Abstract
Context:
Methodological shortcomings often compromise investigations into the effects of primary somatostatin-analog treatment on tumor size in acromegaly. There are also limited data for the long-acting lanreotide formulation.
Objective:
The aim of the study was to better characterize the effects of primary lanreotide Autogel treatment on tumor size in patients with GH-secreting macroadenomas.
Design:
PRIMARYS was a 48-week, multicenter, open-label, single-arm study.
Setting:
The study was conducted at specialist endocrine centers.
Patients:
Treatment-naïve acromegalic patients with GH-secreting macroadenomas participated in the study.
Intervention:
Lanreotide Autogel 120 mg was administered sc every 28 days (without dose titration).
Outcome Measures:
The primary endpoint was the proportion of patients with clinically significant (≥20%) tumor volume reduction (TVR) at week 48/last post-baseline value available using central assessments from three readers. The null hypothesis (H0) for the primary endpoint was that the proportion with TVR was ≤55%. Secondary endpoints included: TVR at other time points, GH and IGF-1, acromegalic symptoms, quality of life (QoL), and safety.
Results:
Sixty-four of 90 (71.1%) patients completed the study. Clinically significant TVR at 48 weeks/last post-baseline value available was achieved by 62.9% (95% confidence interval, 52.0, 72.9) of 89 patients in the primary analysis (intention-to-treat population; H0 not rejected) and 71.9–75.3% in sensitivity (n = 89) and secondary analyses (n = 63) (H0 rejected). At 12 weeks, 54.1% had clinically significant TVR. Early and sustained improvements also occurred in GH and IGF-1, acromegalic symptoms, and QoL. No patients withdrew due to gastrointestinal intolerance.
Conclusions:
Primary treatment with lanreotide Autogel, administered at 120 mg (highest available dose) without dose titration, in patients with GH-secreting macroadenomas provides early and sustained reductions in tumor volume, GH and IGF-1, and acromegalic symptoms, and improves QoL.
Long-acting somatostatin analogs are established treatments for patients with acromegaly after unsuccessful pituitary surgery (1). Meanwhile, primary therapy with these agents is recommended principally for a subgroup of patients with larger tumors when a surgical cure is unlikely (2), and additionally if surgery is refused or contraindicated (1, 3, 4). Studies have adequately demonstrated that primary treatment with octreotide long-acting release (LAR) and lanreotide Autogel improves symptoms and attenuates or normalizes GH and IGF-1 hypersecretion (5, 6). Although reducing the tumor burden is also critical to therapeutic success in this setting, the evidence for tumor-shrinkage effects with long-acting somatostatin analogs is less compelling. Moreover, there are indications that biochemical and tumor-shrinkage effects are dissociated in acromegaly (7, 8). Accordingly, it is particularly important to have robust studies specifically designed to investigate the effects of somatostatin analogs on tumor size. Such studies should be undertaken with treatment-naïve patients, using tumor size as the primary endpoint, and with efforts made to reduce variability in tumor measurements. Few studies to date incorporate these features (7, 9–11). Many are also compromised by mixed-treatment settings (eg, variable doses) and patient populations (eg, micro- and macroadenomas), and by selecting patients responsive to somatostatin analogs. Meta-analyses based on these studies are then additionally hampered by methodological heterogeneity among studies, such as assessment intervals, study durations, treatment formulations, and study populations. Against this background of heterogeneity, authors of a recent meta-analysis concluded that first-line octreotide treatment may produce tumor shrinkage in up to two-thirds of patients (9). It seems likely that lanreotide has a comparable effect (10), although to date fewer studies have investigated its effects on tumor size.
The aim of the PRIMARY treatment of macroadenomas in acromegaly with Somatuline (PRIMARYS) study was to better characterize the effects of primary lanreotide Autogel on tumor size. Accordingly, this was a prospective study with change in tumor volume as the primary endpoint and pituitary magnetic resonance imaging (MRI) scans read centrally using rigorous prespecified methodologies to minimize measurement variability. Treatment was started and continued the highest available dose to improve dataset homogeneity and investigate the time to onset of effects. Patients were treatment-naïve, and all had GH-secreting macroadenomas.
Patients and Methods
Patients
Treatment-naïve men and women (age, 18–75 y) with acromegaly were eligible if mean GH levels (mean of five samples taken at 10- to 15-min intervals for patients with diabetes mellitus) or nadir GH (assessed by oral glucose tolerance test for all other patients) was > 1 μg/L and IGF-1 levels were above age- and sex-matched normal ranges (GH and IGF-1 levels analyzed centrally). All participants had macroadenomas (diameter ≥ 10 mm measured centrally by MRI) and no visual field defects (assessed using Goldmann and automated visual field perimeter examinations). Patients were not selected based on sensitivity to somatostatin analogs.
Patients were excluded if they had undergone or were likely to require pituitary surgery or radiotherapy; had been treated with a somatostatin analog, dopamine agonist, or GH receptor antagonist; or were likely to require these agents (other than lanreotide Autogel 120 mg). They were also excluded if there was cosecretion of prolactin at > 100 μg/L, optic nerve disease or any visual abnormality with risk of worsening during the study, acute/chronic severe renal insufficiency (glomerular filtration rate < 30 mL/min/1.73 m2), medical conditions that may have interfered with the study, allergy to gadolinium (MRI contrast agent), or hypersensitivity to lanreotide or drugs with similar chemical structure; and if they had taken/were scheduled to take an unlicensed drug in the previous 30 days. Women were excluded if they were not using acceptable methods of contraception or were pregnant or lactating.
Patients were withdrawn at any time if there was evidence of new visual field abnormalities or other safety concerns (withdrawal at investigator's discretion), insufficient IGF-1 response (IGF-1 level reduction < 10% at week 24 vs baseline or investigator's judgment that the response was inadequate), or postbaseline prolactin > 100 μg/L (for patients with baseline levels of 20–100 μg/L).
Study design and interventions
This 48-week, open-label, single-arm phase 3b study was conducted in 27 specialist endocrine centers in nine countries (Belgium, Czech Republic, Finland, France, Germany, Italy, The Netherlands, Turkey, United Kingdom) between May 20, 2008, and February 13, 2012. Twelve lanreotide Autogel injections were administered by deep sc injection, one every 28 days, using the highest available dose (120 mg) throughout the study (ie, no dose titration). Injections other than those taking place during study visits could be administered in the patient's home as part of the patient's normal care. The study is registered with ClinicalTrials.gov (NCT00690898) and EudraCT (2007–000155–34).
Patients provided written informed consent before study start, and the trial was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and all local regulatory requirements. Before trial initiation, the protocol, its amendments, consent form, study questionnaires, and the patient information leaflet were approved by institutional review boards. Protocol amendments occurring during the study are summarized in the Appendix (published as Supplemental Data on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Assessments and outcome measures after 12, 24, and 48 weeks
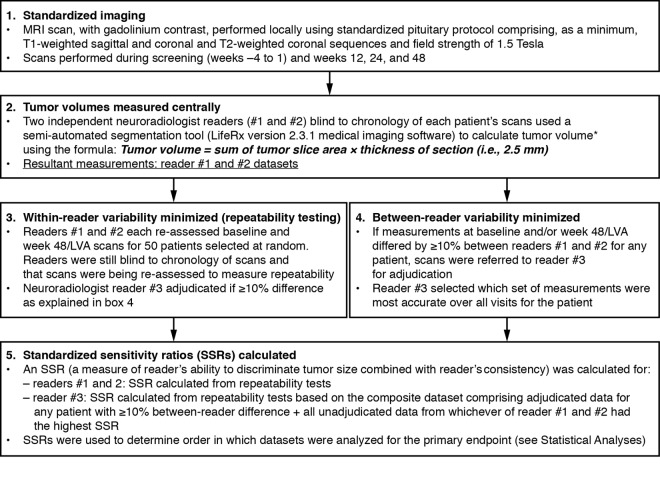
Study visits were conducted during the 4-week screening period and weeks 1 (baseline), 12, 24, and 48 (and early withdrawal, if applicable). MRI scans were performed during screening (used as baseline value for tumor volume) and weeks 12, 24, and 48 (and early withdrawal, if applicable). Tumor volumes were measured centrally by three neuroradiologists blind to the chronology of patients' scans using prespecified methods, including computer modeling of tumor volumes, to ensure consistent and unbiased measurements (Figure 1). Assessments did not incorporate measures of tumor extension.
Figure 1.
Prespecified methodology used to ensure consistent and unbiased evaluations of tumor volume. *, Reader defined the regions of interest on images of the first and final slices of the tumor, with Life Rx software determining regions of interest on images of intervening slices. Details of how the SSRs were calculated are reported in the Supplemental Data.
Hormone levels were determined centrally from blood samples taken under fasting conditions and before injections. IGF-1 levels were assessed from a single sample at each visit using a RIA (Esoterix/LabCorp Endocrine Sciences) with a lower limit of detection of 7.7 μg/L, a lower limit of quantitation of 15 μg/L, intra-assay precision of 5.3–14.1%, and interassay precision of 7.2–17.0%. Five consecutive samples taken at 10- to 15-min intervals were used to determine a mean GH level for each visit, and prolactin levels were assessed at each postscreening visit if the screening value was > 20 μg/L. GH and prolactin levels were measured with simultaneous one-step immunoenzymatic assays (Access Ultrasensitive GH and prolactin assays; Beckman Coulter Inc). The GH assay had a lower limit of detection of 0.002 μg/L, intra-assay precision of 1.9–3.8%, and interassay precision of 2.73–3.85%. For the prolactin assay, corresponding values were 1.0 μg/L, 2.9–4.6%, and 3.04–4.32%, respectively.
Acromegalic symptoms (headache, excessive perspiration, fatigue, soft tissue swelling, arthralgia) were rated by patients at each visit on a scale ranging from 0 to 8 (0, no symptoms; 8, severe incapacitating symptoms). Quality of life (QoL) was determined from patient-reported assessments at each postscreening visit using the AcroQoL questionnaire (except Finland and Turkey, where validated translations were not available).
Safety assessments included adverse events (AEs) collected throughout the study and physical examination and vital signs assessed at all visits. Hematology and biochemistry were assessed from blood samples at screening and week 48/premature withdrawal, with fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) levels assessed additionally at weeks 12 and 24. Gallbladder ultrasound was performed at screening and week 48/premature withdrawal, with additional examinations at any time in case of possible biliary symptoms.
The primary endpoint was the proportion of patients with ≥ 20% reduction in tumor volume at week 48 (or last postbaseline value available [LVA]). The null hypothesis (H0) for the primary endpoint was that the proportion is ≤ 55%, an arbitrary threshold in line with studies published at the time PRIMARYS was designed (7). Secondary efficacy endpoints included: tumor volume at weeks 12 and 24; GH and IGF-1 levels; proportions of patients achieving GH ≤ 2.5 and ≤ 1.0 μg/L and/or normalized IGF-1 (ie, within age- and sex-matched normal ranges); prolactin (for patients with baseline values of 20–100 μg/L); proportions of patients with improved/unchanged/worsened acromegaly symptoms vs baseline; change in AcroQoL scores; and safety.
Statistical analyses
The sample size required (n = 86) was calculated using A'Hern's exact method for single-stage designs (12) and assuming an H0 (null hypothesis) that the proportion of responders is ≤ 55% (with alternative hypothesis that the proportion is ≥ 72%), providing an α risk of 2% with 90% power. Enrollment of 100 patients was needed to obtain evaluable data for 86 patients.
A standardized sensitivity ratio (SSR) for tumor volume measurements was calculated for each reader (for detailed mathematical rationale and derivation, see Supplemental Data). In brief, the SSR measured readers' consistencies (based on SD values for each patient in repeatability tests) combined with sensitivities (mean absolute differences between final and baseline measures). The primary analysis for the primary endpoint was then conducted with the reader dataset with the highest SSR, using the intention-to-treat (ITT) population (patients receiving at least one injection of study medication and with at least one baseline efficacy assessment for the primary endpoint). The robustness of the primary analysis was examined by analyzing ITT data from the other two readers (sensitivity analyses). A secondary analysis was also performed using the per-protocol (PP) population (patients from the ITT population without major protocol deviations) for the reader with the highest SSR.
Descriptive statistics were used for secondary efficacy and safety endpoints. Secondary efficacy analyses were based only on the ITT population, except the proportions of patients reaching both GH and IGF-1 targets, which were also analyzed for the PP population. Safety analyses were based on the safety population (patients receiving at least one injection of study medication). Although missing data were not imputed, LVA assessments were compiled for all efficacy endpoints. Statistical analyses were performed using Statistical Analysis System software version 9.2 (SAS Institute, Inc).
Results
Patient disposition and baseline characteristics
Ninety patients received treatment, and 64 (71.1%) completed the study (Figure 2). Five patients experienced a total of five treatment-emergent AEs leading to premature withdrawal; of these, alopecia, intracranial hypotension (cerebral fluid leakage), and hypertension were considered treatment-related (hypophysectomy and increased IGF-1 level were not considered treatment related and were later coded as insufficient IGF-1 response).
Figure 2.
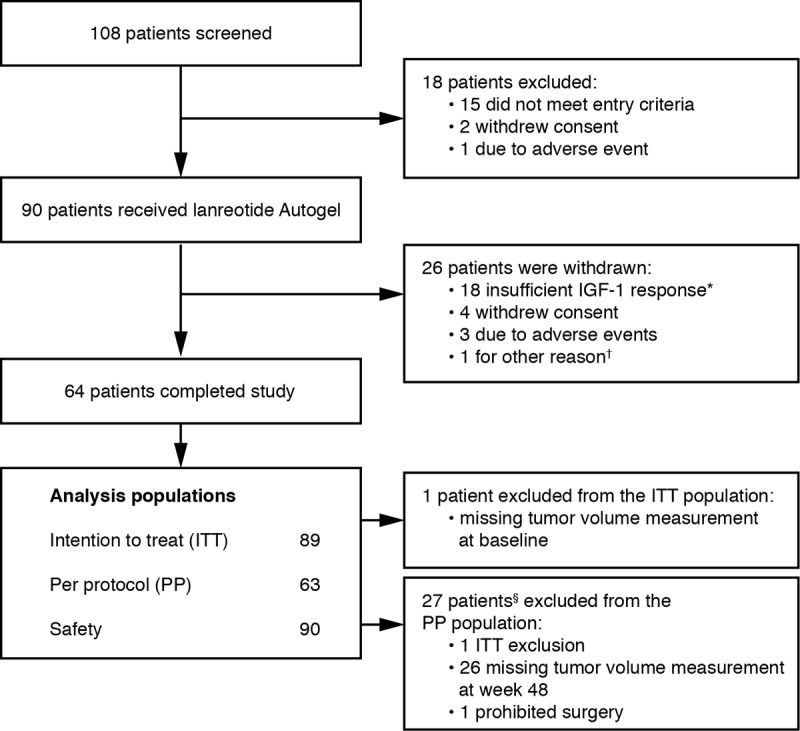
Flow of patients through the study. *, IGF-1 levels decreased by < 10% at week 24 vs baseline (as defined in the protocol) or investigator's judgment of insufficient response; †, patient not able to attend due to mobility issues; §, one patient had two major protocol violations.
Baseline characteristics are shown in Table 1. Nineteen patients (21.1%) had a history of diabetes mellitus at baseline. Of 87 patients undergoing baseline gallbladder ultrasound, four (4.6%) had sludge and six (6.9%) had gallstones. Compared with the ITT population, patients withdrawing because of an insufficient IGF-1 response had similar baseline characteristics but were slightly younger, and a greater proportion had a history of diabetes mellitus (6 of 18 patients; 33.3%).
Table 1.
Baseline Demographic and Disease Characteristics
| Characteristics | |
|---|---|
| No. of patients | 90 |
| Age, y | 49.5 (12.4) |
| Men:women, n (%) | 43 (47.8):47 (52.2) |
| BMI, kg/m2 | 27.7 (4.6) |
| Time since acromegaly diagnosis, da | 121.2 (149.9) |
| Maximum tumor diameter, mm | 19.0 (7.1) |
| Median (Q1–Q3) | 18.1 (13.9–21.9) |
| Maximum tumor volume, mm3 | 2739 (3263) |
| Median (Q1–Q3) | 1677 (867–3422) |
| GH level, μg/L | 15.0 (18.8) |
| Median (Q1–Q3) | 8.5 (4.0–16.6) |
| IGF-1 level, μg/L | 810 (300) |
| Median (Q1–Q3) | 785 (596–994) |
Abbreviation: Q1–Q3, quartiles 1–3. Data are expressed as mean (SD), unless stated otherwise, from the safety population.
Relative to baseline; n = 71.
Efficacy
Primary endpoint: proportions of patients with ≥ 20% reduction in tumor volume at week 48/LVA
Overall, 76% of tumor volume measurements were adjudicated. Reader no. 2 dataset had the highest SSR (0.65) and was used for primary ITT and secondary PP analyses; reader no. 1 and no. 3 datasets (SSRs, 0.57 and 0.52, respectively) were used for ITT sensitivity analyses.
The proportion of patients achieving ≥ 20% tumor volume reduction after 48 weeks/LVA was 62.9% with the primary ITT analysis, 75.3 and 71.9% with ITT sensitivity analyses, and 74.6% with the secondary PP analysis (Figure 3A). H0 was not rejected for the primary analysis because the lower confidence interval (CI) just included the arbitrary 55% threshold; H0 was rejected, however, for sensitivity and secondary analyses (Figure 3A). Tumor volume increased from baseline in nine patients; only two of these had increases > 20% (Figure 3B). (Two of the nine patients had IGF-1 changes between baseline and LVA < 10% [7.9% decrease; 9.8% increase]; by contrast, the range of IGF-1 reductions in the remaining seven patients was 20.8–71.4%.)
Figure 3.
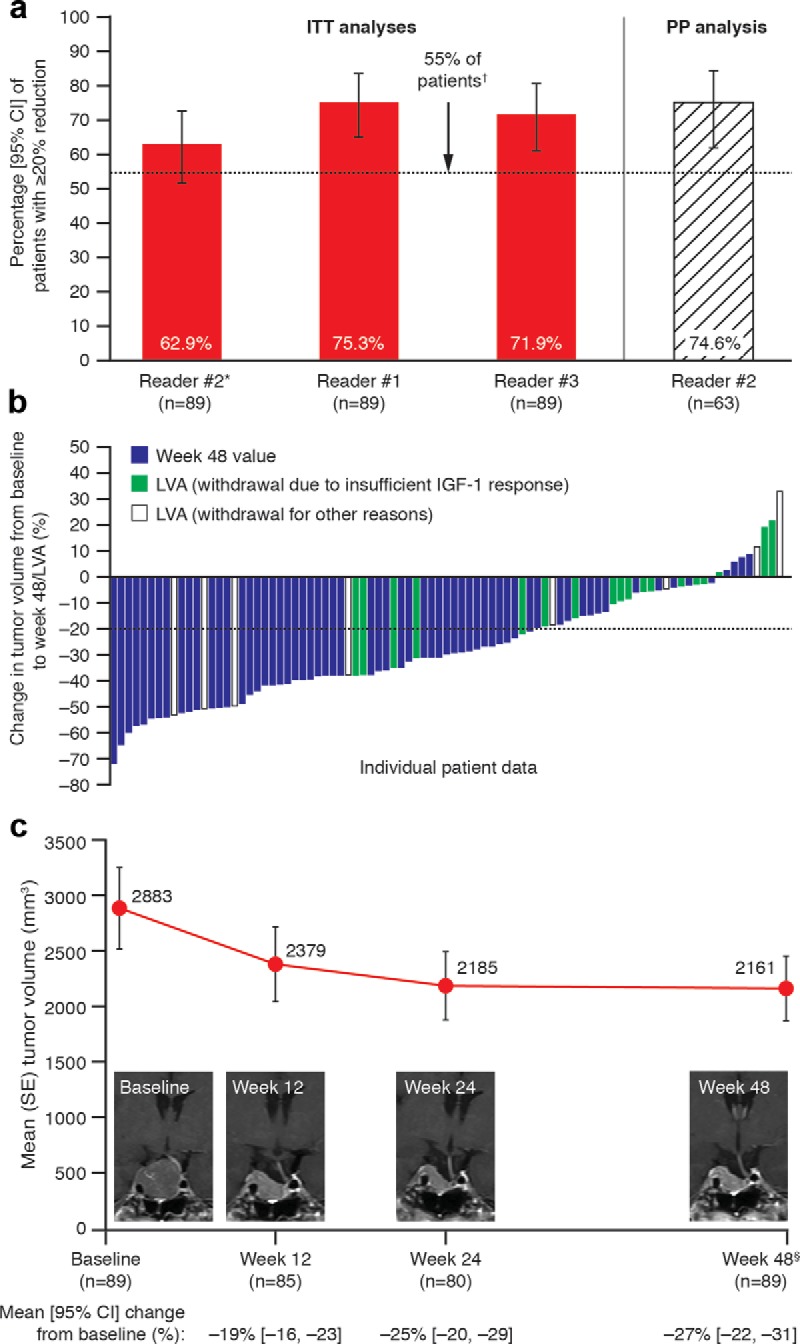
Reductions in tumor volume. A, Analyses of the proportions of patients with ≥ 20% reduction in tumor volume from baseline to week 48/LVA (primary endpoint; ITT and PP populations). B, Tumor volume reductions for individual patients (ITT population). C, Time course of changes in tumor volume reductions (ITT population) with an illustrative MRI series from one patient. The four patients with greatest increases in tumor volumes (all based on LVA measurements) in panel B had IGF-1 changes ranging from +9.8% to −40.9%. The patient with the greatest increase in tumor volume (33.1%) discontinued in the study at week 24 after discussions about the increase with the investigator. *, Primary analysis based on highest SSR; †, proportions and 95% CIs for each reader dataset were compared with the predetermined threshold of 55%; §, data are from week 48 or LVA.
Secondary endpoints
The proportion of patients with ≥ 20% tumor volume reduction was 54.1% (95% CI, 43.0, 65.0%) at week 12 and 56.3% (44.7, 67.3%) at week 24 (reader no. 2 dataset). Mean tumor volumes and levels of GH and IGF-1 (and prolactin in patients with baseline levels of 20–100 μg/L) were greatly reduced at week 24 vs baseline, and reductions were maintained until study end (Figure 3C and Table 2). For tumor volume, mean reductions from baseline were 20% by week 12, 25% by week 24, and 27% by study end. Corresponding mean GH reductions were 62.1, 64.6, and 70.9%, respectively, and mean IGF-1 reductions were 43.8, 47.4, and 56.7%, respectively. The proportions of patients achieving GH and/or IGF-1 targets increased over time (Figure 4). Of 18 patients withdrawn because of “an insufficient IGF-1 response” (in the investigator's opinion or because IGF-1 reduction at week 24 was < 10%), the IGF-1 decrease was in fact >10% at week 24 for three patients and at the early withdrawal visit for four additional patients. Tumor volume changes for these seven patients were in the same range as those for the remaining 11 patients.
Table 2.
GH, IGF-1, and Prolactin Levels at Each Assessment Point
| Baseline | Wk 12 | Wk 24 | Wk 48 | LVA | |
|---|---|---|---|---|---|
| n | 89 | 85 | 78 | 63a | 89a |
| GH level | |||||
| Mean (SE), μg/L | 15.0 (2.0) | 3.6 (0.6) | 2.9 (0.4) | 2.4 (0.5) | 3.3 (0.5) |
| Mean [95% CI] change from baseline, % | – | −62.1 [−70.5, −53.6] | −64.6 [−72.3, −57.0] | −70.9 [−79.2, −62.5] | −61.2 [−69.5, −52.9] |
| IGF-1 level | |||||
| Mean (SE), μg/L | 804 (31) | 446 (31) | 406 (28) | 335 (27) | 428 (29) |
| Mean [95% CI] change from baseline, % | – | −43.8 [−50.1, −37.5] | −47.4 [−53.6, −41.2] | −56.7 [−62.1, −51.3] | −46.0 [−52.0, −40.1] |
| Prolactin level, nb | 21 | 20 | 20 | 12 | 21 |
| Mean (SE), μg/L | 48.0 (5.3) | 31.0 (4.0) | 30.6 (4.4) | 27.5 (4.2) | 31.3 (4.0) |
| Mean [95% CI] change from baseline, % | – | −18.0 [−26.9, −9.1] | −18.6 [−28.1, −9.1] | −17.3 [−29.0, −5.6] | −16.7 [−24.3, −9.2] |
Data are from the ITT population –, not available.
Missing IGF-1 data for one patient.
For patients with baseline prolactin levels of 20–100 μg/L.
Figure 4.
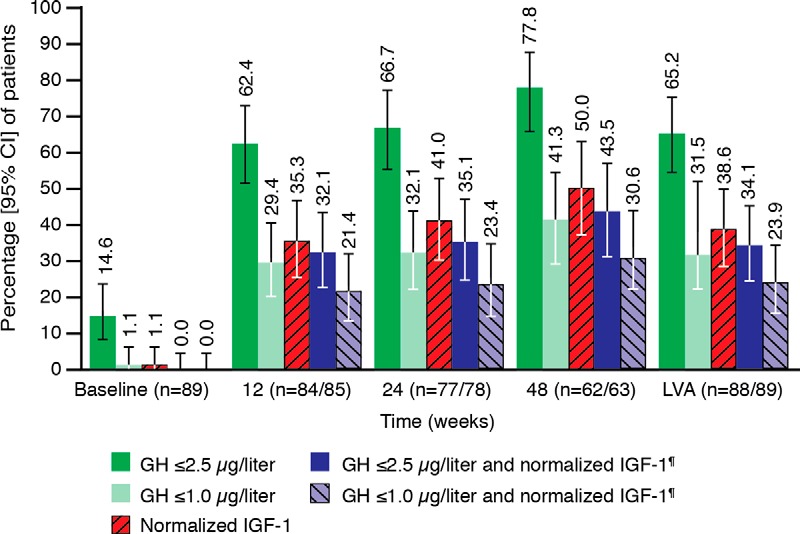
Proportions of patients reaching biochemical targets for GH and IGF-1 levels (secondary endpoints, ITT populations). *, The proportions of patients reaching the GH and IGF-1 combined endpoints were the same for the PP population. Normalized IGF-1 defined as IGF-1 levels within age- and sex-matched normal ranges.
Of the acromegalic symptoms assessed, headache was the least severe at baseline (mean [SD] score, 2.8 [2.6]), but nonetheless improved during the study (proportion of patients with improvements, 38.7–46.9% across visits). Greater proportions of patients experienced improvements in the other symptoms (55.1–68.5% across symptoms and visits). The global AcroQoL score improved from a mean (SD) baseline of 56.2 (16.1) (n = 84) by 7.8 (9.3) (n = 82) by the third injection and by 9.5 (12.7) (n = 59) at study end (corresponding LVA values, 7.7 [12.2]; n = 84).
Safety
A total of 340 treatment-emergent AEs were reported for 73 (81.1%) patients. Most of these patients experienced mild or moderate AEs (Supplemental Data). Sixty-two patients (68.9%) experienced treatment-related AEs, the most common of which was diarrhea (38.9%) (most common AE class was gastrointestinal disorders, 63.3%). Three patients experienced AEs leading to withdrawal (see Patient disposition and baseline characteristics). Of 22 serious AEs, only intracranial hypotension (cerebral fluid leakage) was considered treatment-related, and only syncope occurred in more than one patient (Supplemental Data).
There was no overall trend for any laboratory parameter. Only four abnormal values were considered clinically significant: decreased hemoglobin, thrombocytopenia, increased serum glutamate pyruvate transaminase, and increased γ-glutamyl transferase levels. FPG and HbA1c levels were compared with American Diabetes Association threshold values for categories of glycemic control (13). Thirty-four patients had normal baseline FPG levels; at week 48, 12 of these had levels classified as impaired fasting glucose (5.6–6.9 mmol/L) and two as diabetic (≥7.0 mmol/L; week 48 data missing, n = 7). Of 43 patients with impaired fasting glucose at baseline, three had levels classified as diabetic and four as normoglycemic at week 48 (week 48 data missing, n = 14). Similarly, of 25 patients with normal baseline HbA1c levels, nine had HbA1c levels considered “at risk for diabetes” (5.7–6.4%), and none had levels classified as diabetic (≥6.5%) at week 48 (week 48 data missing, n = 8). Of 42 patients with “at risk” baseline HbA1c levels, none had levels classified as diabetic, and three had levels considered normoglycemic at week 48 (data missing at week 48, n = 12). Gallbladder ultrasound revealed new sludge in eight of 60 patients (13.3%) and new cholelithiasis in 15 of 61 patients (24.6%). No clinically significant changes occurred in vital signs.
Discussion
Confidence in previous studies examining tumor-shrinkage effects with somatostatin analogs is undermined by significant methodological shortcomings and study design heterogeneity. In early studies, for example, assessments of tumor size were hindered by poor image resolution (7). More recently, standard formulae (14, 15) or geometric approximations (16) have commonly been used to estimate tumor volume, but these methods lack precision for larger, more irregularly shaped tumors. Generally, little has been done to address the persistent problem of intra- and inter-reader variability in tumor measurements. In contrast, the PRIMARYS methodology was rigorous, particularly regarding efforts to minimize variability in tumor-size measurements (Figure 1). Moreover, the patient population was homogeneous in terms of treatment (starting and continuing with the highest available dose) and disease (GH-secreting macroadenomas). PRIMARYS showed that primary treatment with lanreotide Autogel 120 mg every 28 days provides clinically significant reductions (≥20%) in tumor volume in 62.9% of patients at 1 year. These reductions were evident in 54.1% of patients by the first postbaseline visit at 3 months, suggesting that the response at 3 months is broadly predictive of the longer-term response. For some individuals, the tumor volume reduction was very marked indeed. Of the few patients with a tumor volume increase, only two had an accompanying IGF-1 nonresponse (that is, an IGF-1 decrease < 10% between baseline and LVA). Overall, the study also confirmed previous observations in this setting regarding improvements in GH and IGF-1 levels (34% of patients achieved both GH ≤ 2.5 μg/L and IGF-1 normalization) and acromegalic symptoms (5). QoL changes with primary lanreotide Autogel have hitherto received limited attention. PRIMARYS showed that QoL improvements occur as early as 3 months and are maintained for up to 1 year. Importantly, despite starting treatment at the highest dose, no patients withdrew because of gastrointestinal intolerance.
A previous meta-analysis, necessarily based on studies varying greatly in design, suggested that first-line octreotide may produce tumor shrinkage in up to two-thirds of patients (9). The data from PRIMARYS for lanreotide Autogel accord well with this estimate. The PRIMARYS data are also broadly consistent with individual studies most closely approximating PRIMARYS in design. At 1 year, 77% of 26 patients (20 with macroadenomas) receiving lanreotide Autogel 120 mg every 4–8 weeks had tumor volume reductions of ≥ 25% (5), whereas 72% of 60 patients with macroadenomas receiving octreotide LAR 10–30 mg achieved ≥ 20% reduction (6). Investigations into tumor-shrinkage effects with the newer somatostatin analog pasireotide are at an earlier stage. In a phase 2 study with the sc formulation in a mixed primary and adjunct therapy setting, seven of 14 treatment-naïve patients with macroadenomas had ≥ 20% reduction in tumor volume after 3 months (17). Full publication of further studies with the long-acting formulation are awaited to better characterize tumor-shrinkage effects.
As anticipated, the beneficial effects of primary lanreotide Autogel treatment on GH and IGF-1 hypersecretion, the cardinal symptoms of acromegaly, and QoL in PRIMARYS resonate with data from previous studies involving either this agent or octreotide LAR (5, 6, 18). The tolerability profile of lanreotide Autogel in PRIMARYS was also as expected. Although impaired glucose tolerance and frank diabetes mellitus are common acromegaly complications (1), there has been speculation that somatostatin analogs may have an additional adverse effect on glucose metabolism. A meta-analysis of lanreotide and octreotide data in acromegaly, however, indicated that modifications of glucose homeostasis induced by these agents may have an overall minor clinical impact (19). Data from PRIMARYS corroborate these findings. There were also no concerns due to using the highest available lanreotide Autogel dose (120 mg) throughout the study because AEs overall were generally consistent with those from other studies (20, 21). Initial GH, IGF-1, and safety data for the newer somatostatin analog pasireotide contrast somewhat from data for lanreotide Autogel and octreotide LAR. In a mixed primary and adjunct therapy setting, pasireotide LAR afforded superior biochemical control to octreotide LAR (31 vs 19% of patients, respectively, achieving GH < 2.5 μg/L and normalized IGF-1 levels) (22). However, early reports indicate that hyperglycemia incidence and severity are increased (22).
As with all trials, there are limitations to the PRIMARYS study. The H0 for the primary endpoint, that the proportion of tumor volume responders was ≤ 55%, was not rejected for the primary analysis. However, the 55% threshold was based on studies published at the time PRIMARYS was designed (7); none of these trials used the same design, and populations were commonly a mixture of patients with micro- and macroadenomas, possibly enriching study populations with responders. Moreover, tumor volume measurements are greatly influenced by inter- and intra-reader variabilities (23). Although considerable efforts were made to minimize variabilities in PRIMARYS, with three-quarters of scan measurements for the primary endpoint adjudicated because they differed by ≥ 10%, we cannot rule out that residual variability influenced the findings. Confidence that a large proportion of patients will show clinically significant tumor volume reduction with lanreotide Autogel in clinical practice should nonetheless be high: the H0 was rejected for all three sensitivity analyses of the primary endpoint, despite confounding factors affecting tumor volume assessments. Although the open-label uncontrolled nature of the study may also have affected outcomes, the case for using such a design is strengthened when, as in PRIMARYS, the primary endpoint is measured objectively and the probability of no treatment effect (as judged by previous studies) is low. The use of the highest available lanreotide Autogel dose and the absence of dose titration contributed to dataset homogeneity and seemed not to have affected treatment tolerability. It is possible, however, that variation in patients' individual sensitivities, such as occurs with biochemical responses (20), would have allowed some patients to achieve a tumor volume response at lower doses (60 or 90 mg). It bears repetition also that PRIMARYS elucidates the clinical effects of primary therapy with lanreotide Autogel 120 mg specifically in patients with macroadenomas. The body of evidence from other studies indicates that microadenoma volume reduction also occurs with either primary lanreotide Autogel or octreotide LAR (9, 10). However, transsphenoidal surgery affords higher biochemical cure rates for patients with smaller adenomas (2) and is therefore generally the first-line treatment. Nevertheless, the early reductions in tumor volume in PRIMARYS, when starting with the highest lanreotide Autogel dose licensed for acromegaly, could be of potential benefit to patients with optic chiasma compression. Such patients were not included in PRIMARYS; any future study of this patient group would require careful safety monitoring.
In summary, PRIMARYS underscores the potential of lanreotide Autogel 120 mg as a primary treatment for patients with acromegaly and GH-secreting macroadenoma. Using a robust methodology, the study confirms that clinical benefits include early and sustained reductions in tumor volume. QoL improvements are also apparent, alongside known benefits of improved biochemical control and amelioration of symptoms. A PRIMARYS extension study is ongoing to characterize the longer-term efficacy and safety of lanreotide Autogel 120 mg as a primary treatment.
Acknowledgments
We thank the patients and investigators involved in the PRIMARYS study.
Clinical trial registration numbers: ClinicalTrials.gov (NCT00690898) and EudraCT (2007–000155–34).
The authors accept direct responsibility for this paper and are grateful for writing assistance provided by Watermeadow Medical (supported by Ipsen). The study was sponsored by Ipsen Pharma.
* PRIMARYS Investigators: Belgium, L. Van Gaal Luc; Czech Republic, J. Marek; Finland, P. Nuutila, M. Välimäki; France, C. Ajzenberg, F. Borson-Chazot, T. Brue, P. Caron, O. Chabre, P. Chanson, C. Cortet Rudelli, B. Delemer, J.-M. Kuhn, A. Tabarin; Germany, K. Badenhoop, C. Berg, S. Petersenn, C. Schöfl, J. Schopohl; Italy, S. Cannavò, A. Colao, L. De Marinis; The Netherlands, A. Stades, A. J. Van der Lely; Turkey, P. Kadioğlu; United Kingdom, J. S. Bevan, D. Flanagan, P. Trainer.
Disclosure Summary: P.J.C. is a consultant and speaker for Ipsen and Novartis, and an advisory board member for Ipsen. J.S.B. is a consultant and study investigator for Ipsen, and an advisory board member for Novartis and ViroPharma. S.P. has received lecture fees and participated in advisory boards for Ipsen, Novartis, and Pfizer. D.F. has received lecture fees from Ipsen and grant support from Ipsen and has consulted for Novartis. A.T. is a consultant for Ipsen, Novartis, and HRA Pharma and has received lecture fees from Novartis and HRA Pharma. G.P. has no conflicts of interest to declare. P.M. and A.C. are employed by Ipsen.
Footnotes
- AE
- adverse event
- CI
- confidence interval
- FPG
- fasting plasma glucose
- H0
- null hypothesis
- HbA1c
- glycated hemoglobin
- ITT
- intention-to-treat
- LAR
- long-acting release
- LVA
- last post-baseline value available
- MRI
- magnetic resonance imaging
- PP
- per protocol
- QoL
- quality of life
- SSR
- standardized sensitivity ratio.
References
- 1. Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011;17(suppl 4):1–44 [DOI] [PubMed] [Google Scholar]
- 2. Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol. 2005;152:379–387 [DOI] [PubMed] [Google Scholar]
- 3. Melmed S, Colao A, Barkan A, et al. ; Acromegaly Consensus Group. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509–1517 [DOI] [PubMed] [Google Scholar]
- 4. Giustina A, Bronstein MD, Casanueva FF, et al. Current management practices for acromegaly: an international survey. Pituitary. 2011;14:125–133 [DOI] [PubMed] [Google Scholar]
- 5. Colao A, Auriemma RS, Rebora A, et al. Significant tumour shrinkage after 12 months of lanreotide Autogel-120 mg treatment given first-line in acromegaly. Clin Endocrinol (Oxf). 2009;71:237–245 [DOI] [PubMed] [Google Scholar]
- 6. Mercado M, Borges F, Bouterfa H, et al. ; SMS995B2401 Study Group. A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol (Oxf). 2007;66:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bevan JS. Clinical review: the antitumoral effects of somatostatin analog therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:1856–1863 [DOI] [PubMed] [Google Scholar]
- 8. Resmini E, Dadati P, Ravetti JL, et al. Rapid pituitary tumor shrinkage with dissociation between antiproliferative and antisecretory effects of a long-acting octreotide in an acromegalic patient. J Clin Endocrinol Metab. 2007;92:1592–1599 [DOI] [PubMed] [Google Scholar]
- 9. Giustina A, Mazziotti G, Torri V, Spinello M, Floriani I, Melmed S. Meta-analysis on the effects of octreotide on tumor mass in acromegaly. PLoS One. 2012;7:e36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazziotti G, Giustina A. Effects of lanreotide SR and Autogel on tumor mass in patients with acromegaly: a systematic review. Pituitary. 2010;13:60–67 [DOI] [PubMed] [Google Scholar]
- 11. Melmed S, Sternberg R, Cook D, et al. A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:4405–4410 [DOI] [PubMed] [Google Scholar]
- 12. A'Hern RP. Widening eligibility to phase II trials: constant arcsine difference phase II trials. Control Clin Trials. 2004;25:251–264 [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36 (Suppl 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colao A, Ferone D, Marzullo P, et al. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab. 2001;86:2779–2786 [DOI] [PubMed] [Google Scholar]
- 15. Baldelli R, Colao A, Razzore P, et al. Two-year follow-up of acromegalic patients treated with slow release lanreotide (30 mg). J Clin Endocrinol Metab. 2000;85:4099–4103 [DOI] [PubMed] [Google Scholar]
- 16. Bevan JS, Atkin SL, Atkinson AB, et al. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor size. J Clin Endocrinol Metab. 2002;87:4554–4563 [DOI] [PubMed] [Google Scholar]
- 17. Petersenn S, Schopohl J, Barkan A, et al. ; Pasireotide Acromegaly Study Group. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010; 95:2781–2789 [DOI] [PubMed] [Google Scholar]
- 18. Colao A, Cappabianca P, Caron P, et al. Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: a randomized, open-label, multicentre study. Clin Endocrinol (Oxf). 2009;70:757–768 [DOI] [PubMed] [Google Scholar]
- 19. Mazziotti G, Floriani I, Bonadonna S, Torri V, Chanson P, Giustina A. Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J Clin Endocrinol Metab. 2009;94:1500–1508 [DOI] [PubMed] [Google Scholar]
- 20. Chanson P, Borson-Chazot F, Kuhn JM, Blumberg J, Maisonobe P, Delemer B. Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly. Clin Endocrinol (Oxf). 2008;69:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melmed S, Cook D, Schopohl J, Goth MI, Lam KS, Marek J. Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide Autogel therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary. 2010;13:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bronstein M, Sheppard M, Freda P, et al. Pasireotide LAR is significantly more effective than octreotide LAR at providing biochemical control in patients with acromegaly: results of a 12-month randomized, double-blind, multicenter, phase III study. Endocr Rev. 2012;33:OR49–3 [Google Scholar]
- 23. Bahurel-Barrera H, Assie G, Silvera S, Bertagna X, Coste J, Legmann P. Inter- and intra-observer variability in detection and progression assessment with MRI of microadenoma in Cushing's disease patients followed up after bilateral adrenalectomy. Pituitary. 2008;11:263–269 [DOI] [PubMed] [Google Scholar]