Abstract
Introduction
To compare the potential beneficial effects on markers of myocardial injury (troponin T) and renal function between sedation with sevoflurane vs propofol after cardiac surgery using extracorporeal cardiopulmonary bypass.
Methods
A prospective study with sequential selection of patients undergoing coronary or coronary and valve cardiac surgery. Intraoperative anesthesia consisted in sevoflurane and remifentanil, while in the postoperative period patients were divided in two groups to receive sedation with either sevoflurane through the AnaConDa© system or propofol. The patients were sedated during a minimum of 120minutes. Markers of myocardial injury and plasmatic creatinine were measured 4, 12, 24, and 48hours after surgery.
Results
Data from 129patients, 62sedated with propofol and 67with sevoflurane, were analyzed. The analysis of the troponin T levels showed differences 12 and 48 hours after admission. Mean values at 12hours were 0.89 (standard deviation 0.55) µg.L-1 in the propofol group and 0.69 (standard deviation 0.40) µg. L-1in the sevoflurane group (p = 0.026). TnT levels at 48hours were 0.60 (standard deviation 0.46) µg.L-1in the propofol group and 0.37 (standard deviation 0.26) µg.L-1in the sevoflurane group (p = 0,007). No differences were found in the groups in the creatinine levels before discharge.
Conclusions
The post-operative sedation with sevoflurane after cardiac surgery with cardiopulmonary bypass is a valid alternative to propofol. It does not increase the number of side effects related to kidney damage in patients with no prior renal disease, leading to reduced troponin T levels 12and 48hours after admission.
Keywords: sedation, cardiac surgery, postoperative care, sevoflurane, propofol
Introduction
The first descriptions of the anti-ischemic effects of volatile anesthetics date back to 1976, when Bland and Lowestein [1] reported that halothane decreased ST segment changes in dogs. Clinically, the cardioprotective effects of volatile anesthetics have mainly been studied in cardiac surgery, differentiating between cardiac preconditioning [2] and postconditioning [3] depending on the time of administration of the anesthetic agent. The effects on myocardium of these forms of administration of volatile anesthetics have been controversial and less conclusive than those reported at experimental level [4]. However, the most significant results depend on the time when the anesthetic gas is administered: these are more evident when it is given throughout the surgical procedure, decreasing the cardiac biomarker levels and improving the myocardial function [5,6,7,8,9].
Today, the use of volatile anesthetics is no longer conditioned by the availability of an anesthesia station with a vaporizer. The development of the Anaesthetic Conserving Device, which may be described as a minivaporizer consisting of an activated charcoal membrane that allows for anesthetic absorption and reuse [10], permits the use of anesthetic gases (sevoflurane and isoflurane) with the usual ventilators in the critical care units. This makes possible the use of volatile anesthetics during the postoperative period [11].
The purpose of this study is therefore to assess whether the extended administration of a volatile anesthetic (sevoflurane) during the postoperative period has beneficial effects on markers of myocardial injury (troponin T) when compared with a sedation with propofol.
Methods
The study was approved by the Ethics Committee of Leon University Hospital, Leon, Spain on 24 May 2011. Written consent for study participation was obtained from 144patients undergoing cardiac surgery with cardiopulmonary bypass (CPB).
This was a prospective study conducted in a tertiary hospital. Sequentially selected patients were alternatively assigned to the study groups. Sample size calculation for an expected standard deviation (SD) in both groups of 0.7, to detect a means difference in troponin T (cTnT) levels of 0.5µg.L1(95% confidence level, 80% power), was 63patients per group. 72 patients per group were enrolled to provide for potential losses.
Inclusion and exclusion criteria. Inclusion criteria were age over 18years, coronary or mixed (valve + coronary) surgery using CPB, and a minimum sedation period of 120minutes.
Patients with a history of malignant hyperthermia, propofol allergy, surgery without CPB, urgent surgery, and cryoablation surgery were excluded. Finally, patients with preoperative creatinine levels higher than 1.5mg.dL-1in a blood sample drawn within 24hours of surgery were excluded from the study.
Intraoperative period. Patients were pre-medicated with a benzodiazepine the night before the procedure and with intramuscular morphine (35mg) in the morning of surgery. In accordance with the standard procedure for cardiac surgery with CPB, anesthesia was induced using midazolam (0.07mg.kg-1), fentanyl (4µg.kg-1), etomidate (3.5mg.kg-1), and cisatracurium (0.2mg.kg-1). Remifentanil and sevoflurane were used to maintain anesthesia, with a target bispectral index (BIS) value ranging from 40to 60. During CPB, sevoflurane was used with the same target BIS.
Postoperative period. Interventions in the intensive care unit (ICU) included:
- Propofol (P) sedation group. Propofol infusion at 2mg.kg-1.h-1, with a loading dose of 1mg.kg-1if required to achieve a BIS ranging from 60to 80. The maintenance rate was adjusted between 1and 4mg.kg-1h-1to maintain BIS values ranging from 60 to 80. Remifentanyl 0.050.1µg.kg-1min-1was concomitantly administered.
- Sevoflurane (S) sedation group. Sedation with sevoflurane through the Anesthetic Conserving Device (AnaConDa©, Sedana Medical, Sundbyberg, Sweden), administered at an infusion rate ranging from 3and 8mL.h-1, to a target BIS ranging from 60to 80. Remifentanil 0.050.1µg.kg-1min-1was concomitantly administered.
This group also underwent end-tidal sevoflurane monitoring through a MaxTm© monitor, maintaining a value ranging from 0.5to 1as target end-tidal sevoflurane sedation.
Criteria for extubation were a discharge of less than 100mL through the thoracic drainages for two consecutive hours, with a bladder temperature of at least 36.5ºC.
Before extubation, both groups were administered ondansetron, morphine (35mg), and acetaminophen.
Variables recorded included age, sex, weight (kg), height (cm), type of surgery (coronary, mixed), operating time (min), CPB time (min), EuroSCORE II, preoperative ejection fraction (normal, depressed, severely depressed), sedation group, sedation time, creatinine (mg.dL1), CK, CK/MB, cTnT at 4, 12, 24, and 48hours after the admission in the ICU, creatinine before surgery, at admission in the hospital, 4, 12, and 24hours after the admission in the ICU and at hospital discharge, requirement of inotropics, vasoconstrictors, or vasopressors during the stay in the ICU, arrhythmia during ICU stay, time of stay in the ICU, length of postoperative stay, and patient vital status 30days after surgery.
Statistical analysis. The Normal distribution of data was verified using a Kolmogorov-Smirnov test. The demographic variables, operating and hospital stay times, and study variables were analyzed using a Student’s t test for independent data. The use of vasoactive agents and the occurrence of cardiac arrhythmia were analyzed using a Chi- square test. The SPSS 17.0statistical software (SPSS Inc., Chicago, IL) was used for analysis.
Results
144patients were initially enrolled in the study. 15 patients were excluded:10patients in the propofol group (5 patients had a preoperative creatinine >1.5 mg.dL-1, 3 patients underwent cryoablation and 2 patients needed urgent surgery) and 5 patients in the sevoflurane group (3 patients had a preoperative creatinine >1.5 mg.dL-1 and 2 patients underwent a cryoablation). Therefore, the data from 129patients, 62in the propofol group and 67in the sevoflurane group, were finally analyzed.
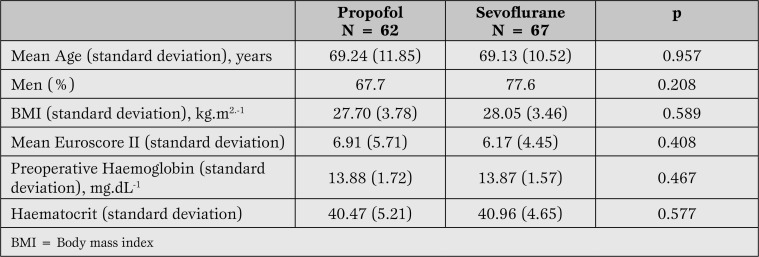
There were no differences between the groups in demographic and surgical risk data reflected by EuroSCORE II (Table 1).
Table 1.
Demographic data, surgical risk expressed by EuroSCORE tool, and preoperative haemoglobin and haematocrit.
Coronary bypass surgery was performed in 46.8% of patients in the propofol group and in 49.3% of patients in the sevoflurane group. All other surgical procedures were mixed (coronary + valve surgery). The ejection fraction, as estimated by cardiac catheterization, was normal in 79% of patients in the propofol group and in 74.6% of patients in the sevoflurane group. It was severely depressed in 4.8% and 1.5% of patients in the propofol group and in the sevoflurane group respectively.
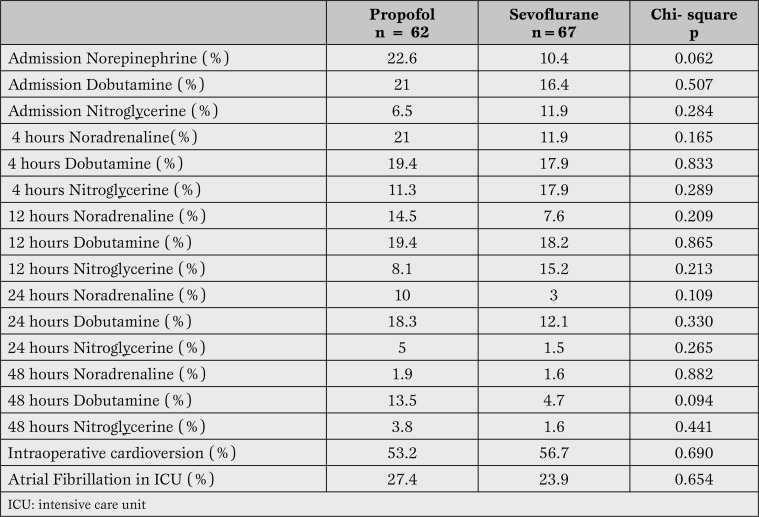
During the stay in the recovery room, no differences were found among the groups in the proportion of patients who needed the administration of norepinephrine, dobutamine, or intravenous vasodilating agents. There were no differences in the number of patients who developed an atrial fibrillation during this period (Table 2).
Table 2.
Comparisonbetween the useof dobutamine, norepinephrine, nitroglycerine andatrial fibrillation developmentduringthe stay in theintensive care unit. We did not find anystatistically significant differences inthecut-off pointsat admission,4, 12, 24 and 48hours of monitoring.
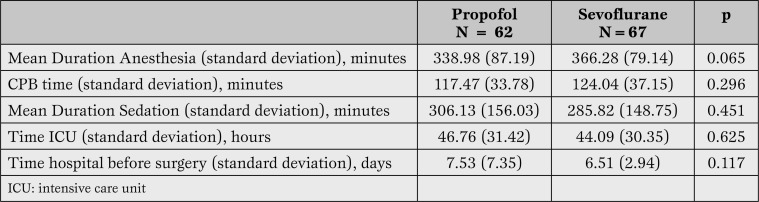
There were no differences among the groups in the in traoperative time, the time from induction of to recovery from anesthesia, or the time of extracorporeal circulation (Table 3).
Table 3.
Mean duration of anesthesia, CPB time, sedation, time in ICU and time betweensurgery andhospital discharge.
The proportion of patients who required cardioversion after removal of CPB was 53.2% in the propofol group and 56.7% in the sevoflurane group, with a mean of 1.06(SD 1.30) shocks in the first group and 1.10(SD 1.38) in the second group (p = 0.866).
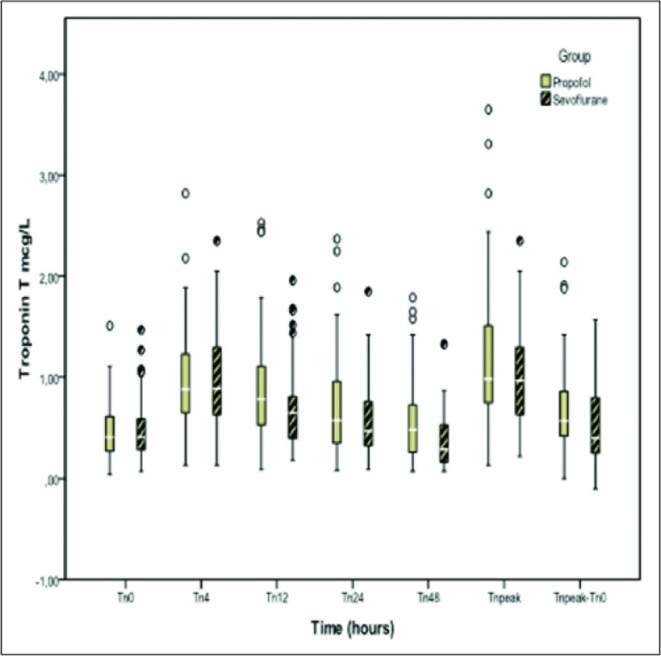
With regards to the objective of the study, differences in TnT levels were found at 12hours, with a mean of 0.89 (SD 0.55) µg.L-1 in the propofol groupversus 0.69 (SD 0.40) µg.L-1in the sevoflurane group, with a difference between means of 0.19(95% CI 0.02to 0.34), p = 0.026. There were differences at 48hours with a mean TnT level in the propofol group of 0.60 (SD0.46) µg.L-1 versus 0.37 (SD 0.26) µg.L-1in the sevoflurane group, with a difference between means of 0.22(95% CI 0.06to 0.39), p= 0.007.
The difference between the peak level of TnT and the levelof TnT at the time of admission(shown as TnT peak - TnT 0 in Figure 1) was also different in each group: 0.67(SD 0.44) with propofol and 0.51(SD 0.36) with sevoflurane, with a difference between means of 0.16(95% CI 0.07to 0.19), p = 0.027.
Figure1.
TroponinTon admission,4, 12, 24 and 48hours in patientssedated withpropofoland sevoflurane. ItalsoshowsthepeaktroponinTand the difference betweenthepeakvalue oftroponinTand troponinon admission. Statistically significant differences at 12 (p=0.026) and 48 hours (p=0.007). TnT peak - TnT 0 was also statistically different p=0.027.
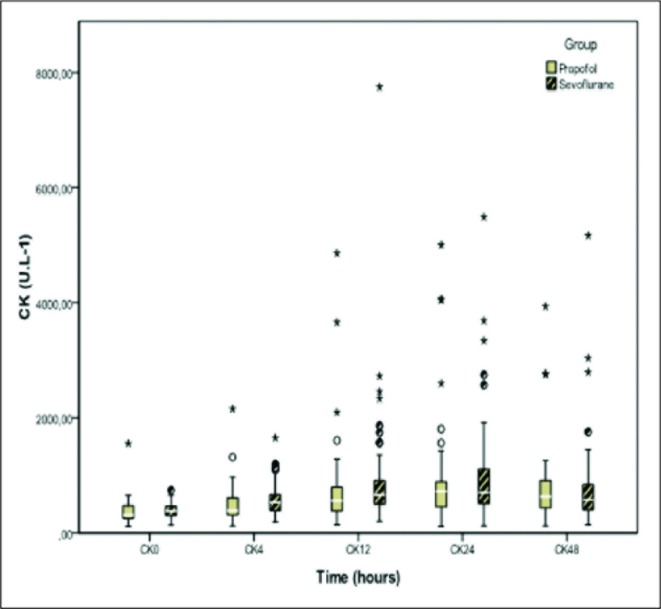
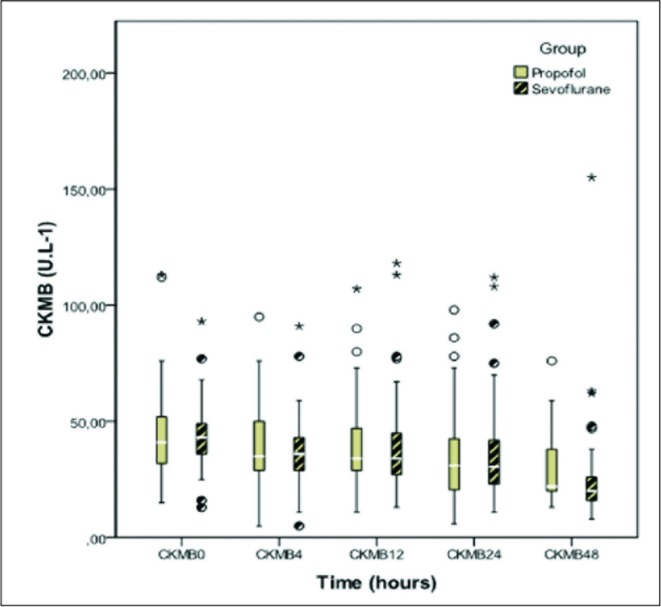
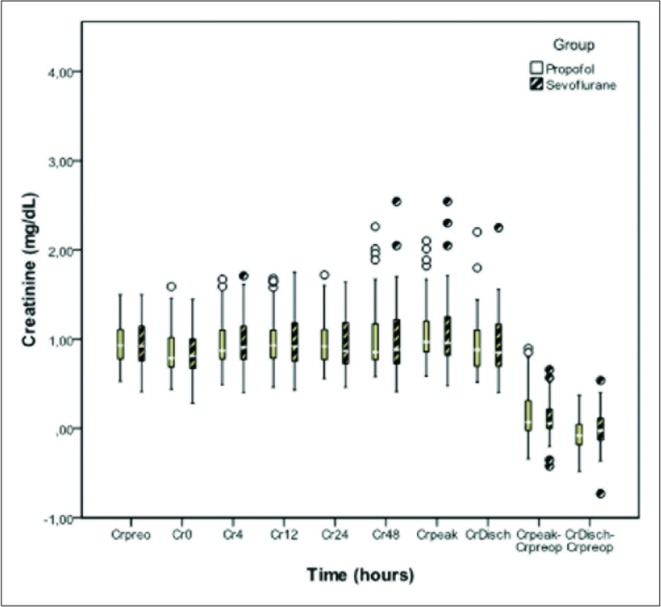
No differences were seen between the groups in the levels of CK, CKMB, and creatinine at the time of admission or 4, 12, 24, and 48hours after admission (Figures 2, 3, and 4).
Figure 2.
Creatine kinaseon admission,4, 12, 24 and 48hours in patientssedated withpropofol andsevoflurane. No statistically significant differences in any of the monitoring points. CK = Creatine kinase.
Figure 3.
Creatine kinase MB fraction on admission, 4, 12, 24 and 48 hours of intensive care unit stay in patients sedated with propofol and sevoflurane. No statistically significant differences in any of the monitoring points. CK MB = Creatine kinase MB fraction
Figure 4.
Preoperative creatinine values at admission, 4, 12, 24 and 48 hours of intensive care unit stay in patients sedated with propofol sevoflurane. It also shows peak creatinine (Crpeak) values ??at hospital discharge (CrDisch), and differences between preoperative (Crpreop) and peak value and high preoperative value in both groups. No statistically significant differences in any of the monitoring points.
The levels of plasmatic creatinine before surgery and at hospital discharge, the difference between the peak and preoperative creatinine and the difference between the plasmatic creatinine at hospital discharge and before surgery showed no differences between the groups (Figure 4).
No differences were found among the groups in the length of hospital stay and in the stay from surgery to discharge. No deaths occurred in any of the groups in the 30days following surgery.
Discusson
In patients undergoing coronary revascularization surgery, either alone or combined with valvular surgery, the continuous postoperative sedation with sevoflurane decreased TnT levels at 12 and 48 ;hours after surgery when compared with the administration of propofol during sedation.
These data disagree with those reported by Hellstrom et al [12], who found no differences in the levels of TnT 12 hours after coronary surgery alone. Prior studies have shown that the raise in the TnT levels following cardiac surgery with cardiopulmonary bypass was associated to increased mortality and longer ICU stay [13, 14]. By contrast, no statistically significant differences were seen in our study in terms of length of ICU or hospital stay, which should lead us to question whether the differences (0.19 and 0.22 µg.L1) found in our study have a clinical - and not just statistical - impact. In any case, attention should be called to the trend we identified (1 day of difference in the length of stay from surgery to hospital discharge favoring patients sedated with sevoflurane). Such difference was not statistically significant, possibly because of an inadequate sample size for this objective, but it would agree with the studies where troponin was found to be a predictor for surgical outcome.
According to these findings, we would like to emphasize the differences obtained in the time of postoperative sedation, which is longer in the propofol group (mean difference 20 minutes), although they are not statistically significant and would not cause any change in the TnT plasmatic levels. The shorter time until recovery after sedation with sevoflurane, as obtained in previous studies, could be an influencing factor for these results [15].
We must remember that cardiac surgery constitutes a suitable, but still suboptimal model for the study of potential cardioprotective effects of anaesthetic agents [16]. The perioperative morbidity has decreased significantly, so it becomes increasingly difficult to prove that an additional protective measure will result in a change in perioperative morbidity and mortality [17] because we would need larger sample sizes.
Different meta-analyses [18, 19] have shown that the use of anesthetic agents decreases the use of vasopressors. The statistical analysis of our study sample showed no significant quantitative differences in the use of norepinephrine, dobutamine, and nitroglycerin for the first 48 hours after admission. However, the analysis of the data did show a trend to a decreased use of both norepinephrine and dobutamine and to an increased use of nitroglycerin in the sevoflurane group. This trend may condition the levels of TnT, and makes us wonder whether the use of vasopressors and vasodilators might be conditioned by the sedative agent used. It should be noted that there were no target mean blood pressure levels considered in the design of the study, and that the use of vasopressors was a decision of the anesthesiologist in charge. The use of vasopressor or vasodilator agents was recorded, but not the drug doses.
Renal toxicity is a critical aspect when assessing long-term exposure to sevoflurane. In the 70s, following marketing of methoxyflurane, it was postulated that a plasma level of this drug>50 µmol.L-1resulted in kidney damage [20]. After that, it was questioned whether this threshold of 50 µmol.L1 could be extrapolated from all anesthetic gases [21, 22]. Damage caused by methoxyflurane was partly attributed to intrarenal metabolism, which does not occur with other anesthetic gases such as sevoflurane. Levelsofinorganic fluorine remains high until 48 hours after administration of sevoflurane. Data reported here show creatinine levels as a marker of kidney damage during the first 48 hours, with a biochemical measurement available on the day of hospital discharge. There was no difference among the two groups in the creatinine levels or in the peak creatinine levels at admission. A creatinine level > 1.5 mg.dL-1within 24 hours of surgery was an exclusion criterion. This, combined with a mean pre- operative hemoglobin > 13 mg.dL-1 (another determinant factor for the occurrence of acute renal failure after cardiac surgery [23]), would be the reason for the low incidence of acute renal failure and for the fact that no patient required extrarenal clearance procedures. The CPB time, the other factor especially associated to renal failure, did not differ among the groups. Sevoflurane may therefore be used in patients with prior kidney damage to assess its results in this patient group.
Continuous administration of sevoflurane in the postoperative period is permitted by the AnaConDa® (Anesthetic Conserving Device) system, which has an efficiency in terms of sevoflurane consumption similar to a circular circuit with fresh gas at 1.5 L.min-1 [24].
The AnaConDa® may be used with any ICU ventilator, and it has been shown to be a valid alternative to a conventional vaporizer when no anesthesia station is available [25]. A critical aspect in the use of this device is environmental pollution, which respects the limits established by the National Institute for Occupational Safety and Health of 2 ppm during a working day [26]. The safe use, in terms of minimal contamination, of the AnaConDa® with a scavenging system in the ICU environments (values not exceeded 1 ppm) has been described [27]. Moreover, its safe use without any scavenging system has been demonstrated [28], making this unnecessary according to some authors [29].
In terms of efficiency, the direct costs of the use of sevoflurane as sedative agent are significantly lower when compared to midazolam [30]. When compared to propofol, there is no difference in costs between them in short-time sedation, when only the drugs are considered [31]. The sedation with sevoflurane is more expensive if we add the costs of the dispositive (AnaConda©). To date, there is not any study evaluating the indirect costs of both strategies in terms of time of sedation, time of mechanical ventilation or length of stay in the ICU.
The use of the AnaConDa® device is remarkably simple: it can be filled using a specific connection and the anesthetic can be administered through a syringe pump. The infusion can be easily modified to achieve the desired level of sedation, so that it can be monitored by the nursing staff with a minimum waste of time [32].
The study limitations include the sequential selection of patients, who were alternatively enrolled into each study group. A double-blind study was not conducted because of the characteristics of drugs administered for sedation; only the part related to statistical analysis was blinded. With regards to the use of vasoactive drugs, these were only administered according to the invasive blood pressure and the central venous oxygen saturation; no invasive monitors were used, and no postoperative echocardiography was performed to assess cardiac function.
In conclusion, continuous sedation with sevoflurane in the postoperative period of coronary or mixed (valvularand coronary) surgery may not only be considered as a valid alternative to propofol, butit might alsodecrease cardiac TnT levels. No differences were found in renal function when levels of creatinine were measured.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Marcos-Vidal JM, González R, Garcìa C, Soria C, Galiana M, De Prada B. Sedation with sevoflurane in postoperative cardiac surgery: influence on troponin T and creatinine values. Heart, Lung and Vessels. 2014; 6(1): 33-42.
References
- Bland JH, Lowenstein E. Halothane-induced decrease in experimental myocardial ischemia in the non-failing canine heart. Anesthesiology. 1976;45:287–293. doi: 10.1097/00000542-197609000-00006. [DOI] [PubMed] [Google Scholar]
- Julier K, da Silva R, García C. et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: adouble-blinded, placebo-controlled multicenter study. Anesthesiology. 2003;98:1315–1327. doi: 10.1097/00000542-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Pagel PS. Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth. 2008;22:753–765. doi: 10.1053/j.jvca.2008.03.005. [DOI] [PubMed] [Google Scholar]
- De Hert SG. Anesthetic preconditioning: how important is it in today's cardiac anesthesia? J Cardiothorac Vasc Anesth. 2006;20:473–476. doi: 10.1053/j.jvca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- De Hert SG, ten Broecke PW, Mertens E. et al. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology. 2002;97:42–49. doi: 10.1097/00000542-200207000-00007. [DOI] [PubMed] [Google Scholar]
- De Hert SG, Cromheecke S, ten Broecke PW. et al. Effects of propofol, desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology. 2003;99:314–323. doi: 10.1097/00000542-200308000-00013. [DOI] [PubMed] [Google Scholar]
- Conzen PF, Fisher S, Detter C. et al. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing offpump coronary artery bypass surgery. Anesthesiology. 2003;99:826–833. doi: 10.1097/00000542-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Bein B, Renner J, Caliebe D. et al. Sevoflurane but not propofol preserves myocardial function during minimally invasive direct coronary artery bypass surgery. Anesth Analg. 2005;100:610–616. doi: 10.1213/01.ANE.0000145012.27484.A7. [DOI] [PubMed] [Google Scholar]
- De Hert SG, Van der Linden PJ, Cromheecke S. et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299–310. doi: 10.1097/00000542-200408000-00009. [DOI] [PubMed] [Google Scholar]
- Soukup J, Schärff K, Kubosch K. et al. State of the art: sedation concepts with volatile anesthetics in critically ill patients. J Crit Care. 2009;24:535–544. doi: 10.1016/j.jcrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Sturesson LW, Jonahsson A, Bodelsson M. et al. Wash-in kinetics for sevoflurane using a disposable delivery system (AnaConDa) in cardiac surgery patients. Br J Anaesth. 2009;102:470–476. doi: 10.1093/bja/aep019. [DOI] [PubMed] [Google Scholar]
- Hellstrom J, Owall A, Bergstrom J. et al. Cardiac outcome after sevoflurane verus propofol sedation following coronary bypass surgery: a pilot study. Acta Anaesthesiol Scand. 2011;55:460–470. doi: 10.1111/j.1399-6576.2011.02405.x. [DOI] [PubMed] [Google Scholar]
- Mohammed AA, Agnihotri AK, van Kimmenade RR. et al. Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation. 2009;120:843–850. doi: 10.1161/CIRCULATIONAHA.108.837278. [DOI] [PubMed] [Google Scholar]
- Nesher N, Alghamdi AA, Singh SK. et al. Troponin after cardiac surgery: a predictor or a phenomenon? Ann Thorac Surg. 2008;85:1348–1354. doi: 10.1016/j.athoracsur.2007.12.077. [DOI] [PubMed] [Google Scholar]
- Hellström J, Öwal A, Sackey PV. Wake-up times following sedation with sevoflurane versus propofol after cardiac surgery. Scand Cardiovasc J. 2012;46:262–268. doi: 10.3109/14017431.2012.676209. [DOI] [PubMed] [Google Scholar]
- De Hert SG, Preckel B, Hollmann MW. et al. Drugs mediating myocardial protection. Eur J Anaesthesiol. 2009;26:985–995. doi: 10.1097/EJA.0b013e32832fad8b. [DOI] [PubMed] [Google Scholar]
- De Hert SG. Is anaesthetic caridioprotection clinically relevant? Another futile search for a magic bullet? Eur J Anaesthesiol. 2011;28:616–617. doi: 10.1097/EJA.0b013e3283497d00. [DOI] [PubMed] [Google Scholar]
- Symons JA, Myles PS. Myocardial protection with volatile anaesthetic agents during coronary artery bypass surgery: A metanalysis. Br J Anaesth. 2006;97:127–136. doi: 10.1093/bja/ael149. [DOI] [PubMed] [Google Scholar]
- Landoni G, Biondi-Zoccai GG, Zangrillo A. et al. Desflurane and sevoflurane in cardiac surgery: a meta-analysis of randomize clinical trials. J Cardiothorac Vasc Anesth. 2007;21:502–511. doi: 10.1053/j.jvca.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Cousins MJ, Mazze RI. Methoxyflurane nephrotoxicity. A study of dose response in man. JAMA. 1973;325:1611–1616. doi: 10.1001/jama.225.13.1611. [DOI] [PubMed] [Google Scholar]
- Rohm KD, Mengistu AM, Boldt J. et al. Renal integrity in sevoflurane sedation in the Intensive Care Unit with the Anesthetic-Conserving Device: A comparison with intravenous propofol sedation. Anesth Analg. 2009;108:1848–1854. doi: 10.1213/ane.0b013e3181a1988b. [DOI] [PubMed] [Google Scholar]
- Spencer EM, Willatts SM, Prys-Roberts C. Plasma inorganic fluoride concentrations during and after prolonged (>24 hr) isoflurane sedation. Effects on renal function. Anesth Analg. 1991;73:731–737. doi: 10.1213/00000539-199112000-00010. [DOI] [PubMed] [Google Scholar]
- Karkouti K, Wijeysundera DN, Yau TM. Acute kidney injury after cardiac surgery: focus on modificable risk factors. Circulation. 2009;119:459–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- Tempia A, Olivei M, Calza E. et al. The anesthetic conserving device compared with convetional circle system used under differentt flow conditions for inhaled anesthesia. Anesth Analg. 2003;96:1056–1061. doi: 10.1213/01.ANE.0000050558.89090.95. [DOI] [PubMed] [Google Scholar]
- Soro M, Badenes R, García-Pérez ML. et al. The accuracy of the anesthetic conserving device (AnaConDa) as an alternative to the classical vaporizer in anesthesia. Anesth Analg. 2010;111:1176–1179. doi: 10.1213/ANE.0b013e3181f4db38. [DOI] [PubMed] [Google Scholar]
- Migliari M, Bellari G, Rona R. et al. Short-term evaluation of sedation with sevoflurane administered by the anesthetic conserving device in critically ill patients. Intensive Care Med. 2009;35:1240–1246. doi: 10.1007/s00134-009-1414-7. [DOI] [PubMed] [Google Scholar]
- Pickworth T, Jerath A, DeVine R. et al. The scavenging of volatile anesthetic agents in the cardiovascular intensive care enviorement: a technical report. Can J Anaesth. 2013;60:38–43. doi: 10.1007/s12630-012-9814-5. [DOI] [PubMed] [Google Scholar]
- Sackey PV, Martling CR, Nise G, Radell P. Ambient isoflurane pollution and isoflurane consumption during intensive care unit sedation with the Anesthetic Conserving Device. Crit Care Med. 2005;33:585–590. doi: 10.1097/01.ccm.0000156294.92415.e2. [DOI] [PubMed] [Google Scholar]
- Marbini HD, Palayiwa E, Chantler J. Active gas scavenging is unnecessary when using the AnaConDa volatile agent delivery system. Intensive Care Soc. 2009;10:26–28. [Google Scholar]
- Bisbal M, Arnal JM, Passelac A. et al. Efficacy, safety and cost of sedation with sevoflurane in the intensive care unit. Ann Fr Anesth Reanim. 2011;30:335–341. doi: 10.1016/j.annfar.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Röhm KD, Wolf MW, Schöllhorn T. et al. Short-term sevoflurane sedation using the Anaesthetic Conserving Device after cardiothoracic surgery. Intensive Care Med. 2008;34:1683–1689. doi: 10.1007/s00134-008-1157-x. [DOI] [PubMed] [Google Scholar]
- Sackey PV, Martling CR, Granath F. et al. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med. 2004;32:2241–2246. doi: 10.1097/01.ccm.0000145951.76082.77. [DOI] [PubMed] [Google Scholar]