Abstract
AIM: To verify that the T stage has greater weight than the N stage in the staging of colorectal cancer.
METHODS: Open data from the Surveillance, Epidemiology, and End Results program were reviewed and analyzed according to the T stage, N stage, and patients’ observed survival (OS). The relative weights of the T and N stages were calculated by multiple linear regressions based on their impact on survival. Risk scores for 25 TN categories were then calculated from the T and N stage relative weights, and a rearranged tumor node metastasis (TNM) staging system was proposed via a cluster analysis of the TN scores.
RESULTS: Both T and N stages significantly affect the OS of patients with colorectal cancer. Moreover, the T stage has greater weight than the N stage in the TNM staging system of colorectal cancer. For colon cancer, the relative T and N stage weights were 0.58 and 0.42, respectively, and for rectal cancer, the relative T and N stage weights were 0.61 and 0.39, respectively. On the basis of cluster analysis of the TN scores, T1N1a was classified to stage I, and T2N1a-1b and T1N1b-2a were classified to stage II in our revised TNM staging system for both colon and rectal cancer. For colon cancer, T4bN0 was classified to stage IIIa, but for rectal cancer, it was classified to stage IIIb.
CONCLUSION: As the T stage affects colorectal cancer survival more significantly than the N stage, the TNM staging should be revised by relative T stage weight.
Keywords: Colorectal cancer, Neoplasm staging, Cluster analysis, Survival analysis, Observational study
Core tip: The 7th edition of the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system for colorectal cancer can not predict survival linearly by stage. We propose that the T stage has greater weight than the N stage, more especially in rectal cancer than in colon cancer. Moreover, in this article, we propose a revised scheme for the 7th edition of the AJCC TNM staging system. In our revised scheme, T4bN0 is classified to stage IIIa in colon cancer, but to stage IIIb in rectal cancer. This is the first attempt to revise the established TNM staging system for colorectal cancer by shaking the keystone of present classification based on the lymph nodes status.
INTRODUCTION
The American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system is widely used to predict the prognosis for patients with colorectal cancer and to guide adjuvant therapy after potentially curative surgery. The 7th edition of the AJCC TNM staging system was published in 2010[1]. Patients with colorectal cancer, which directly invades or is adherent to other organs or structures, have poorer prognoses. As a result, stage T4 was stratified to T4a and T4b, and patients with T4bN0 lesions were reclassified from stage IIb to IIc. Similarly, T1-2N2 was moved from stage IIIc to IIIa/IIIb. These changes reflect the fact that the T stage affects survival in colorectal cancer patients more significantly than previously believed.
The most obvious drawback of the AJCC TNM staging system is that the relative weighting of the N stage is over-estimated. Except for patients in stage IV, all patients with lymph node involvement are defined as stage III. However, data from the Surveillance, Epidemiology, and End Results (SEER) program has shown that the 5-year observed survival (OS) of stage IIIa patients (T1-2N1 and T1N2a) matches that of stage I patients[2,3]. On the other hand, stage IIc patients have a poorer prognosis, equivalent to that of stage IIIb patients (Figure 1). In other words, the 7th edition of the AJCC TNM staging system fails to predict survival linearly by stage. In a study in which survival data for 1165 Japanese colorectal cancer patients were calculated according to 7th edition of the AJCC TNM staging system, it was found that after stage I, patients in stage IIIa unexpectedly showed the best prognosis[4].
Figure 1.
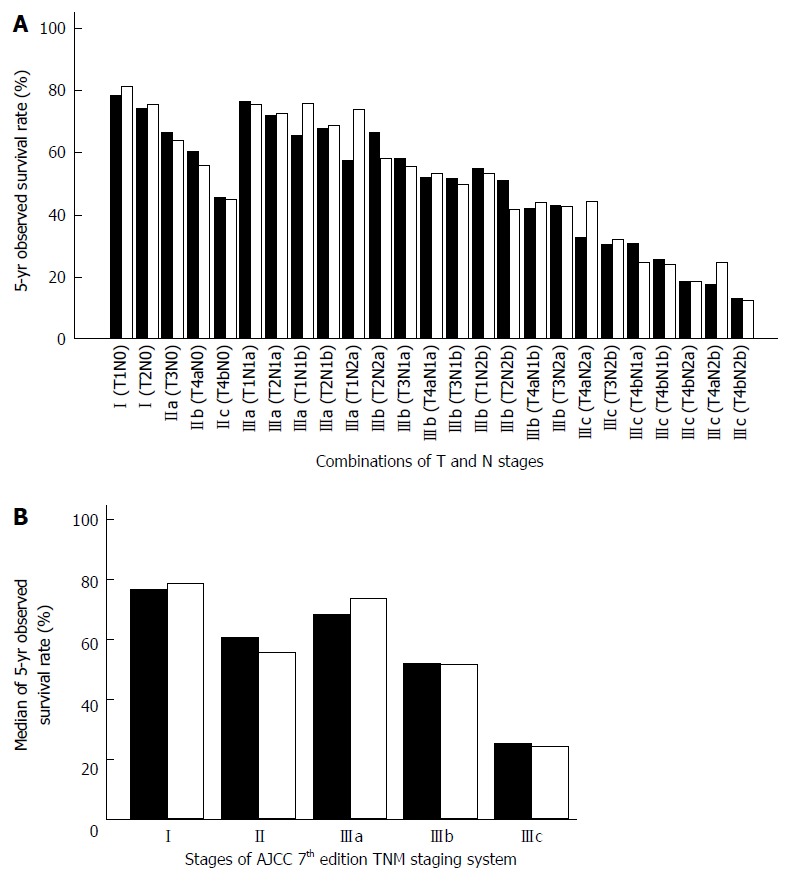
Relationship between survival of colorectal cancer patients and stages of the American Joint Committee on Cancer 7th edition tumor node metastasis staging system. A: The 5-year observed survival rate of colorectal cancer patients according to 25 combinations of T and N stages of the American Joint Committee on Cancer (AJCC) 7th edition tumor node metastasis (TNM) staging system; B: The median 5-year observed survival rate of colorectal cancer patients for overall stages (I, II, IIIa, IIIb and IIIc) according to the 7th edition of the AJCC TNM staging system. Colon cancer is represented by the solid black grids. Rectal cancer is represented by the white grids (Surveillance, Epidemiology, and End Results open data).
As we were concerned that the T stage may have a greater impact on the survival of colorectal cancer patients following potentially curative surgery than that proposed in the updated 7th edition of the AJCC TNM staging system, we analyzed open SEER data further to clarify the impact of different T and N stage weights on survival. In doing so, we propose a revised scheme for the 7th edition of the AJCC TNM staging system.
MATERIALS AND METHODS
Open SEER population-based data from 1992 to 2004 were reviewed[2,3], and data on colon cancer and rectal cancer were analyzed separately. Patients with stage 0 and IV disease were excluded from the study. 5-year OS rate data were extracted according to 25 combinations of the T stage (1 = T1, 2 = T2, 3 = T3, 4 = T4a, and 5 = T4b) and N stage (0 = N0, 1 = N1a, 2 = N1b, 3 = N2a, and 4 = N2b). Stage N1c (tumor deposit) was also excluded from the study because no data were available.
As T and N stage scores are independent variables, and 5-year OS rates are dependent variables, three-dimensional (3D) scatter plots were constructed to demonstrate the relationships of the T stage, N stage and OS. In addition, multiple linear regressions were calculated to elucidate the quantitative relationships of these parameters. For example:
OS = (c-b1 × T - b2 × N) × 100%
where c is the survival constant, and b1 and b2 are the mean coefficients of regression of T and N. According to the coefficients of regression, we get:
αOS/αT = b1, αOS/αN = b1
This means that when the T stage changes by one unit, the relative influence on OS is b1, and when the N stage changes by one unit, the relative influence on OS is b2. Therefore, the relative influence on OS of the T stage and N stage is b1:b2. Since OS is only influenced by 2 indicators, T and N, according to the relative influence on OS of T and N, we can calculate the normalization weights of the indicators T and N. For example, the weight of T is:
WT = b1/(b1 + b2) × 100%
and the weight of N is:
WN = 1 - WT.
The scores of TN combinations are the scores of each stage multiplied by its weight. For example, TN scores for T4aN0 = WT × 4 + WN × 0, and TN scores for T2N2b = WT × 2 + WN × 4 (WT is the weight of T, and WN is the weight of N). This concept was derived from a comprehensive evaluation of all the available data, which balances the various indicators. Using this method, the scores of 25 TN combinations of T stage and N stage were calculated.
Subsequently, we used cluster analysis (also called group analysis; a statistical analysis method for studying the classification of samples or indicators[5]) of the TN scores to rearrange the TNM staging system.
Statistical analysis
All statistical analyses were performed using SPSS® version 16.0. A P value < 0.05 was considered statistically significant.
RESULTS
Multiple linear regressions of T stage, N stage and OS
Both T and N stages significantly affect the OS of patients with colorectal cancer. The 3D scatter plots of T and N stages and 5-year OS for colon cancer are shown in Figure 2A and B. The multiple regression equation for colon cancer is: OS = (97.432 - 10.56T - 7.812N) × 100%. The relative weight of T = 0.58, and the relative weight of N = 0.42. These calculations indicate that the T stage affects colon cancer survival more significantly than the N stage.
Figure 2.
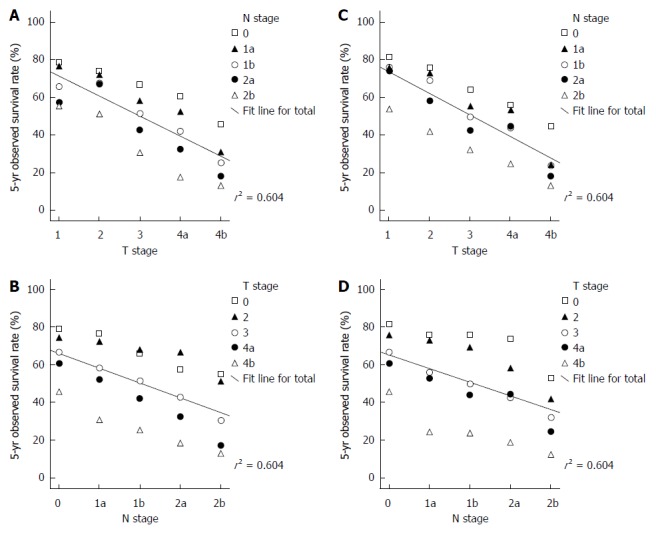
Scatter plots for T stage, N stage and the 5-year observed survival of colorectal cancer patients. A: Highlight of the T stage of colon cancer; B: Highlight of the N stage of colon cancer; C: Highlight of the T stage of rectal cancer; D: Highlight of the N stage of rectal cancer.
The 3D scatter plots of T and N stages and 5-year OS for rectal cancer are shown in Figure 2C and D. The multiple regression equation for rectal cancer is: OS = (99.108 - 11.356T - 7.194N) × 100%. The relative weight of T = 0.61 and the relative weight of N = 0.39. Thus, the T stage appears to have greater weight in rectal cancer than in colon cancer.
TN scores and cluster analysis
The 25 combinations of T and N stages and the corresponding 5-year OS of colon cancer and rectal cancer are shown in Tables 1 and 2, respectively. According to the TN scores, the TNM staging system can be rearranged to stage I (TN score ≤ 1.0), stage II (1.0 < TN score ≤ 2.0), stage IIIa (2.0 < TN score ≤ 3.0), stage IIIb (3.0 < TN score ≤ 4.0), and stage IIIc (TN score > 4.0). The proposed TNM staging system according to these TN scores fits well with the 5-year OS of colorectal cancer patients after potentially curative surgery (Figures 3 and 4). The summary of our proposed TNM staging system is shown in Table 3.
Table 1.
Tumor node scores and cluster analysis for colon cancer
| AJCC 7th ed TNM stage | TN combinations | Patients (n) | TN score | 5-yr OS | Proposed TNM stage | TN combinations | Patients (n) | TN score | 5-yr OS |
| I | T1N0 | 10930 | 0.58 | 78.7% | I | T1N0 | 10930 | 0.58 | 78.7% |
| T2N0 | 12931 | 1.16 | 74.3% | T1N1a | 643 | 1.00 | 76.7% | ||
| IIa | T3N0 | 40338 | 1.74 | 66.7% | II | T2N0 | 12931 | 1.16 | 74.3% |
| IIb | T4aN0 | 5020 | 2.32 | 60.6% | T1N1b | 325 | 1.42 | 65.8% | |
| IIc | T4bN0 | 3088 | 2.90 | 45.7% | T2N1a | 1270 | 1.58 | 72.1% | |
| IIIa | T1N1a | 643 | 1.00 | 76.7% | T3N0 | 40338 | 1.74 | 66.7% | |
| T2N1a | 1270 | 1.58 | 72.1% | T1N2a | 77 | 1.84 | 57.4% | ||
| T1N1b | 325 | 1.42 | 65.8% | T2N1b | 896 | 2.00 | 67.7% | ||
| T2N1b | 896 | 2.00 | 67.7% | IIIa | T3N1a | 8759 | 2.16 | 58.2% | |
| T1N2a | 77 | 1.84 | 57.4% | T1N2b | 27 | 2.26 | 55.0% | ||
| IIIb | T2N2a | 300 | 2.41 | 66.6% | T4aN0 | 5020 | 2.32 | 60.6% | |
| T3N1a | 8759 | 2.16 | 58.2% | T2N2a | 300 | 2.41 | 66.6% | ||
| T4aN1a | 1311 | 2.74 | 52.2% | T3N1b | 9107 | 2.58 | 51.7% | ||
| T3N1b | 9107 | 2.58 | 51.7% | T4aN1a | 1311 | 2.74 | 52.2% | ||
| T1N2b | 27 | 2.26 | 55.0% | T2N2b | 95 | 2.84 | 51.0% | ||
| T2N2b | 95 | 2.84 | 51.0% | T4bN0 | 3088 | 2.90 | 45.7% | ||
| T4aN1b | 1460 | 3.16 | 42.1% | T3N2a | 5331 | 3.00 | 42.8% | ||
| T3N2a | 5331 | 3.00 | 42.8% | IIIb | T4aN1b | 1460 | 3.16 | 42.1% | |
| IIIc | T4aN2a | 982 | 3.58 | 32.5% | T4bN1a | 845 | 3.32 | 30.6% | |
| T3N2b | 3235 | 3.42 | 30.4% | T3N2b | 3235 | 3.42 | 30.4% | ||
| T4bN1a | 845 | 3.32 | 30.6% | T4aN2a | 982 | 3.58 | 32.5% | ||
| T4bN1b | 929 | 3.74 | 25.4% | T4bN1b | 929 | 3.74 | 25.4% | ||
| T4bN2a | 730 | 4.16 | 18.3% | T4aN2b | 671 | 4.00 | 17.5% | ||
| T4aN2b | 671 | 4.00 | 17.5% | IIIc | T4bN2a | 730 | 4.16 | 18.3% | |
| T4bN2b | 653 | 4.58 | 12.9% | T4bN2b | 653 | 4.58 | 12.9% |
OS: Observed survival. AJCC: American Joint Committee on Cancer; TNM: Tumor node metastasis.
Table 2.
Tumor node scores and cluster analysis for rectal cancer
| AJCC 7th ed TNM stage | TN combinations | Patients (n) | TN score | 5-yr OS | Proposed TNM stage | TN combinations | Patients (n) | TN score | 5-yr OS |
| I | T1N0 | 3348 | 0.61 | 81.4% | I | T1N0 | 3348 | 0.61 | 81.4% |
| T2N0 | 6613 | 1.22 | 75.7% | T1N1a | 274 | 1.00 | 75.7% | ||
| IIa | T3N0 | 10615 | 1.83 | 64.0% | II | T2N0 | 6613 | 1.22 | 75.7% |
| IIb | T4aN0 | 818 | 2.44 | 55.7% | T1N1b | 170 | 1.39 | 75.9% | |
| IIc | T4bN0 | 769 | 3.05 | 44.7% | T2N1a | 923 | 1.61 | 72.7% | |
| IIIa | T1N1a | 274 | 1.00 | 75.7% | T1N2a | 62 | 1.78 | 73.8% | |
| T2N1a | 923 | 1.61 | 72.7% | T3N0 | 10615 | 1.83 | 64.0% | ||
| T1N1b | 170 | 1.39 | 75.9% | T2N1b | 641 | 2.00 | 68.9% | ||
| T2N1b | 641 | 2.00 | 68.9% | IIIa | T1N2b | 24 | 2.17 | 53.2% | |
| T1N2a | 62 | 1.78 | 73.8% | T3N1a | 2758 | 2.22 | 55.4% | ||
| IIIb | T2N2a | 302 | 2.39 | 58.2% | T2N2a | 302 | 2.39 | 58.2% | |
| T3N1a | 2758 | 2.22 | 55.4% | T4aN0 | 818 | 2.44 | 55.7% | ||
| T4aN1a | 218 | 2.83 | 53.2% | T3N1b | 3029 | 2.61 | 49.7% | ||
| T3N1b | 3029 | 2.61 | 49.7% | T2N2b | 120 | 2.78 | 41.7% | ||
| T1N2b | 24 | 2.17 | 53.2% | T4aN1a | 218 | 2.83 | 53.2% | ||
| T2N2b | 120 | 2.78 | 41.7% | T3N2a | 1964 | 3.00 | 42.5% | ||
| T4aN1b | 262 | 3.22 | 43.9% | T4bN0 | 769 | 3.05 | 44.7% | ||
| T3N2a | 1964 | 3.00 | 42.5% | IIIb | T4aN1b | 262 | 3.22 | 43.9% | |
| IIIc | T4aN2a | 199 | 3.61 | 44.3% | T3N2b | 1791 | 3.39 | 32.0% | |
| T3N2b | 1791 | 3.39 | 32.0% | T4bN1a | 201 | 3.44 | 24.4% | ||
| T4bN1a | 201 | 3.44 | 24.4% | T4aN2a | 199 | 3.61 | 44.3% | ||
| T4bN1b | 222 | 3.83 | 24.0% | T4bN1b | 222 | 3.83 | 24.0% | ||
| T4bN2a | 156 | 4.22 | 18.5% | T4aN2b | 198 | 4.00 | 24.5% | ||
| T4aN2b | 198 | 4.00 | 24.5% | IIIc | T4bN2a | 156 | 4.22 | 18.5% | |
| T4bN2b | 152 | 4.61 | 12.3% | T4bN2b | 152 | 4.61 | 12.3% |
OS: Observed survival; AJCC: American Joint Committee on Cancer; TNM: Tumor node metastasis.
Figure 3.
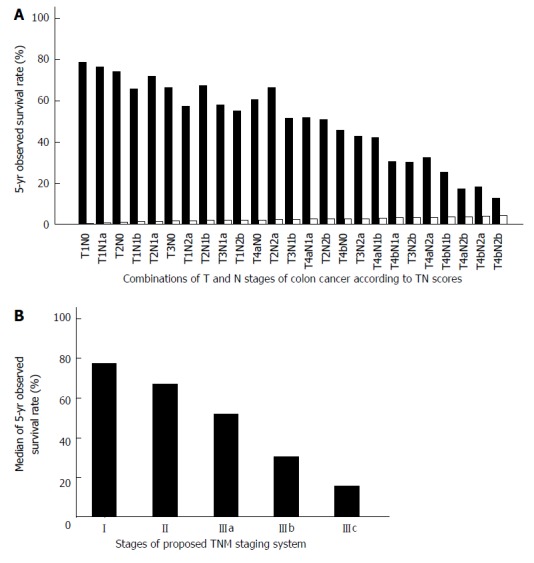
Relationship between survival of colon cancer patients and stages of the revised tumor node metastasis staging system. A: The 5-year observed survival rate of colon cancer patients for combinations of T and N stages according to TN scores. The observed survival rate is represented by the solid black grids. TN scores are represented by the white grids; B: The median 5-year observed survival rate of colon cancer patients for the proposed tumor node metastasis (TNM) staging system according to TN scores.
Figure 4.
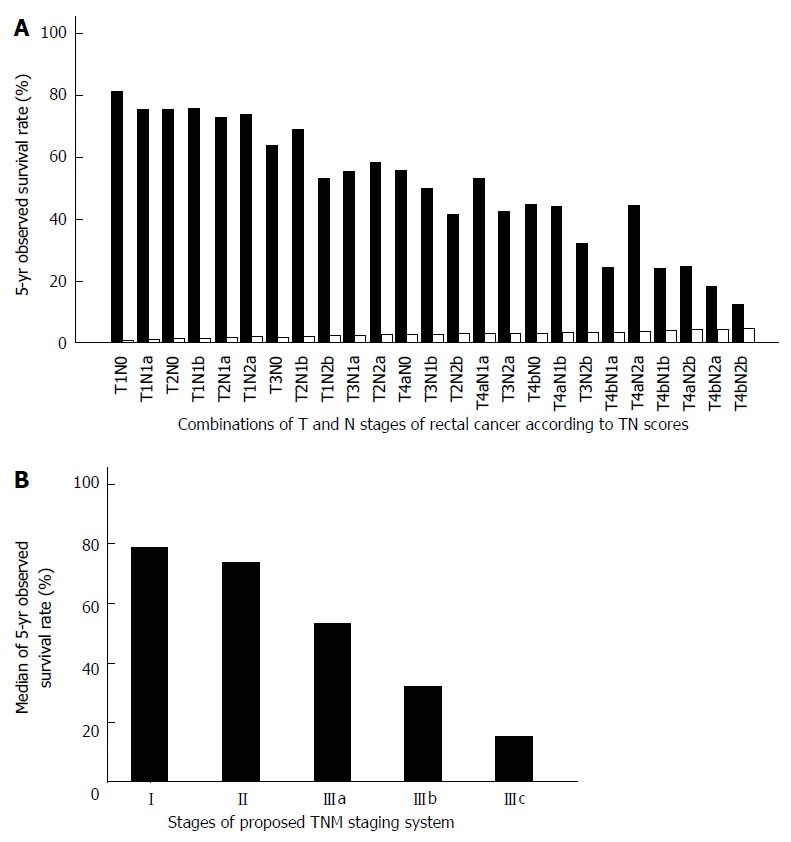
Relationship between survival of rectal cancer patients and stages of the revised tumor node metastasis staging system. A: The 5-year observed survival rate of rectal cancer patients for combinations of T and N stages according to TN scores. The observed survival rate is represented by the grey grids. TN scores are represented by the white grids; B: The median 5-year observed survival rate of rectal cancer patients for the proposed tumor node metastasis (TNM) staging system according to TN scores.
Table 3.
Summary of proposed tumor node metastasis staging system
| Proposed TNM stage for colon cancer | TN combinations | Proposed TNM stage for rectal cancer | TN combinations |
| I | T1N0-1a | I | T1N0-1a |
| II | T1N1b-2a | II | T1N1b-2a |
| T2N0-1b | T2N0-1b | ||
| T3N0 | T3N0 | ||
| IIIa | T1N2b | IIIa | T1N2b |
| T2N2a-2b | T2N2a-2b | ||
| T3N1a-2a | T3N1a-2a | ||
| T4aN0-1a | T4aN0-1a | ||
| T4bN0 | |||
| IIIb | T3N2b | IIIb | T3N2b |
| T4aN1b-2b | T4aN1b-2b | ||
| T4bN1a-1b | T4bN0-1b | ||
| IIIc | T4bN2a-2b | IIIc | T4bN2a-2b |
TNM: Tumor node metastasis.
DISCUSSION
The present study and Mori’s report[4] both found that the 7th edition of the AJCC TNM staging system cannot accurately predict the survival of patients with colorectal cancer, especially for stages IIc and IIIa[4]. It is easy to misinterpret the prognosis of IIc as being better than that of IIIa. This defect of the current TNM staging system originates from the inherent notion that lymph node metastases (N stage) affect the prognosis more significantly than local invasion (T stage), and this opinion is reflected in the current classification of stages II and III colorectal cancer. Although patients with T4bN0 have a lower 5-year survival rate than many stage IIIa/b patients, they are still currently classified as stage IIc. Recent data have shown that adjuvant chemotherapy improves both the progression-free survival and overall survival of patients with stage II colon cancer[6], implying that there must be some problems with the existing staging system, especially in the identification of stages II and III.
There are 2 considerations that may be the root of the problem. Firstly, the TNM staging system traditionally relies on anatomical staging. The current system omits survival benefit because of the advances in surgery and adjuvant therapy in recent decades. As is well known, chemotherapy with oxaliplatin and fluorouracil and adjuvant radiotherapy have significantly improved the prognosis of patients with stage II/III colorectal cancer[7]. The data on which the 7th edition of the AJCC staging system for colorectal cancer were based were derived from the SEER outcome data of 1998-2002, well before the findings of adjuvant trials in stages II and III became available, and they certainly do not reflect current practice and prognosis[8,9].
Secondly and more importantly, our data indicate that the weight of the N stage has been over-estimated, and that this has been accompanied by an under-estimation of the T stage. This traditional concept needs to be reconsidered and correlated with contemporary survival data. The widespread application of complete mesocolic excision (CME) and total mesorectal excision (TME) standardized colorectal cancer surgery, with their greater lymph node yields, has reduced loco-regional recurrences and thereby improved survival rates[10,11]. On the other hand, patients with locally advanced (especially T4) tumors have a higher risk of local recurrence and peritoneal and distant metastases, resulting in poorer outcomes[12].
The findings of the present study support the hypothesis that the weight of T stage has been under-estimated in colorectal cancer patients. The relative weights of the T and N stages were 0.58 and 0.42, respectively, in colon cancer, and 0.61 and 0.39, respectively, in rectal cancer. To confirm that the T stage should carry more weight in the TNM staging system, our study calculated 25 categories of TN scores according to different T/N weightings. The survival rate decreased with increasing TN scores with good linear relationships. In addition, the proposed rearrangement of TNM stages according to the TN scores also showed good linear relationships with survival. Consequently, the traditional classification system, which relied more on the N stage, needs to be revised to place more emphasis on the T stage.
It is worth noting that the T stage has even greater weight in rectal cancer than in colon cancer, which probably indicates a higher risk and worse local recurrence consequences in rectal cancer. As a result, we propose that T4bN0 should be reclassified to stage IIIa for colon cancer, but to stage IIIb for rectal cancer. In addition, T1N1a should be reclassified to stage I, rather than stage IIIa in the 7th edition of the AJCC TNM staging system, and T2N1a-1b and T1N1b-2a should be reclassified to stage II, rather than stage IIIa in the 7th edition of the AJCC TNM staging system. Our proposed rearrangement of the TNM staging system reflects the significance of the T stage in colorectal cancer and abandons the rigid classification by lymph node status, similar to the TNM staging system for gastric cancer[13]. It should be noted, however, that there may be potential biases arising from the SEER database, because the survival of patients can also be affected by factors such as surgical procedures, adjuvant therapies, and the number of lymph nodes, etc. Consequently, the reliability of our findings should be validated not only with regard to individual SEER data and other datasets, but also with regard to other staging systems, such as the 5th edition of the TNM staging system, which is used in Europe[4,14,15].
As mentioned above, patients who previously were classified as stage IIIa (T1-2N1 and T1N2a) should now be reclassified as stage I or II. As the use of adjuvant therapy remains problematic for patients with colon cancer, information on treatment approaches was omitted in our study. In this regard, there are 2 factors to be considered. Firstly, stage IIIa patients receive adjuvant therapy to improve their prognosis. Secondly, patients who have a good prognosis do not need either adjuvant therapy or intensive adjuvant therapy. Considering that patients with both stages II and III rectal cancer previously received adjuvant radiochemotherapy, some stage IIIa patients still have a better prognosis than those in stage II. It is speculated that some stage IIIa colorectal cancer patients have a naturally good prognosis, no matter which treatment is offered. As stage III patients traditionally receive adjuvant therapy, this issue should be clarified in future trials.
The question as to how to classify tumor deposits was not considered in this study. Peri-tumor deposits first emerged as prognostic indicators in the 5th edition of the AJCC TNM staging system in 1997[14]. A tumor nodule > 3 mm in diameter in the perirectal or pericolic adipose tissue, without histologic evidence of residual lymph node tissue, is classified as a regional lymph node metastasis (N category). A tumor nodule up to 3 mm in diameter is classified within the T category. These definitions are called the 3 mm rule. In the 6th edition of the AJCC TNM staging system published in 2002, the 3 mm rule was replaced by the contour rule[16]. A tumor nodule without histologic evidence of a residual lymph node is now classified within the N category if the nodule has the form and smooth contour of a lymph node. If the nodule has an irregular contour, it is classified within the T category. In the 7th edition of the AJCC TNM staging system, the definition of tumor deposits is left to the discretion of the pathologist[1]. Colorectal cancer with an adjacent tumor deposit but no lymph node metastasis is now classified as N1c. The definition and classification of tumor deposits have kept changing in recent editions of TNM staging systems on the basis of expert consensus instead of high level evidence. There is some evidence to support the view that the 5th edition of TNM staging system is the best choice to define peri-tumoral tumor deposits in colorectal cancer[17,18].
The average number of lymph nodes within the SEER dataset was not well defined. Of the 109953 colon cancer cases in the SEER dataset, 13 or more lymph nodes were harvested in 37% of patients. As a result, it is easy to see how understaging can occur within the SEER dataset, especially for stage II patients. However, in terms of the average number of lymph nodes, the SEER dataset is equivalent to other databases. For example, in US hospital data, more than 60% of hospitals (792/1296) failed to archive a compliance benchmark for the 12-node measure[19]. A nationwide population-based study in the Netherlands showed that the median number of lymph nodes harvested in colon cancer was only 8[20]. However, the latest study based on SEER data shows that the detection of fewer lymph nodes does not result in understaging. Although the proportion of patients with 12 or more lymph nodes has increased over the period 1988 to 2008 (from 34.6% in 1988-1990 to 73.6% in 2006-2008, P < 0.001), this has not resulted in a significant overall increase in the proportion of node-positive cases (40% in 1988-1990 vs 42% in 2006-2008, P = 0.53). Therefore the “upstaging” hypothesis as the primary basis for improved survival in patients with more lymph nodes is questionable[21].
It is difficult to evaluate the accurate stage for many patients with advanced rectal cancer who receive neoadjuvant chemoradiotherapy. While some cases of rectal cancer will respond and downstage, a stratified analysis was not possible in this study because adjuvant and palliative treatments are anfractuous between patients, and also because fewer patients received neoadjuvant treatment at the time the SEER data were collected (1998-2002). As a result, rectal cancer patients who did or did not receive neoadjuvant treatment were included together in this study without stratification. There are 3 reasons supporting the lack of stratification in this study. Firstly, the outcomes of rectal cancer after preoperative treatment are decided by the post-treatment pathologic stage rather than preoperative stage[22]. Secondly, pretreatment stages are not absolutely accurate, even when magnetic resonance imaging (MRI) or endorectal ultrasound have been employed[23]. Thirdly, the proposed TNM staging system for rectal cancer in this study is remarkably similar to that for colon cancer, but with no stratification for treatment. T4b is the exception in rectal cancer. Although our study showed that T4b has greater weight in rectal cancer than in colon cancer, it is not clear whether this is an intrinsic fact or a fault due to the lack of stratification for treatment. As mentioned above, the influence of treatment is difficult to assess considering the various treatments, especially after cancer recurrences. Prospective, randomized clinical trials comparing the prognosis of patients with high T stages but negative lymph nodes and patients with low T stages and low-positive N stages who received the same adjuvant therapy may be helpful to clarify this problem. It is worth noting that the MERCURY study found that postoperative ypT stage and circumferential resection margin (CRM), but not the post-treatment N status, were important predictors of the outcome of locally-advanced rectal cancer after neoadjuvant therapy[24,25]. This finding also implies that the T stage may affect the outcome of rectal cancer more significantly than the N stage. In the future, a separate staging system should be made for rectal cancer which emphasizes the T stage and the CRM status.
In conclusion, in the present study, we found that the T stage affects colorectal cancer survival more significantly than the N stage. Therefore, it is reasonable to stratify TNM stages according to relative T and N weightings. In our proposed rearrangement of the TNM staging system, T4bN0 should be reclassified as stage IIIa in colon cancer and stage IIIb in rectal cancer, while patients previously classified as IIIa (T1-2N1 and T1N2a) should be reassigned to stage I or II. Our proposed TNM staging system based on relative T and N weightings should be examined in future prospective, randomized controlled trials and stratified studies.
ACKNOWLEDGMENTS
All authors state that there are no financial or personal relationships with any persons or organizations to disclose. We gratefully thank Jerry J Lou and Prof. Qun Lu from UCLA for their revisions of this manuscript.
COMMENTS
Background
The American Joint Committee on Cancer (AJCC) TNM staging system is widely used to predict the prognosis for patients with colorectal cancer and to guide adjuvant therapy after potentially curative surgery. The 7th edition of the AJCC TNM staging system for colorectal cancer, which was published in 2010, cannot predict survival linearly by stage. For example, the 5-year observed survival of stage IIIa patients (T1-2N1 and T1N2a) matches that of stage I patients. On the other hand, stage IIc patients have a poorer prognosis, equivalent to that of stage IIIb patients.
Research frontiers
In the 7th edition TNM staging system, stage T4 was stratified to T4a and T4b, and patients with T4bN0 lesions were reclassified from stage IIb to IIc. These changes reflect the fact that the T stage affects survival in colorectal cancer patients more significantly than previously believed.
Innovations and breakthroughs
Authors found that for colon cancer, the relative T and N stage weights were 0.58 and 0.42, respectively, and for rectal cancer, the relative T and N stage weights were 0.61 and 0.39, respectively. It appears that T stage has greater weight in rectal cancer than in colon cancer (which would be consistent with the greater risk of local recurrence seen with rectal cancer). Moreover, the authors propose a revised scheme for the 7th edition tumor node metastasis (TNM) staging system. Consequently, T4bN0 is classified to IIIa in colon cancer, but to IIIb in rectal cancer. It is the first try to revise established TNM staging system for colorectal cancer by shaking the keystone of lymph nodes status (N stage).
Applications
In the present study, authors found that the T stage affects colorectal cancer survival more significantly than the N stage. Therefore, it is reasonable to stratify TNM stages according to relative T and N weightings in future revision of the TNM staging system.
Terminology
Cluster analysis, also called group analysis, is a statistical analysis method for studying the classification of samples or indicators. In this study, the TNM staging system is rearranged according to the cluster analysis results of TN scores.
Peer review
The authors analyzed the relationship between the survival and stages of colorectal cancer using AJCC 7th edition TNM staging system. They found the 7th edition TNM staging system for colorectal cancer cannot predict survival linearly by stage, but the relative weight of T stage has more impact on patients survival based on multiple linear regression analysis. Even the criteria used in the TNM system have varied over time according to the different editions that AJCC and UICC have released, one aim for adopting a global standard is to give an indication of prognosis and assist in the evaluation of the results of treatment. To predict the survival of colorectal cancer more accurate, more and new factors should be introduced into the evaluation system. The authors found the relative weight of T/N stage is a factor that could predict survival effectively. It is novel for TNM staging system.
Footnotes
P- Reviewers: Lu F, Tepes B S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Edge SB, Byrd DR, Compton , CC , Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 2.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256–263. doi: 10.1200/JCO.2009.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori T. A comparison of the new (planned) TNM classification and Japanese general rule for staging colorectal cancer. Cancer Invest. 2010;28:387–392. doi: 10.3109/07357900903287055. [DOI] [PubMed] [Google Scholar]
- 5.Frades I, Matthiesen R. Overview on techniques in cluster analysis. Methods Mol Biol. 2010;593:81–107. doi: 10.1007/978-1-60327-194-3_5. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie S, Nelson R, Mailey B, Lee W, Chung V, Shibata S, Garcia-Aguilar J, Kim J. Adjuvant chemotherapy improves survival in patients with American Joint Committee on Cancer stage II colon cancer. Cancer. 2011;117:5493–5499. doi: 10.1002/cncr.26245. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi L, Morelli F, Cinieri S, Santini D, Silvestris N, Fazio N, Orlando L, Tonini G, Colucci G, Maiello E. Adjuvant colon cancer chemotherapy: where we are and where we’ll go. Cancer Treat Rev. 2010;36 Suppl 3:S34–S41. doi: 10.1016/S0305-7372(10)70018-9. [DOI] [PubMed] [Google Scholar]
- 8.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 9.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 10.Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354–364; discussion 364-365. doi: 10.1111/j.1463-1318.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 11.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 12.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute. Available from: http://seer.cancer.gov/csr/1975_2008/
- 13.National Comprehensive Cancer Network. Gastric Cancer Version 2, 2011. Available from: http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
- 14.Fleming ID, Cooper JS, Henson DE. AJCC Cancer Staging Manual. 5th ed. Philadelphia, PA: Lippincott Raven; 1997. [Google Scholar]
- 15.Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, Cottier B, Poston G. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page D, Fleming ID. AJCC Staging Handbook. 6th ed. New York: Springer; 2002. [Google Scholar]
- 17.Quirke P, Williams GT, Ectors N, Ensari A, Piard F, Nagtegaal I. The future of the TNM staging system in colorectal cancer: time for a debate? Lancet Oncol. 2007;8:651–657. doi: 10.1016/S1470-2045(07)70205-X. [DOI] [PubMed] [Google Scholar]
- 18.Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HC, Påhlman L, Brown JM, Guillou PJ, Quirke P. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011;29:2487–2492. doi: 10.1200/JCO.2011.34.6429. [DOI] [PubMed] [Google Scholar]
- 19.Bilimoria KY, Bentrem DJ, Stewart AK, Talamonti MS, Winchester DP, Russell TR, Ko CY. Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst. 2008;100:1310–1317. doi: 10.1093/jnci/djn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elferink MA, Siesling S, Visser O, Rutten HJ, van Krieken JH, Tollenaar RA, Lemmens VE. Large variation between hospitals and pathology laboratories in lymph node evaluation in colon cancer and its impact on survival, a nationwide population-based study in the Netherlands. Ann Oncol. 2011;22:110–117. doi: 10.1093/annonc/mdq312. [DOI] [PubMed] [Google Scholar]
- 21.Parsons HM, Tuttle TM, Kuntz KM, Begun JW, McGovern PM, Virnig BA. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA. 2011;306:1089–1097. doi: 10.1001/jama.2011.1285. [DOI] [PubMed] [Google Scholar]
- 22.Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB, Goodman KA, Minsky BD, Wong WD, Weiser MR. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113:57–64. doi: 10.1002/cncr.23516. [DOI] [PubMed] [Google Scholar]
- 23.Fleming FJ, Påhlman L, Monson JR. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum. 2011;54:901–912. doi: 10.1007/DCR.0b013e31820eeb37. [DOI] [PubMed] [Google Scholar]
- 24.Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 25.Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]


