are transmitting to a data receiver worn by the subject during ambulatory monitoring with data sensed at a frequency of 434 MHz. The pH is accurate to within ± 0.5 units and pressure measurements are accurate to ± 5 mmHg below 100 mmHg. After completion of the study, data was downloaded from the receiver to a compatible computer (Dell, Bracknell, United Kingdom) via a USB docking station and was analysed using semi-automated pressure analysis software (MotiliGI; Given Imaging Ltd, Yoqneam, Israel).
Compartmental transit times
The position of the various physiological landmarks within the GI tract, were determined from the physiological traces by two independent experienced investigators (Scott SM, Hobson AR) thus identifying gastric emptying, exit from the ileum into the right colon and excretion of the capsule. Disagreement regarding landmark locations was resolved by further review. The regional transit times were defined according to method proposed by Sarosiek et al[25] and anchored around stereotypical pH changes. Briefly, gastric emptying time (GET) was defined as the time between the ingestion of capsule and a sharp abrupt, pH rise (> 3 pH units) from gastric baseline to a pH > 4.0, marking the passage of the capsule from the acidic antrum to the relative alkaline environment of the duodenum. Small bowel transit time (SBTT) was defined as the time from which the WMC left the stomach until it arrived at the cecum as denoted by a pH drop of at least 1 pH unit, observed at least 30 min after GET and persisting for a minimum of 10 min. Colonic transit time (CTT) was defined as the elapsed time from the WMC accession at the ICJ until the capsule’s exit from the body. The exit of the capsule from the body was determined either by an abrupt cessation data being recorded in conjunction with a subject’s report of passing the capsule coinciding with a bowel movement entry in the subject’s activity diary or an abrupt drop in temperature as the WMC exits the body. Thus, whole gut transit time (WGTT) was defined as the time from ingestion to excretion of the WMC.
Motility measures
In addition to measuring transit times, the WMC also measures intraluminal pressure across the GI tract thereby measuring frequency of contractions and amplitude of contractions. Motility measures are presented as area under the curve (AUC), anchored around the pH landmarks. Sixty-minute epochs were used to quantify antral, duodenal, ileal, caecal and distal colonic motility.
Caecal pH, magnitude of pH drop across the ileocaecal valve and caecal contractility
Caecal pH was defined as the fall in pH from the stable ileal peak to its nadir value as the WMC passed from the ileum into the cecum, as per the method defined by Zarate et al[22]. The Δchange was derived from the caecal nadir to the stable ileal peak. Caecal contractility was derived from the AUC for 1-h post passage through the ICJ, see Figure 1.
Figure 1.
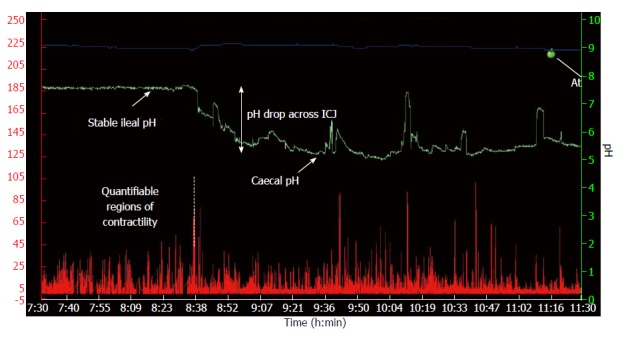
A typical wireless motility capsule trace demonstrating temperature (blue line), pH (green line) and contractility (red line) across the ileo-caecal junction. The pH drop was defined as the difference between the stable ileal pH and the caecal pH nadir. ICJ: Ileo-caecal junction.
Statistical analysis
Data distribution was analysed using the D’Agostino-Pearson omnibus K2 normality test[26]. Results of quantitative data are presented either as median with interquartile ranges, for non-normally distributed data, or mean ± standard deviation (SD) and range for parametric data. Categorical data were summarised as the percentage of the group total. For quantitative data, differences between the groups were assessed using the Student’s t-test. Correlational analyses were performed using Pearson’s correlation. Two-tailed tests were used throughout. P < 0.05 was adopted as the statistical criterion. All analyses were performed using proprietary software (GraphPad Prism 5, CA, United States).
RESULTS
Participant characteristics
Sixteen female patients (median age 31 years, range 24-52 years) and 16 age, sex matched healthy controls (median age 38.5 years, range 21-74) completed the study.
Compartmental transit times
There were no appreciable differences in GET, SBTT, CTT or WGTT between patients and controls, see Table 1.
Table 1.
A comparison of the regional transit times between patients and healthy controls
| Transit time (min) | Patients (mean ± SD) | Controls (mean ± SD) | P value |
| GET | 290 ± 76 | 306 ± 55.9 | 0.86 |
| SBTT | 305 ± 14.5 | 270 ± 25.9 | 0.24 |
| CTT | 1443 ± 192.8 | 1861 ± 263.7 | 0.21 |
| WGTT | 2039 ± 202 | 2437 ± 279.4 | 0.26 |
GET: Gastric emptying time; SBTT: Small bowel transit time; CTT: Colonic transit time; WGTT: Whole gut transit time.
Motility comparisons
There were no appreciable differences in antral, duodenal, ileal, caecal and colonic motility between patients and controls, see Table 2.
Table 2.
A comparison of the regional motility pattern, given by areas under the curve, between patients and healthy controls
| Motility (AUC) | Patients (mean ± SD) | Controls (mean ± SD) | P value |
| Antral | 3590 ± 708.3 | 4710 ± 850.1 | 0.32 |
| Duodenal | 3909 ± 919 | 6000 ± 1310 | 0.20 |
| Ileal | 13414 ± 2203 | 12679 ± 2131 | 0.81 |
| Cecal | 4071 ± 531.2 | 5176 ± 878 | 0.29 |
| Recto-sigmoid | 20504 ± 4583 | 11894 ± 215 | 0.09 |
AUC: Areas under the curve.
Regional pH comparisons
There were no appreciable differences in ileal pH between patients and controls, 7.7 ± 0.1 vs 7.6 ± 0.1, P = 0.17. However, caecal pH was significantly lower in patients in comparison to controls, 5.12 ± 0.05 vs 6.16 ± 0.15, P < 0.0001.
Δchange ileo-caecal:caecal pH and relationship of caecal pH to caecal contractility
Δ%change ileo-caecal:caecal pH was significantly higher in patients compared to controls (-33.8% ± 0.84 vs -18.7 ± 1.5, P < 0.0001), see Figure 2. For the whole cohort, there was a moderate correlation between caecal pH and right colonic contractility (r = 0.54, P = 0.002), see Figure 3.
Figure 2.
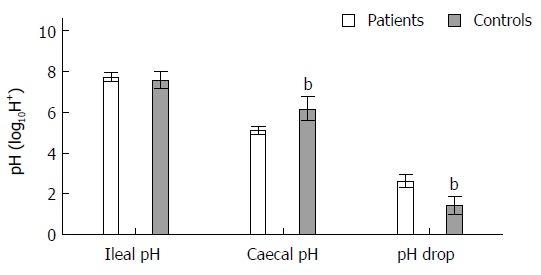
Differences (mean ± SD) in ileal, caecal and ileo-caecal junction pH drop between patients and controls. Caecal pH significantly lower in patients than in controls and pH drop across the ICJ was lower in patients than in controls. bP < 0.01 vs patients group. ICJ: Ileo-caecal junction.
Figure 3.
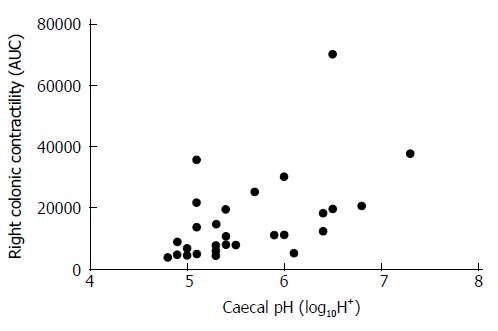
Caecal pH and caecal contractility was positively correlated (r = 0.54, P = 0.002). AUC: Area under the curve.
DISCUSSION
In this study, we have demonstrated that patients with lower abdominal symptoms typically associated with, but not limited to, conditions such as IBS, have a significantly lower caecal pH compared to controls. This relatively acidic environment is maintained by fermentation and subsequent SCFA production. In addition, we have observed that excessive fermentation in the cecum is correlated with a reduction in caecal contractility.
The measurement of bacterial fermentation products demonstrate marked regional differences in their production across the colon such that SCFA concentrations are greatest in the caecum (127 mmol/L) falling progressively in the transverse (117 mmol/L) and distal colon (90 mmol/L)[17]. These differences in SCFA concentration indicate that fermentation occurs maximally within the right colon, presumably where concentrations of the substrate, arriving from the small bowel, are at their highest[27]. Studies in IBS patients, in which bloating and distension are prevalent symptoms, have demonstrated alterations in colonic fermentation[28,29]. Mortensen et al[28] observed that SCFA concentrations are increased in diarrhoea predominant-IBS (IBS-D) and decreased in constipation predominant IBS (IBS-C) although Treem and colleagues reported conflicting results in IBS-C[29]. These observations have engendered the application of a diverse array of sophisticated molecular and culture independent approaches to the evaluation of the GI microbiota in FGID. For instance, Tana et al[30] determined SCFA concentrations using high-performance gas chromatography, and found that IBS patients had significantly higher concentrations, which were associated with increased GI symptoms and quality of life burden. A recent important study by Jeffery et al[31] performed a detailed pyrosequencing analysis of faecal microbiota composition and demonstrated two species specific subtypes of IBS, independent of symptom based classification derived from the Rome III criteria. The first of these showed a microbial composition similar to normal whereas the second was characterized by an increase in Firmicutes-associated taxa in association with a relative depletion of Bacteroides-related taxa. The implication of this data is that in future GI microbial enterotyping may facilitate stratifications of IBS sub-populations. However, at the present time such methods have limited practicality as a routine clinical biomarker as they are resource and labour intensive[32]. However, given that a raised Firmicutes:Bacteroides has been positively correlated with increased concentrations of SCFA in a pre-clinical human model[33], an alluring speculation is that the measurement of caecal pH, using the WMC, may provide an attractive, readily available, surrogate marker obviating many of the limitations of the current microbial enterotyping techniques whilst also assessing for motility disorders.
Another potential application of measuring fermentation by caecal pH may enable the early identification of patients in whom a particular treatment may be more efficacious, particularly given recent laudable progress in therapeutic interventions. Nevertheless, given the heterogeneous nature of FGID populations, it is not surprising that the degree of therapeutic gain conferred by these advances is somewhat variable. In this respect, two areas where considerable furtherance has been made are those of non-absorbable antibiotics and dietary modifications. Firstly, Pimentel et al[34] reported the pooled results of two phase 3, double-blind, placebo-controlled trials comparing adequate global relief of symptoms and bloating in IBS patients without constipation who were randomly assigned to received either rifaximin, a minimally absorbed oral broad spectrum antimicrobial agent, or placebo. Patients treated with rifaximin had a significant reduction in global symptoms of IBS and it can be derived that at 3 mo the numbers needed to treat (NNT) weekly bloating symptoms is approximately 10. Secondly, the quantity of poorly absorbed short chain carbohydrates, which exert an intraluminal osmotic effect and are rapidly fermented by bacteria, entering the colon can be modified by dietary restriction[35]. Collectively these short chain carbohydrates are known as fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and reducing their intake has proven successful in reducing bloating and abdominal pain in IBS patients, with an approximate NNT with respect to the former of 3[36]. However, these novel and emerging interventions are notwithstanding concerns regarding implications for healthcare expenditure, short and long-term safety and patient compliance[35,37,38]. The absolute practical utility of caecal pH measurement as a marker of fermentation remains to be fully determined but it may facilitate the identification of FGID patients who may preferentially benefit from antibiotic or dietary interventions.
In humans, 90%-95% of SCFA are composed of acetate, propionate and butyrate and are plurifunctional through their contribution to the maintenance of mucosal integrity[39], stimulation of salt and water absorption[40], regulation of colonic mucosal blood flow[18] as well as having anti-carcinogenic[41] and immuno-modulatory properties[42]. Moreover, SCFAs may also act as intra-luminal chemical stimuli modifying GI motility but whether these effects are stimulatory or inhibitory is unclear and varies according to different experimental paradigms, species and the region of the GI tract being considered[43]. In humans, intra-colonic SCFA infusion has not been shown to influence motility although it is plausible to suggest that the infusion concentrations used were insufficient to activate any putative sensorimotor mechanisms[44]. Our results demonstrate, in an ambulatory relatively physiological setting, a correlation between pH and caecal contractility. Therefore in this situation it is possible that heightened fermentation in the cecum with associated with elevated concentrations of SCFA, inhibit proximal colonic motor activity potentially leading to a degree of stasis, or “caecoparesis.” In an animal model, Dass et al[43] investigated the hypothesis that SCFA may modulate colonic motility through the G protein-coupled receptors. Interestingly they demonstrated that SCFA enhanced neuronally mediated contractions of rat distal colon yet increased the frequency of peristaltic contractions in guinea-pig terminal ileum. These data therefore suggest differential regional actions of SCFA such that there is inhibition of motility in the right colon followed by a pro-motility effect in the more distal colon. Combined with increased fermentation in the cecum leading to distension, caecoparesis may be the long sort after alteration in motor function which differentiates IBS patients from healthy subjects and explains why IBS preferentially experience pain in the right colon and upper abdomen in response to balloon distension[45]. Whilst we were unable to accurately assess segmental colonic transit times using the WMC (due to technical limitations discussed earlier), further studies with techniques such as MRI may help to prove this concept further.
The role of SCFA in modulating visceral perception and nociception has been afforded considerable interest. Whilst several investigators have examined the effect of intra-colonic instillation of butyrate, results are conflicting. Bourdu et al[46] showed that the administration of butyrate enemas in a rat model caused a sustained, dose-dependent increase in sensitivity to colorectal distension, in the absence of demonstrable microscopic or histological abnormality in the colonic mucosa, closely mimicking what is seen in a proportion of IBS patients. In studies of healthy human volunteers the converse effect has been demonstrated where the administration of butyrate rectal enemas, at physiologically relevant concentrations, caused a dose-dependent decrease in rectal sensitivity[47]. However, a pertinent fundamental limitation of this type of study remains as to whether distal colonic administration of SCFA, even in physiologically relevant concentrations, alters the composition in the proximal colon where SCFA concentration is at its highest as retrograde colonic spread of rectal enemas is irregular and formulation dependent[48]. Whether these data, derived from a small group of healthy volunteers, are applicable to larger cohorts of community-based patients with a FGID currently is uncertain.
This study is not without significant limitations. Firstly, we did not actively screen for diabetes mellitus, thyroid dysfunction or smoking through HbA1c, thyroid stimulating hormone or serum cotinine respectively. Secondly, the control group was marginally older, although this difference did not reach statistical significance. Thirdly, this was an unselected sample, SIBO not actively screened for and being a retrospective analysis. In addition, our findings were unexpected. A further valid criticism is the lack of dietary control but as all subjects had fasted for at least 12 h prior to the study, and subsequently for a further 6 h after the standardized test meal, by the time the WMC capsule reached the cecum, it is improbable, although not impossible, that further intake could influence GI microbiota, fermentation and thus caecal pH[49]. Nevertheless, whilst further validation needed, the overall concept presented herein plausible concept in IBS/functional bloating.
In conclusion the measurement of caecal pH using the WMC provides a quantifiable biomarker of fermentation. In future, this may be used to sub-classify patients with a broad spectrum of FGID and identify those that may benefit most from antibiotic and dietary interventions providing novel insights into the pathophysiology of lower GI symptoms and mechanism of actions of novel treatments.
ACKNOWLEDGMENTS
A preliminary form of this data was presented in abstract form at the British Society of Gastroenterology Annual Meeting 2013, abstract number BSG13-1656.
COMMENTS
Background
Irritable bowel syndrome is a common disorder of gastrointestinal function whose pathophysiology is incompletely understood.
Research frontiers
There is an increasing body of evidence to suggest that the gastrointestinal microbiota play a significant role in the genesis of symptoms in irritable bowel syndrome. Within the colon, the microbiota ferments a number of substrates whose main by products are short chain fatty acids. To date, the measurement of short chain fatty acids in humans has been invasive and technically difficult.
Innovations and breakthroughs
In this study, authors have utilised wireless motility capsule technology to measure the pH as it traverses the gastrointestinal tract. They have demonstrated that patients with lower abdominal symptoms typically associated with, but not limited to, conditions such as irritable bowel syndrome, have a significantly lower caecal pH compared to controls. This relatively acidic environment is maintained by fermentation and subsequent short chain fatty acid production. In addition, they have observed that excessive fermentation in the cecum is correlated with a reduction in caecal contractility.
Applications
By using such methodology, it may be possible to stratify patients with irritable bowel syndrome in based on their caecal pH. For instance, patients with a low caecal pH, thereby suggesting heightened fermentation and thus bacterial load, may preferentially benefit from antibiotic and “substrate lowering” dietary interventions.
Terminology
The gastrointestinal microbiota is a complex symbiotic ecosystem, whose interactions with the host are complex and largely remain to be fully characterized. This microbiota, in the right colon, ferment substrate arriving from the proximal gastrointestinal tract whose end products are short chain fatty acids.
Peer review
It is an interesting and innovative paper of great clinical impact.
Footnotes
Supported by A Grant from the SmartPill Corporation
P- Reviewers: Casadesus D, Guslandi M S- Editor: Cui XM L- Editor: A E- Editor: Liu XM
References
- 1.Jiang X, Locke GR, Choung RS, Zinsmeister AR, Schleck CD, Talley NJ. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57:756–763. doi: 10.1136/gut.2007.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Houghton LA, Lea R, Morris J, Reilly B, Whorwell PJ. Bloating and distention in irritable bowel syndrome: the role of visceral nsation. Gastroenterology. 2008;134:1882–1889. doi: 10.1053/j.gastro.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X, Locke GR, Zinsmeister AR, Schleck CD, Talley NJ. Health care seeking for abdominal bloating and visible distention. Aliment Pharmacol Ther. 2009;30:775–783. doi: 10.1111/j.1365-2036.2009.04080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuteja AK, Talley NJ, Joos SK, Tolman KG, Hickam DH. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008;103:1241–1248. doi: 10.1111/j.1572-0241.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 5.Maxton DG, Morris JA, Whorwell PJ. Ranking of symptoms by patients with the irritable bowel syndrome. BMJ. 1989;299:1138. doi: 10.1136/bmj.299.6708.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Talley NJ. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10:134–141. doi: 10.1038/nrgastro.2013.14. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol. 2009;104:1998–2004. doi: 10.1038/ajg.2009.251. [DOI] [PubMed] [Google Scholar]
- 9.Park HJ, Jarrett M, Cain K, Heitkemper M. Psychological distress and GI symptoms are related to severity of bloating in women with irritable bowel syndrome. Res Nurs Health. 2008;31:98–107. doi: 10.1002/nur.20237. [DOI] [PubMed] [Google Scholar]
- 10.Haderstorfer B, Psycholgin D, Whitehead WE, Schuster MM. Intestinal gas production from bacterial fermentation of undigested carbohydrate in irritable bowel syndrome. Am J Gastroenterol. 1989;84:375–378. [PubMed] [Google Scholar]
- 11.Maratka Z. Abdominal bloating and distension in functional gastrointestinal disorders - epidemiology and possible mechanisms. Aliment Pharmacol Ther. 2008;27:713–714; author reply 714. doi: 10.1111/j.1365-2036.2008.03616.x. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 13.Issa B, Wafaei NA, Whorwell PJ. Abdominal bloating and distension: what is the role of the microbiota. Dig Dis Sci. 2012;57:4–8. doi: 10.1007/s10620-011-1834-4. [DOI] [PubMed] [Google Scholar]
- 14.Young VB, Schmidt TM. Overview of the gastrointestinal microbiota. Adv Exp Med Biol. 2008;635:29–40. doi: 10.1007/978-0-387-09550-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 16.Shen Q, Zhao L, Tuohy KM. High-level dietary fibre up-regulates colonic fermentation and relative abundance of saccharolytic bacteria within the human faecal microbiota in vitro. Eur J Nutr. 2012;51:693–705. doi: 10.1007/s00394-011-0248-6. [DOI] [PubMed] [Google Scholar]
- 17.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31:1391–1394. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis SJ, Heaton KW. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut. 1997;41:245–251. doi: 10.1136/gut.41.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson BW, Meldrum SJ, Riddle HC, Brown RL, Sladen GE. pH profile of gut as measured by radiotelemetry capsule. Br Med J. 1972;2:104–106. doi: 10.1136/bmj.2.5805.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarate N, Mohammed SD, O’Shaughnessy E, Newell M, Yazaki E, Williams NS, Lunniss PJ, Semler JR, Scott SM. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1276–G1286. doi: 10.1152/ajpgi.00127.2010. [DOI] [PubMed] [Google Scholar]
- 23.Tillisch K, Labus JS, Naliboff BD, Bolus R, Shetzline M, Mayer EA, Chang L. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005;100:896–904. doi: 10.1111/j.1572-0241.2005.41211.x. [DOI] [PubMed] [Google Scholar]
- 24.Drossman DA. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean, Va. Degnon Associates; 2006. [Google Scholar]
- 25.Sarosiek I, Selover KH, Katz LA, Semler JR, Wilding GE, Lackner JM, Sitrin MD, Kuo B, Chey WD, Hasler WL, et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther. 2010;31:313–322. doi: 10.1111/j.1365-2036.2009.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Agostino RB, Stephens MA. Goodness-of-fit techniques. New York: M. Dekker; 1986. [Google Scholar]
- 27.Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen PB, Andersen JR, Arffmann S, Krag E. Short-chain fatty acids and the irritable bowel syndrome: the effect of wheat bran. Scand J Gastroenterol. 1987;22:185–192. doi: 10.3109/00365528708991878. [DOI] [PubMed] [Google Scholar]
- 29.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519, e114-115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 31.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 32.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Zanten GC, Knudsen A, Röytiö H, Forssten S, Lawther M, Blennow A, Lahtinen SJ, Jakobsen M, Svensson B, Jespersen L. The effect of selected synbiotics on microbial composition and short-chain fatty acid production in a model system of the human colon. PLoS One. 2012;7:e47212. doi: 10.1371/journal.pone.0047212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–717. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 36.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 37.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35; quiz 36. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 38.Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233–238. doi: 10.1097/MCG.0b013e31802cbc2f. [DOI] [PubMed] [Google Scholar]
- 39.Kripke SA, Fox AD, Berman JM, Settle RG, Rombeau JL. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr. 1989;13:109–116. doi: 10.1177/0148607189013002109. [DOI] [PubMed] [Google Scholar]
- 40.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–1507. [PubMed] [Google Scholar]
- 41.Boutron-Ruault MC, Marteau P, Lavergne-Slove A, Myara A, Gerhardt MF, Franchisseur C, Bornet F, Eripolyp Study Group. Effects of a 3-mo consumption of short-chain fructo-oligosaccharides on parameters of colorectal carcinogenesis in patients with or without small or large colorectal adenomas. Nutr Cancer. 2005;53:160–168. doi: 10.1207/s15327914nc5302_5. [DOI] [PubMed] [Google Scholar]
- 42.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM, Sanger GJ. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 44.Kamath PS, Phillips SF, O’Connor MK, Brown ML, Zinsmeister AR. Colonic capacitance and transit in man: modulation by luminal contents and drugs. Gut. 1990;31:443–449. doi: 10.1136/gut.31.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swarbrick ET, Hegarty JE, Bat L, Williams CB, Dawson AM. Site of pain from the irritable bowel. Lancet. 1980;2:443–446. doi: 10.1016/s0140-6736(80)91885-1. [DOI] [PubMed] [Google Scholar]
- 46.Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, Dechelotte P, Bommelaer G, Eschalier A, Ardid D. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology. 2005;128:1996–2008. doi: 10.1053/j.gastro.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 47.Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21:952–e76. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 48.Otten MH, De Haas G, Van den Ende R. Colonic spread of 5-ASA enemas in healthy individuals, with a comparison of their physical and chemical characteristics. Aliment Pharmacol Ther. 1997;11:693–697. doi: 10.1046/j.1365-2036.1997.00199.x. [DOI] [PubMed] [Google Scholar]
- 49.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]


