Abstract
Introduction
Even though advantages of ultrasound line placement seem obvious, many countries do not have easy access to such technology. This study aims to compare the degree of difficulty in central venous line placement with or without ultrasound and the incidence of complications, and to establish the effect of the operator’s degree of training.
Methods
The study included 257 patients that required central venous catheterization during the study period. Patients were divided into groups according to the operator’s experience: expert group (over 70 central accesses performed before the study) (n=152) and in-training or non-expert group. Procedures were randomized to “without ultrasound” (n=80 expert and 54 non-expert) and “with ultrasound" (n=72 expert and 51 non-expert).
Results
Catheter placements were more successful in the “expert” and in the “with ultrasound” than in the “non-expert” (88% vs 79%; p=0.04) or in the “without ultrasound” groups (91% vs 78%; p=0.005). Incidence of complications was 11.7%, with no significant difference among “with ultrasound” (8.1%) and “without ultrasound” (14.9%) groups. However, the “non-expert” group had fewer complications with the use of ultrasound (7.8% vs 24%).
Conclusions
Ultrasound reduces the incidence of complications when placement is performed by inexperienced operators. Centers with residents should emphasize the necessity of ultrasound for central line catheterization. Training in ultrasound might be of paramount importance in the effectiveness of the technique.
Keywords: intensive care unit, anesthesia, ultrasound, central venous line
Introduction
Central venous catheterization is a procedure frequently performed in intensive care units. Indications include hemodynamic monitoring, total parenteral nutrition, renal replacement therapy and medication prescription, among others. It is estimated that 5,000,000 central venous lines (CVLs) are placed each year in the USA [1]. The rate of mechanical complications during the procedure ranges between 6% and 19% [2,3,4], representing between 250,000 and 1,000,000 mechanical complications per year.
CVLs are usually inserted in the internal jugular vein (IJV), the subclavian vein, and the femoral vein. Subclavian access potentially presents an higher risk of pneumothorax [2,3,4] while femoral CVL displays a higher risk of artery puncture [5, 6] and of infectious complications [2] when compared to the jugular approach.
Anatomically, the IJV is in an anterolateral position with respect to the internal carotid artery (ICA), covered by the sternocleidomastoid muscle (SCM). It ends behind the internal edge of the clavicular bundle of the SCM, near the medial end of the clavicle when it joins the subclavian vein to form the venous brachiocephalic trunk [7]. Traditionally, IJV catheterization has been performed taking these anatomical landmarks into account, particularly in relation with the SCM [8]. The first description of percutaneous access to the IJV in critical care dates back to 1966 [9]; different approaches have been described since: anterior [10], central [11] and posterior [12].
Although the anatomical landmark techniques have been validated by the above-mentioned studies, they present mechanical complications during insertion, such as accidental ICA puncture, local hematoma and pneumothorax, at an incidence of 3% to 10% for ICA and/or local hematoma, and 0.8% to 2.4% for pneumothorax [3, 13,14,15]. Other less frequent complications involve nerve injury, such as lesions of the recurrent laryngeal nerve [16], cervical sympathetic chain [17], and brachial plexus [18]; accidental deaths during the procedure have also been reported [13, 19]. Additionally, the IJV approach by anatomical landmarks displays a variable incidence of failure, ranging from 2% to 35% [13, 15, 20, 21]. Many studies have identified the factors associated with complications during CVL placement by anatomical landmarks, namely operator’s experience, site of placement, number of attempts, patient’s body mass index (BMI), or CVL placement in an emergency situation [13, 14, 22, 23].
In 1978, Ullman et al. reported the advantages of localizing the IJV by means of Doppler ultrasound before its catheterization [24]. Later on, Legler et al. published a prospective randomized study on central venous catheterization with Doppler ultrasound versus the technique by landmarks, reporting a higher success rate and lower rate of complications with the former [25]. Bond et al. gave an account of the first IJV catheterization with bi-dimensional ultrasound [26]: through this technique, the neck structures and the advancement of the puncture needle can be directly visualized. Mallory et al. published a prospective randomized study indicating a higher rate of success and a lower number of attempts and of immediate complications for IJV catheterization with bi-dimensional ultrasound versus the anatomical landmarks technique [20]. Additionally, they reported that patients in whom the CVL by anatomical landmarks failed could be catheterized under ultrasound through the same puncture site. These results have been confirmed by many consequent prospective randomized studies, in which a success rate of 98% to 100% has been reported, together with a rate of complications <5%, a decrease in the time used to perform a CVL of <50%, and a reduction in the number of punctures to <50% compared to the anatomical landmarks technique [15, 18, 21, 27,28,29].
Two meta-analyses have been performed with prospective studies comparing the landmarks technique versus the ultrasound-guided technique [30, 31], concluding that the ultrasound-guided technique lead to fewer failures and a lower number of attempts than the traditional technique. In the pediatric setting reports also favor the ultrasound technique over the traditional one [32]. The Agency for Healthcare Research and Quality of the USA [33] and the UK National Institute of Clinical Excellence [34] both recommend CVL catheterization under ultrasound as one of the safe practices to improve patient care. To date, there are no published studies analyzing in a randomized and controlled manner the effects of operator’s experience, both with or without ultrasound, on the efficiency and incidence of complications when performing a CVL.
Despite the obvious advantages of ultrasound line placement, many countries (mainly emerging countries) do not have access to such technology in every operating room. In those cases, it is essential to identify the operators that strongly require ultrasound to guide CVL placement. We hypothesize that non-expert operators must use ultrasound for CVL placement. If ultrasound is not available, it is suggested that only expert operators should perform CVL placement.
This study aims to compare the efficacy and the incidence of complications in CVL placement, with or without ultrasound, depending on the operator’s degree of training or the patient’s neck anatomy. In Uruguay, only one study has analyzed the advantages of ultrasound guided CVL placement focusing on a Nephrology Center of the University Hospital [35].
Methods
This is a prospective and randomized controlled study focused on the placement of CVL in the intensive care unit and operating rooms of the Military Hospital in Montevideo, Uruguay.
Oral or written consent was obtained from the patients or their families in order to include them in this study, which was approved by the Military Hospital Ethics Committee (in Uruguay, oral consent is accepted under special circumstances). Critically ill patients or those that required surgery and a CVL were included; unconscious, intubated, and paralyzed patients were also included. Patients under 18 years old were excluded from the study, as were conscious but non-collaborative patients.
The central IJV approach was used for CVL placement in all cases. Operators were divided into two groups according to their previous experience in CVL placement. An operator was considered an “expert” (E) when he/she had placed more than 70 central accesses by anatomical landmarks over the last 5 years. Anesthesia and intensive care unit residents that had performed less than 70 procedures were included in the “non-expert” (NE) group. Initially two doctors were included in the E group and two in the NE group.
One of the residents surpassed 70 procedures during the study period and was switched to the E group. At the beginning of the study, a basic training course on ultrasound CVL placement was given to both E and NE groups simultaneously.
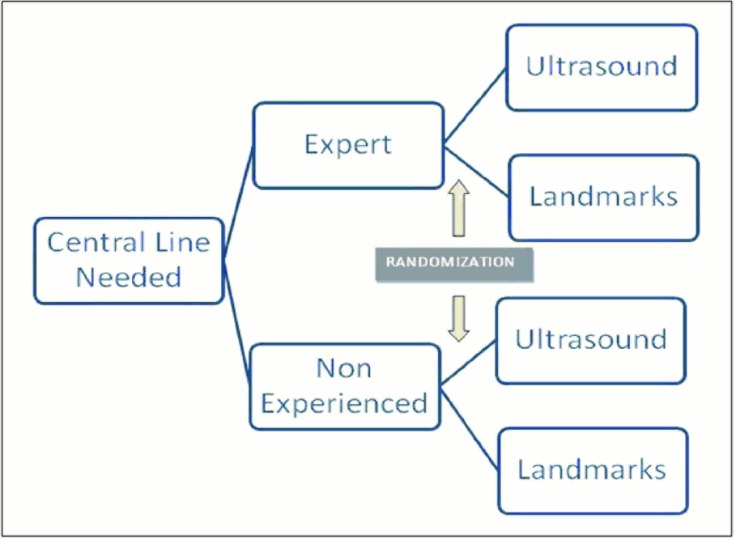
The attending or resident responsible for the patient was the one who performed the CVL. CVLs were only placed if the clinical condition required it (absolutely independently of the study). When the attending or resident informed the authors that a CVL needed to be placed on a patient, he/she was randomly assigned to either the “ultrasound” or the “landmark” groups. Randomization was performed through a computer random number generator, placing the results in sheets inside closed envelopes, which were opened right before CVL placement (Figure 1).
Figure 1.
Study design and randomization process.
The number of patients to be included in this study was calculated through a test for the comparison of two proportions for dichotomy variables based on the average rate of complications of the procedure without ultrasound taken from the literature, which is approximately 12% [3, 13].
The formula:
n=
was applied, with 95% confidence (Z=1.96), accuracy of 3% (d=0.03), a proportion of 12% (p=0.12) and q=0.88.
The number of the sample is 450 patients.
The study was interrupted when an intermediate analysis indicated a statistically significant difference in the complications of the procedure with or without ultrasound in the NE group.
Complications. Mechanical complications were defined as ICA, hematoma in the site of puncture and pneumothorax. ICA was diagnosed by visualization of throbbing bright red blood return through the syringe, local hematoma was diagnosed through inspection and palpation of the puncture site, and pleural complications were diagnosed by thorax radiography.
Success. CVL success was determined when the IJV was catheterized in three or less than three attempts. Catheterization after more than three attempts was defined as failure. After failure was assigned, the operator could opt to perform the puncture on the contralateral side or to use a different approach. Multiple punctures was defined as the occurrence of more than one skin piercing with the puncture needle in order to perform the venous catheterization.
Type of neck. With respect to the patient’s neck features, patients were classified into two groups: normal neck with palpable anatomical landmarks (sternocleidomastoid muscle muscle bundles and carotid pulse) and difficult neck with non-palpable landmarks due to obesity, subcutaneous cell tissue infiltration, previous CVL, or tracheotomy, among others.
Landmark-guided technique. The apex of the triangle of separation between the sternal and clavicle bundles of the SCM was used as an anatomical landmark (Sedillot’s triangle). The IJV was placed in a deep position in that space. For CVL placement, EPSA’s CVL kit (Electroplast S.A., Montevideo, Uruguay) was used. Catheterization was carried out in sterile conditions. Patients were placed in the Trendelenburg position, with the head rotated 45º towards the contralateral side of the puncture. The carotid pulse was identified and the puncture was performed through Sedillot’s triangle apex, directing the needle towards the areola. Once blood aspiration was confirmed, the CVL was placed using Seldinger’s technique. Control of the central catheter position was performed by chest x-ray in all cases.
Ultrasound-guided technique. An expert in ultrasound-guided procedures trained the operators (anesthesiologists and intensivists) in ultrasound-guided IJV cannulation. The need to follow and visualize the needle tip during the procedure was stressed. During the one-week training period, all CVLs needed at the Intensive Care Unit of the Military Hospital were placed by the operators, under supervision, using ultrasound; the number of CVLs placed was similar for all operators: 7 to 9.
A portable ultrasound scanner (Logic Book XP, GE Medical Systems, USA) in bi-dimensional (2D) mode with a linear transducer of 8 Mhz was used in all procedures. The transducer was covered with ultrasound gel and wrapped in a sterile plastic bag. Sterile physiological saline solution was spread on the patient’s skin to eliminate the air interface between the skin and the plastic bag. The transducer was placed in a transverse position to the patient’s neck axis, leveled with the separation of the sternal and clavicle bundles of the SCM, or, if non-palpable, lateral to the visceral axis at the middle point between the lower jaw angle and the clavicle. Briefly, the ICA was identified as a round, throbbing, and incompressible structure. The IJV was identified because it is placed in front and outwards with respect to the ICA, it is compressible, and non-throbbing. Catheterization was attempted after checking for compressibility. In case of finding an incompressible vein (intraluminal thrombus), catheterization was attempted through the contralateral side. Each procedure was carried out by a single operator, who performed the ultrasound scan and the puncture simultaneously (mono-operator). Vein penetration was confirmed objectively when the tip of the puncture needle was visualized inside the vein or when blood return was obtained. Once inside the vein, the CVL was placed using Seldinger’s technique. Control of the central catheter position was performed by thorax radiography in all cases.
Data collection and statistical analyses. Demographic data were collected from all patients: age, sex, BMI, main condition, and mechanical ventilation. From the procedure, the following data were collected: puncture side, number of attempts, success/failure, complications (arterial puncture, hematoma, and/or pneumothorax), and neck features. Data were analyzed by Χ2 independent test through 2×2 tables. In order to include three variables into the analysis, difference of proportions tests were used. The level of significance was established at p=0.05. Demographic data were analyzed with parametric and non-parametric tests.
The variables included in the analysis were type of operator (expert/non-expert), ultrasound scan (yes/no), patient’s neck anatomy (normal/difficult), complications (yes/no), and success in CVL placement (yes/no).
Results
A total of 257 procedures were performed: 152 by the E group (72 with ultrasound scanner and 80 with landmarks) and 105 by the NE group (51 with ultrasound scanner and 54 with landmarks). The difference in the number of procedures performed by the E and NE operators is explained by the higher number of expert operators included in this study.
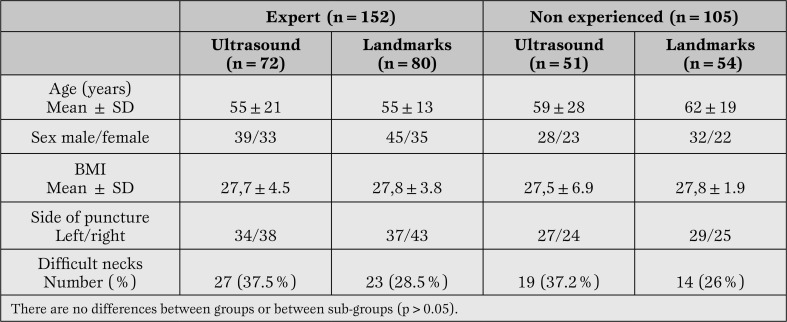
Patients’ characteristics are shown in Table 1.
Table 1.
Characteristics of the patients in the “expert” and “non-expertise” groups.
Although there is a higher percentage of patients with a difficult neckin both the ultrasound and landmark groups, this difference was not statistically significant (p=0.09).
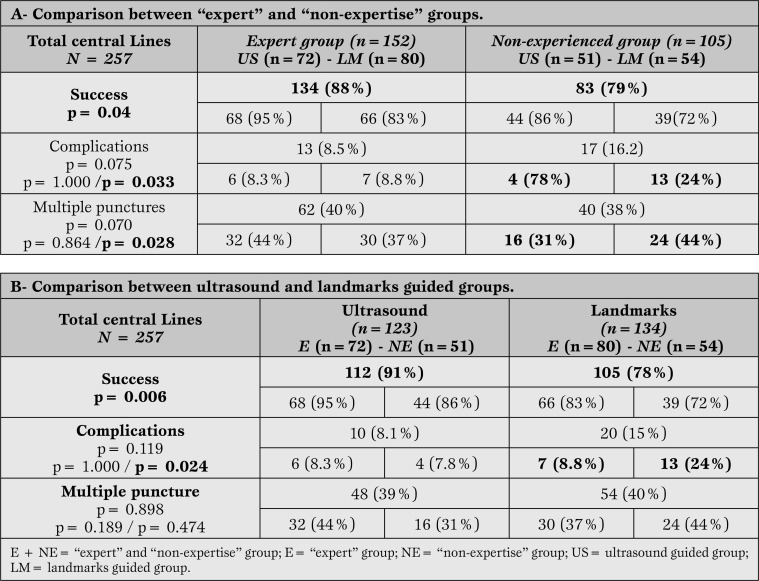
In the E group, 134 successful placements were recorded (88%), 68 with the ultrasound technique (95%) and 66 (83%) with the landmark technique, whereas the NE group was successful in 83 of the placements (79%), 44 (86%) with ultrasound and 39 (72%) without (Table 2-A). The Χ2 independence test showed a statistically significant difference in favor of the expert operators for this variable (p=0.04) (Table 2-A) and an increase in the success rate with the use of ultrasound (p=0.005) (Table 2-B).
Table 2.
OUTCOMES: success, complications and multiple puncture in each group. A- Comparison between expert and non-experienced groups; B- Comparison between ultrasound guided and landmarks guided groups.
A total of 30 complications were recorded (11.67%), of which 21 were ICA punctures requiring local compression, 8 were visible hematomas without identified ICA puncture, and 1 was a case of pneumothorax in the NE group without ultrasound, a complication that was solved by pleural drainage. When analyzing complications according to group, results showed that 13 complications (8.5%) occurred in the E group, 6 of them with ultrasound and 7 without; this difference was not statistically significant with respect to ultrasound scanner use (Table 2-A). The small sample size (the study was interrupted after 257 procedures) caused difficulties in the interpretation of the results.
In the NE group, 17 complications were recorded (16.2%), 4 with ultrasound scanner and 13 without. In this case, there was a significant difference in favor of ultrasound scanner use (p <0.033).
There were more complications in the NE group when ultrasound scanner was not used (Table 2-B).
There were no differences between E and NE groups (Table 2-A) or between the ultrasound and landmark technique (Table 2-B) regarding the number of patients with multiple punctures. Even though in the NE group, the use of ultrasound decrease the number of multiple punctures from 44% to 31% (p=0.028)
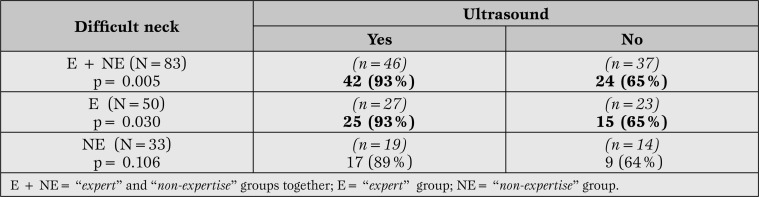
When analyzing the variable type of neck, for difficult necks (n=50), the use of ultrasound lead to success in 92.6% of cases (25 out of 27), while without ultrasound the success rate was 65% (15 out of 23), both for expert operators; this difference was statistically significant (p <0.030) (Table 3).
Table 3.
Success related to ultrasound scan in difficult necks. The rate of success was analyzed separately: in all patients with difficult neck, in non-experienced group and in the expert group.
Two complications were recorded in the ultrasound group (7.4%) and 4 (17.4%) in the landmark group (both for expert operators) (p>0.05). In the non-expertgroup working with difficult necks (n = 33 the success rate was 89% with ultrasound (17 out of 19) and 64% without (9 out of 14). There were 3 complications with the ultrasound scanner (15.8%) and one without (7.1%); no significant differences were found in these cases neither for success nor for complications.
Discussion
Several studies [31, 36,37,38] have demonstrated that the use of ultrasound guidance is clearly cost-effective, not only in terms of reduction of major complications, but primarily regarding the reduction of access time: therefore all operating rooms should have ultrasound guidance. Nevertheless, unfortunately ultrasound is not routinely available in all operating rooms in many emerging countries. Although this study focused on analyzing the advantages of ultrasound, its experimental design allowed the evaluation of the influence of other variables such as operators’ experience (operator-dependent variable) and the type of neck (patient-dependent variable) on the use of the above-mentioned technique. Identification of a target population of patients and/or doctors with an increased benefit in using ultrasound guidance may help emerging countries (without universal availability) to prioritize the use of the technique in certain contexts.
The effectiveness rate in the expert group was 83% with the landmark technique, improving to 95% with ultrasound use. These data are in agreement with previously published papers which show an effectiveness of 87% to 89% without ultrasound [3, 13] and of 97% to 100% with ultrasound [15, 27, 28, 39]. Regarding non-expert operators, comparison with previous studies that include ultrasound scanner use is difficult because most prospective studies included operators that were experts in CVL. The study of this issue involving non-expert or in-training operators is one of the novelties of our work. It is noted that in the present study the puncture technique was performed using an ultrasound scanner in a mono-operator mode, i.e., the operator had to learn to perform both the ultrasound scan and the puncture simultaneously. Mey et al. reported on the efficiency of the ultrasound-guided technique when performed by two operators (one performing the puncture while the other performed the ultrasound scan) [40]. The study showed that the most important factor when performing a puncture under ultrasound guidance is the scanner operator’s experience, since only when this operator was experienced the success rate was >97.1%; on the other hand, if only the operator performing the puncture was experienced or both operators were inexperienced, effectiveness was reduced to between 90.1% and 90.3% [40]. In our study, non-expertoperators succeeded in 72% of the cases without ultrasound and improved to 86% with ultrasound use, which coincides with the above-mentioned results. Slama et al. published a prospective study with inexperienced operators, reporting a 76% effectiveness without ultrasound and 100% with ultrasound while using the two-operators technique with an experienced ultrasound scanner operator [29].
With regards to complications in the NE group, we recorded an incidence of 24% without ultrasound, which is higher than that obtained by Sznajder et al. [3] (14%) and Eisen et al. [13] (10%). With the help of the ultrasound scanner, the incidence of complications in this group decreased to 7.8%, which is similar to that obtained by Mey et al., who reported an incidence of 10% to 17% for inexperienced ultrasound operators and puncture performers [40]. In the case of expert operators, a similar rate of complications was observed both with and without an ultrasound scanner, namely 8.3% and 8.8%, respectively. The rate of complications without ultrasound is similar to that reported by Karaktisos et al. (10.6%) [15], Troianos et al. (8.43%) [27], and Denys et al. (8.3%)[18]; however, we did not observe a decrease in the rate of complications with ultrasound use, as was observed in these studies. This might reflect a lack of experience in the use of ultrasound, since the 8.3% rate of complications can be compared to the 7.8% obtained by the NE group, and is in concordance with the study by Mey et al. highlighting the importance of experience in the ultrasound scan [40]. Therefore, training in ultrasound scanning is essential to achieve higher benefits from the technique.
The most frequently found complications [97% (29/30)], were ICA puncture and/or local hematoma. Although the classical anatomical description of the vein in the anterolateral position with respect to the artery is the most usual, it does not apply to all cases. Gordon et al. reported that the vein was found in anterolateral or lateral position in 72% of cases while in 22% and 5% of cases it was in an anterior position and inside with respect to the artery, respectively [41]. Troianos et al., in a study with over 1,000 patients, found that the vein was overlapping the artery over 75% of its circumference in 54% of the patients [42]. The mere movement of head rotation in a contralateral direction relative to the puncture site, employed as part of the vascular access technique, increases the overlap between both vessels [43]. It has also been observed that the vein is easily compressed by the puncture needle, sometimes going across this blood vessel; along with the overlap observed between the IJV and the ICA, this compression helps to explain the higher rate of arterial puncture when performing the maneuver blindly [44].
Further, we investigated if the absence of palpable landmarks had an influence on the results; the E group was more successful with ultrasound (93% vs 65%), whereas the NE group did not show significant differences. In our opinion, the difficult neck situation posed an additional difficulty for the NE group. Regarding complications in patients with necks considered difficult to puncture, there were no significant differences in either of the groups. We consider that the number of procedures analyzed might be insufficient to detect differences and that a more powerful study (higher patient numbers) may be necessary to detect them.
Limitations
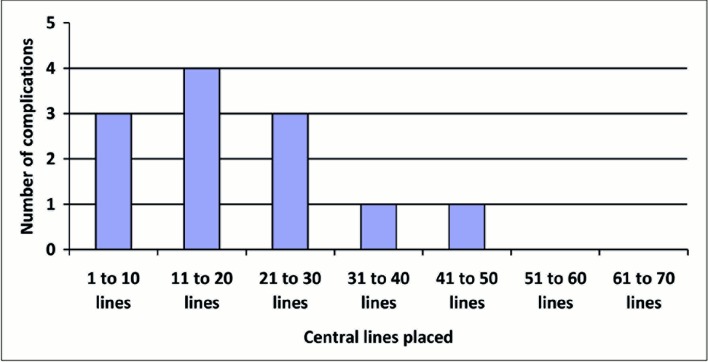
A significant limitation of the current is the unequal number of patients included in the E and NE groups. The cutoff point was decided when half the number of patients estimated in the sample was reached (257 of 450) and the number of patients in each group (E and NE) was not considered in that decision. Another limitation is the threshold of 70 procedures for inclusion of an operator in the E group. This limit may be too high, since an observation a posteriori of our data indicates that for an operator who performed the 70 procedures in the NE group, most complications occurred during the first 30 procedures (Figure 2).
Figure 2.
Number of complications in the 70 procedures performed by a non-experienced operator. Most of the complications (10 of 12) were presented in the first 30 punctures.
It is possible that an operator can be considered an “expert” with only more than 30 CVLs placed; if that is the case, the NE group would actually be a moderately trained group, with smaller differences between the two groups than expected. Even though there is lack of a specifically delineated number of procedures to develop competence in ultrasound CVL cannulation, it has been suggested by experts that a minimum of 10 procedures are required [38]. A recent paper by Nguyen et al. demonstrated that the maximal technical skills score was obtained after 8 procedures [45]. In our study, the “expert” operators in CVL placement were probably not trained in ultrasound techniques well enough, which would explain the same percentage of complications with and without ultrasound in the “expert” group. Nevertheless, this lack of a difference may also be attributed to the small sample size (the study was interrupted in 257 procedures). For a correct interpretation of this result, the study would need to be completed with the total number of patients that were initially calculated in the methods section of the study. Finally, difficult neck with non-palpable landmarks due to obesity, subcutaneous cell tissue infiltration, previous CVL, tracheotomy, or intraluminal thrombus could be considered as exclusion criteria in order to avoid bias in the study, as they have not been evenly distributed between the two studied groups.
Conclusion
Ultrasound scanning facilitates the insertion of central lines and decreases the number of complications. If ultrasound is not available for CVL placement, we highly recommend that experienced operators insert the CVL. Experience in ultrasound scanning has an influence on the results; therefore, operators, no matter whether they are experienced in CVL placement or not, should receive training on ultrasound scanning to improve results.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Rando K, Castelli J, Pratt JP, Scavino M, Rey G, Rocca ME, Zunini G. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart, Lung and Vessels. 2014; 6(1): 13-23.
References
- Raad I. Intravascular-catheter-related infections. Lancet. 1998;351:893–898. doi: 10.1016/S0140-6736(97)10006-X. [DOI] [PubMed] [Google Scholar]
- Merrer J, De Jonghe B, Golliot F, Lefrant J Y, Raffy B, Barre E. et al. Complications of Femoral and Subclavian Venous Catheterization in Critically Ill Patients: A Randomized Controlled Trial. JAMA. 2001;286:700–707. doi: 10.1001/jama.286.6.700. [DOI] [PubMed] [Google Scholar]
- Sznajder J I, Zveibil F R, Bitterman H, Weiner P, Bursztein S. Central Vein Catheterization Failure and Complication Rates by Three Percutaneous Approaches. Archives of Internal Medicine. 1986;146:259–261. doi: 10.1001/archinte.146.2.259. [DOI] [PubMed] [Google Scholar]
- Mansfield P F, Hohn D C, Fornage B D, Gregurich M A, Ota D M. Complications and failures of subclavian vein catheterization. N Engl J Med. 1994;331:1735–1738. doi: 10.1056/NEJM199412293312602. [DOI] [PubMed] [Google Scholar]
- Timsit J F, Farkas J C, Boyer J M, Martin J B, Misset B, Renaud B. et al. Central vein catheter related thrombosis in intensive care patients: Incidence, risk factors and relationship with catheter related sepsis. Chest. 1998;114:207–213. doi: 10.1378/chest.114.1.207. [DOI] [PubMed] [Google Scholar]
- Durbec O, Viviand X, Potie F, Vialet R, Martin C. Lower extremity deep vein thrombosis: A prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997;25:1982–1985. doi: 10.1097/00003246-199712000-00013. [DOI] [PubMed] [Google Scholar]
- Rouvière H. MASSON. 2005. Anatomia Humana. Descriptiva, Topográfica, Funcional, 11th edition. [Google Scholar]
- Defalque R J. Percutaneous Catheterization of the Internal Jugular Vein. Anesthesia and Analgesia. 1974;53:116–121. [PubMed] [Google Scholar]
- Hermosura B, Vanags L, Dickey M W. Measurement of Pressure During Intravenous Therapy. JAMA. 1966;185:321–321. [Google Scholar]
- Mostert J W, Kenny G M, Murphy G P. Safe Placement of Central Venous Catheter Into Internal Jugular Veins. Arch Surg. 1970;101:431–432. doi: 10.1001/archsurg.1970.01340270079021. [DOI] [PubMed] [Google Scholar]
- English I C, Frew R M, Pigott J F, Zaki M. Percutaneous cannulation of the internal jugular vein. Thorax. 1969;24:496–497. doi: 10.1136/thx.24.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman A J, Costley D O. Internal Jugular Venipuncture. JAMA. 1973;223:182–183. [PubMed] [Google Scholar]
- Eisen L A, Narasimhan M, Berger J S, Mayo P H, Rosen M J, Schneider R F. Mechanical Complications of Central Venous Catheters. J Intensive Care Med. 2006;21:40–46. doi: 10.1177/0885066605280884. [DOI] [PubMed] [Google Scholar]
- Ruesch S, Walder B, Tramèr M R. Complications of central venous catheters: Internal jugular versus subclavian access - A systematic review. Crit Care Med. 2002;30:454–60. doi: 10.1097/00003246-200202000-00031. [DOI] [PubMed] [Google Scholar]
- Karakitsos D, Labropoulos N, De Groot E, Patrianakos A P, Kouraklis G, Poularas J. et al. A Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10:162–162. doi: 10.1186/cc5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman M, Potter M, Ethel M, Myint F. Recurrent Laryngeal Nerve Injury: A Complication of Central Venous Catheterization : A Case Report. Angiology. 2004;55:345–346. doi: 10.1177/000331970405500316. [DOI] [PubMed] [Google Scholar]
- Ohlgisser M, Heifetz M. Ohlgisser M, Heifetz M. An injury of the stellate ganglion following introduction of a canula into the inner jugular vein (Horner`s syndrome). Anaesthesist. 1984;33:320–321. [PubMed] [Google Scholar]
- Denys B G, Uretsky B F, Reddy P S. Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark-guided technique. Circulation. 2003;87:1557–1562. doi: 10.1161/01.cir.87.5.1557. [DOI] [PubMed] [Google Scholar]
- Callum K G, Whimster F, Dyet J F, Gaines P A, Gillespie I, Johnston L C. et al. The report of the national confidential enquiry into perioperative deaths for interventional vascular radiology. Cardiovasc Intervent Radiol. 2001;24:2–24. doi: 10.1007/s002700001778. [DOI] [PubMed] [Google Scholar]
- Mallory D L, McGee W T, Shawker T H, Brenner M, Bailey K R, Evans R G. et al. Ultrasound guidance improves the success rate of internal jugular vein cannulation. A prospective, randomized trial. Chest. 1990;98:157–160. doi: 10.1378/chest.98.1.157. [DOI] [PubMed] [Google Scholar]
- Augoustides J G, Diaz D, Weiner J, Clarke C, Jobes D R. Current Practice of Internal Jugular Venous Cannulation in a University Anesthesia Department: Influence of Operator Experience on Success of Cannulation and Arterial Injury. J Cardiothorac Vasc Anesth. 2002;16:567–571. doi: 10.1053/jcan.2002.126949. [DOI] [PubMed] [Google Scholar]
- McGee D C, Gould M K. Preventing Complications of Central Venous Catheterization. N Engl J Med. 2003;348:1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- Maecken T, Grau T. Ultrasound imaging in vascular access. Crit Care Med. 2007;35:178–185. doi: 10.1097/01.CCM.0000260629.86351.A5. [DOI] [PubMed] [Google Scholar]
- Ullman J I, Stoelting R K. Internal Jugular Vein Location with the Ultrasound Doppler Blood Flow Detector. Anesthesia and Analgesia. 1978;57:118–118. doi: 10.1213/00000539-197801000-00024. [DOI] [PubMed] [Google Scholar]
- Legler D, Nugent M K. Doppler localization of the internal jugular vein facilitates central venous cannulation. Anesthesiology. 1984;60:481–482. doi: 10.1097/00000542-198405000-00016. [DOI] [PubMed] [Google Scholar]
- Bond D M, Champion L K, Nolan R. Real-Time Ultrasound Imaging Aids Jugular Venipuncture. Anesth Analg. 1989;68:700–701. [PubMed] [Google Scholar]
- Troianos C A, Jobes D R, Ellison N. Ultrasound-Guided Cannulation of the Internal Jugular Vein. A Prospective, Randomized Study. Anesth Analg. 1991;72:823–826. doi: 10.1213/00000539-199106000-00020. [DOI] [PubMed] [Google Scholar]
- Turker G, Kaya F N, Gurbet A, Aksu H, Erdogan C, Atlas A. Internal jugular vein cannulation: an ultrasound-guided technique versus a landmark-guided technique. Clinics (Sao Paulo). 2009;64:989–992. doi: 10.1590/S1807-59322009001000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama M, Novara A, Safavian A, Ossart M, Safar M, Fagon J Y. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23:916–919. doi: 10.1007/s001340050432. [DOI] [PubMed] [Google Scholar]
- Randolph A G, Cook D J, Gonzales C A, Pribble C G. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24:2053–2058. doi: 10.1097/00003246-199612000-00020. [DOI] [PubMed] [Google Scholar]
- Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C. et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327:361–361. doi: 10.1136/bmj.327.7411.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich C D, Rigby M R, Rosenberg E S, Li R, Roerig P L, Easley K A. et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Crit Care Med. 2009;37:1090–1096. doi: 10.1097/CCM.0b013e31819b570e. [DOI] [PubMed] [Google Scholar]
- Rothschild J. Ultrasound Guidance of Central Vein Catheterization. Evidence report/Technology Assessment N 43. Making Health Care Safer. A critical analysis of patient safety practices. AHRQ. 2001;01-EO5:245–253. [Google Scholar]
- National Institute for Clinical Excellence. National Institute for Clinical Excellence. Guidance on the use of ultrasound locating devices for placing central venous catheters. London: NICE, 2002 [NICE Technology Appraisal No 49]. London2002. [Google Scholar]
- Fernández Cean J M, Orihuela S. Utilidad de la ecograf��a para la cateterizaci�n venosa central en pacientes en hemodi�lisis peri�dica. Revista M�dica del Uruguay. 2002;18:239–243. [Google Scholar]
- Randolph A G, Cook D J, Gonzales C A, Pribble C G. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24:2053–2058. doi: 10.1097/00003246-199612000-00020. [DOI] [PubMed] [Google Scholar]
- Lamperti M, Bodenham A R, Pittiruti M, Blaivas M, Augoustides J G, Elbarbary M. et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38:1105–1117. doi: 10.1007/s00134-012-2597-x. [DOI] [PubMed] [Google Scholar]
- Troianos C A, Hartman G S, Glas K E, Skubas N J, Eberhardt R T, Walker J D. et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society Of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114:46–72. doi: 10.1213/ANE.0b013e3182407cd8. [DOI] [PubMed] [Google Scholar]
- Palepu G B, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Radiol Imaging. 2009;19:191–198. doi: 10.4103/0971-3026.54877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey U, Glasmacher A, Hahn C, Gorschlüter M, Ziske C, Mergelsberg M. et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer. 2003;11:148–155. doi: 10.1007/s00520-002-0399-3. [DOI] [PubMed] [Google Scholar]
- Gordon A C, Saliken J C, Johns D, Owen R, Gray R R. US-guided Puncture of the Internal Jugular Vein: Complications and Anatomic Considerations. J Vasc Interv Radiol. 1998;9:333–338. doi: 10.1016/s1051-0443(98)70277-5. [DOI] [PubMed] [Google Scholar]
- Troianos C A, Kuwik R J, Pasqual J R, Lim A J, Odasso D P. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85:43–48. doi: 10.1097/00000542-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Sulek C A, Gravenstein N, Blackshear R H, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82:125–128. doi: 10.1097/00000539-199601000-00022. [DOI] [PubMed] [Google Scholar]
- Turba U C, Uflacker R, Hannegan C A, Selby J B. Anatomic Relationship of the Internal Jugular Vein and the Common Carotid Artery Applied to Percutaneous Transjugular Procedures. Cardiovasc Intervent Radiol. 2005;28:303–306. doi: 10.1007/s00270-004-0039-z. [DOI] [PubMed] [Google Scholar]
- Nguyen B V, Prat G, Vincent J L, Nowak E, Bizien N, Tonnelier J M. et al. Determination of the learning curve for ultrasound-guided jugular central venous catheter placement. Intensive Care Med. 2013 doi: 10.1007/s00134-013-3069-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]