Abstract
Introduction
Pilot study associating hemodialysis-to-general-anesthesia time interval and post-operative complications in hemodialysis patients to better define a more optimal pre-anesthetic waiting period.
Methods
Pre-anesthetic and 48-hours post-anesthetic parameters (age, gender, body-mass-index, pre-operative ultrafiltrate, potassium, renal disease etiology, hemodialysis sessions per week, Acute Physiology and Chronic Health Evaluation-II score, Portsmouth-Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity, American Society of Anesthesiologists physical status, Johns Hopkins Surgical Classification System Category, surgical urgency, intra-operative fluids, estimated blood loss, post-operative complications) were collected on chronic hemodialysis patients between 11/2009-12/2010. Continuous data were analyzed by Analysis of Variance or t-test. Bivariate data were analyzed by Fisher’s Exact Test. Relative Risks/Confidence Intervals were calculated for statistically significant comparisons (p=0.05). Exclusion criteria were incomplete records, peritoneal dialysis, intra-operative hemodialysis, liver transplant, and cardiopulmonary bypass.
Results
Patients were grouped by dialysis to anesthesia time interval: Group 1 >24 hours, Group 2 7-23.9 hours, Group 3 < 7 hours. Among Surgical Category 3-5 patients, hypotension was more common in Group 3 than Group 1 (63.6% vs 9.2%, p<0.0001, relative risk=6.9, confidence interval=3.0-15.7) or Group 2 (63.6% vs 17.3%, p=0.0002, relative risk=3.7, confidence interval=1.9-7.2). Other complications rates were not statistically significant. Disease and surgical severity scores, preoperative ultrafiltrate, and intra-operative fluids were not different.
Conclusions
Post-anesthetic hypotension within 48 hours was more common in those with < 7 hours interval between dialysis and anesthesia. Therefore, if surgical urgency permits, a delay of ≥7 hours may limit postoperative hypotension. More precise associations should be obtained through a prospective study.
Keywords: general, anesthesia, dialysis, hypotension, time, interval, complications, ultrafiltrate, surgery, post-operative
Introduction
Patients with end-stage kidney disease require dialysis to maintain homeostasis and life. Advances in medical care have made it possible to prolong the life of many dialysis-dependent patients. As a result, more dialysis patients are diagnosed with diseases that benefit from surgery and are considered to be in a general state of health to tolerate such surgeries [1]. Induction and maintenance of general anesthesia and surgery in a patient with derangement of volume status, electrolyte balance, or uremia can be detrimental. Therefore, dialysis may be required prior to general anesthesia. We hypothesize that post-operative complications are more frequent when induction of general anesthesia is very close to completion of hemodialysis. Since anesthesiologists are beginning to take a more active role in the peri-operative management of patients, we focused our attention to the 48 hours immediately post-operative rather than intra-operative. A literature search revealed case reports describing post-operative complications in dialysis-dependent patients who underwent general anesthesia. Though case reports were few, organ hypoperfusion and major arrhythmia were among them [2,3,4,5]. Others have attempted to study intra-operative fluid requirements of hemodialysis patients without addressing timing between hemodialysis and general anesthesia [6]. Though timing of dialysis prior to surgery was recorded in some patients, they were not stratified by times and analyzed.
As anesthesiologists are increasingly asked to involve themselves as peri-operative physicians outside the operating room [7, 8], we submit that preoperative medical management decisions such as timing of hemodialysis and general anesthesia may have consequences after conclusion of the anesthetic. To this end, we conducted a retrospective chart review to elucidate the relationship between the time interval from the end of hemodialysis to the induction of general anesthesia and postoperative complications. We hoped to identify risk factors for and frequency of postoperative complications in these patients.
Historically, hemodialysis alone has been associated with up to a 50% incidence of hypotensive events [9] so it is not surprising that hemodialysis and induction of general anesthesia in rapid succession could be associated with an increase in hypotension as well. Removal of fluid requires ultrafiltration. During ultrafiltration, hydrostatic pressure across the dialysis membrane is increased to push solvent from blood to dialysate. This also tends to carry some solute with it. The net result is a relatively hypovolemic blood compartment with lower oncotic pressure. Lower oncotic pressure leads to further depletion of intravascular volume. Often, this effect can be counterbalanced by instilling sodium into the blood [10,11,12]. The difficulty with this approach is that serum sodium levels regulate thirst and cause patients to drink excessively and gain fluid in the interdialytic period [13,14,15,16]. Therefore, many are not aggressively treated with sodium to maintain oncotic pressure, and there is significant net movement of volume from the intravascular space to the extravascular (intracellular) space [9, 17]. Simultaneous transfer of heat from dialysate to patient also tends to decrease blood pressure through vasodilation [18, 19]. Induction of anesthesia often includes agents with intrinsic myocardial depressant and systemic vasodilatory activity. Furthermore, major surgery imparts a generalized inflammatory state upon the patient, which further exacerbates extravascular (extracellular) movement of volume [20]. These considerations may make post-operative blood pressure regulation difficult, potentially contributing to continuing hypotension, even after the conclusion of the anesthetic.
Methods
This study was approved by the Institutional Review Board of Loma Linda University. Charts from patients with end-stage renal disease managed by chronic hemodialysis [21] who underwent surgery at a single tertiary-care academic teaching hospital (Loma Linda University Medical Center, Loma Linda, CA) between November 1, 2009 and December 31, 2010 were reviewed. Adults maintained on hemodialysis for at least 3 months prior to general anesthesia and who received it for surgery were identified. Patients managed with peritoneal dialysis were excluded. We also excluded those undergoing procedures with fluid shifts in excess of those predicted from preoperative hemodialysis (cardiopulmonary bypass, veno-veno bypass, liver transplantation, and intra-operative hemodialysis). Patients found with incomplete documentation of the timing of hemodialysis were excluded from analysis.
Basic demographic data were gathered. Based on recorded data both Acute Physiology and Chronic Health Evaluation II (APACHE II) [22] and Portsmouth Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (P-POSSUM) [23,24,25] scores were calculated. The time interval between the end of hemodialysis and the induction of general anesthesia was calculated from inpatient and outpatient documentation as available. In cases where outpatient hemodialysis was performed at an independent center and records were not available, this information was determined from the patient’s admitting documentation. Post-operative complications documented after the end of anesthesia were entered into our database. We sought documentation of complications defined as:
- Dysrhythmia - abnormal rhythm resulting in: unscheduled intravenous medication, cardioversion, cardiology consultation, prolonged length of stay, or any one of the other listed complications.
- Hypotension - systolic blood pressure below 85 mmHg causing unscheduled fluid bolus of 1000 mL of crystalloid, 250 mL of 5% albumin, 50 mL of 25% albumin, and/or vasoactive medications.
- Hypertension - elevated blood pressure treated with unscheduled intravenous medication, unscheduled hemodialysis, or other invasive procedure such as heart catheterization.
- Delirium - delirium documented as needing specific medication, procedure, specialist consultation, prolonged length of stay, or causing any other listed complication.
- Electrolyte abnormality - postoperative electrolyte abnormality requiring unscheduled medication, transfer to higher level of care, prolonged length of stay, or causing any other listed complication.
- Re-intubation.
- Advanced Cardiac Life Support - acute clinical decompensation resulting in initiation of rapid response or code blue team or initiation of Advanced Cardiac Life Support resuscitation in a unit that provides for its own rapid response/code blue.
- Death during same hospital stay.
Each complication was noted either present or absent per patient, thus a single patient could have multiple types of complications but the frequency of any single type was not noted more than once during the 48-hour postoperative period. Surgical procedures were classified using the Johns Hopkins Surgical Classification System (JHSCS), based on pre-operative documentation and the proposed procedure [26]. Emergent surgeries were cases documented to need intervention within 2 hours; while urgent surgeries were defined as non-elective cases that could wait at least 2 hours.
Statistical Methods: Preliminary analysis supported grouping of patients based on the documented interval between hemodialysis and general anesthesia into three groups: Group 1 interval >24 hours; Group 2 interval from 7-23.9 hours; or Group 3 interval < 7 hours. Complications were expressed as a percentage of each respective group. The primary outcome measure was the difference in complications between groups. Secondary outcome measures included the incidence of complications related to specific surgical procedures. Analysis was performed using computerized statistical software (GraphPad Prism, GraphPad Software, La Jolla, CA). Continuous data were analyzed with ANOVA or Kruskal-Wallis tests. Bivariate data were analyzed by Fisher’s Test. Relative Risks and Confidence Intervals were calculated for comparisons reaching statistical significance (p=0.05).
Results
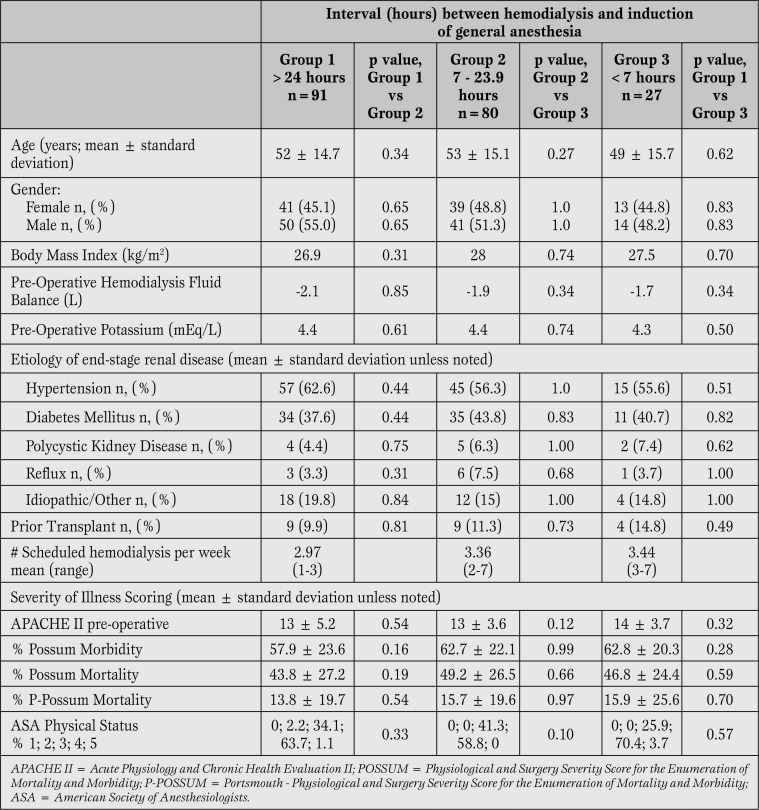
Chart review yielded 238 chronic hemodialysis patients with surgery under general anesthesia between November 2009 and December 2010. Of these, 40 were excluded: in 23 cases surgery involved fluid shifts in excess of those predicted from preoperative hemodialysis and in 17 cases documentation regarding interval between hemodialysis and general anesthesia was incomplete. Demographic and illness scores were not different between groups (Table 1).
Table 1.
Patient Demographics and Severity of Illness Scoring. No differences were found between patients in whom the dialysis to general anesthesia interval was less than 7 hours compared to those with longer dialysis to general anesthesia intervals.
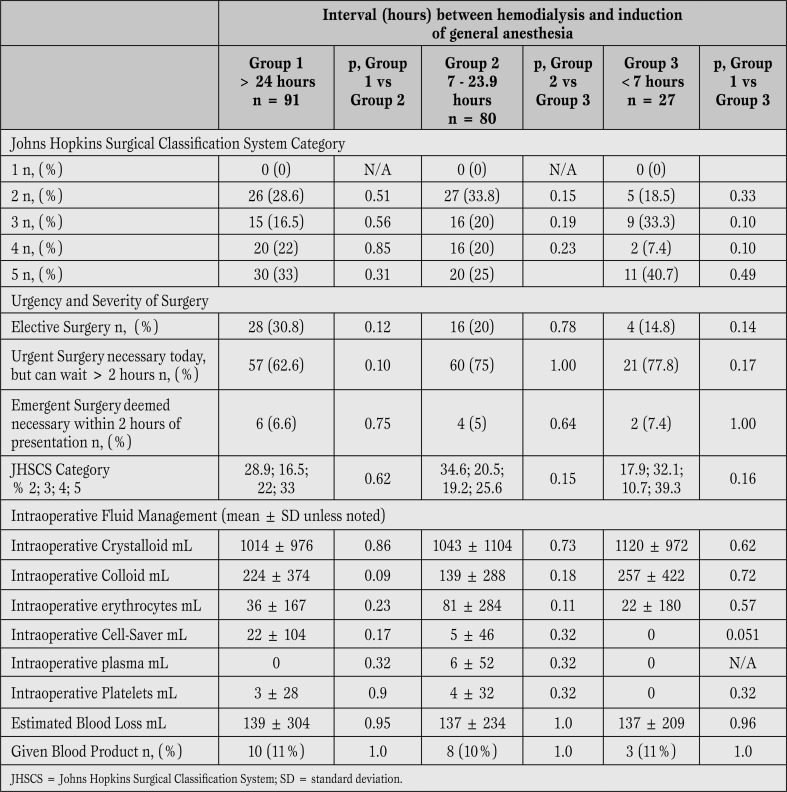
Severity of surgery, urgency of surgery and fluid/blood product administration were also similar between groups (Table 2).
Table 2.
Surgical factors. No differences were found between patients in whom the dialysis to general anesthesia interval was less than 7 hours compared to those with longer dialysis to general anesthesia intervals.
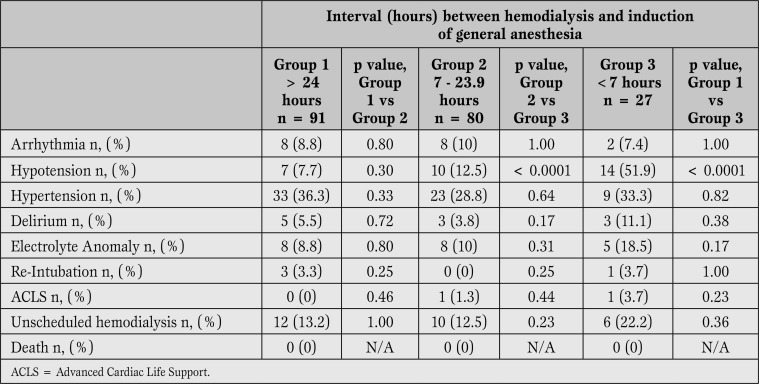
As shown in Table 3, the only difference in complications was postoperative hypotension, which was more common in Group 3 than either Group 1 or 2.
Table 3.
Complications Regardless of Surgical Severity. Hypotension was more common in Group 3 than either Group 1 or Group 3.
Surgery with minimal physiologic perturbations (Category 2) was not associated with increased risk for any postoperative complication, regardless of the interval between completion of hemodialysis and induction of general anesthesia. When analyzing onlysurgical procedures other than minimal (Category 3-5), hypotension was more common in Group 3 than Group 1 (63.6% vs 9.2%, p<0.0001, relative risk=6.9, confidence interval=3.0-15.7) or Group 2 (63.6% vs 17.3%, p=0.0002, relative risk=3.7, confidence interval=1.9-7.2). Postoperative hypotension was also more frequent in Group 3 for both elective (p=0.03) and urgent (p<0.0001) surgeries. Of note, postoperative hypotension was not more common when analyzed by case urgency alone, although the small number of emergent cases (n=12) limits this analysis.
Discussion
Major limitations include the retrospective nature of our study and thus our inability to review the hemodialysis prescriptions of patients dialyzed at independent outpatient hemodialysis centers. Detailed records from outside facilities were sometimes unavailable and the interval from hemodialysis to general anesthesia could only be estimated based on admitting documentation. All of these cases, however, involved patients in whom the interval was clearly longer than 7 and less than 24 hours, thus the inability to calculate a precise interval is unlikely to have affected group assignment. Another limitation related to outpatient hemodialysis is variability between hemodialysis prescriptions. Many factors contribute to the rate and degree of fluid shifts during and following hemodialysis including ultrafiltration rate/amount, dialysate sodium, calcium content, patient’s pre-hemodialysis solute load, etc. Because we limited our study to cases with chronic hemodialysis, however, is likely that most sessions had similar ultrafiltration goals. It is worth noting that intradialytic hypotension may be ameliorated by lengthening treatment duration, thus reducing ultrafiltration rate to allow vascular refilling to better match fluid removal [20]. It may be worthwhile to investigate the use of sustained low-efficiency dialysis methods or continuous renal replacement therapy in high-risk patients. A prospective study with blinding and controls on hemodialysis interval/prescription may provide a better delineation of hemodialysis timing and prescription for outpatients.
Patients in whom the interval between the end of hemodialysis and the induction of general anesthesia for major surgery was less than 7 hours were found to have higher risk of developing post-operative hypotension that requires treatment, independent of surgical urgency. These findings imply that, for major surgery in chronic hemodialysis patients (Category 3 to 5), an interval greater than 7 hours is advisable to avoid post-operative hypotension. Though less emergent cases may be delayed due to risk of hypotension, many emergent procedures cannot be delayed. The post-operative team thus should expect and be prepared to treat hypotension in these patients. On the other hand, patients undergoing low-severity surgery (Category 2) were not found to have greater risk for post-operative hypotension regardless of the interval between hemodialysis and general anesthesia. There a decision to proceed with minor surgery soon after the end of hemodialysis can be made on a patient-by-patient basis. In making these recommendations, anesthesiologists can make a significant impact on patient outcomes well beyond the immediate anesthetic period.
It’s worth noting that our study included very few ASA 5 patients. Since ASA status is more useful for estimating operative mortality than teasing out individual risk factors, we felt that it was a measure with inter-observer variability. The most important limitation of our study was the low number of emergency surgeries included. Whether or not this introduced bias in attributing hypotension to dialysis interval rather than urgency of surgery can be better elucidated by a prospective study using multivariate analysis with sufficient power for emergent cases. Furthermore, such a study could be useful in identifying sub-groups in which our association may hold more true than in others.
Conclusion
It may be advisable to delay elective and urgent induction of general anesthesia for major surgery by about 7 hours after hemodialysis, in order to minimize hypotension. Prospective research with sufficient power should be done to better elucidate subgroups that may be more or less vulnerable to post-operative complications. Such a study would also serve to better define a more exact length of time to wait between hemodialysis and general anesthesia. Pre-operative planning and decisions can make a significant impact on a patient’s care, even well into the post-operative period. Studies analyzing the effect of different dialysis modalities and different ways of coordination of dialysis with surgery may be valuable in finding ways to optimize patient outcome and efficiency.
Footnotes
Source of Suppot Nil.
Disclosures None declared.
Cite as: Deng J, Lenart J, Applegate RL. General anesthesia soon after dialysis may increase postoperative hypotension - A pilot study. Heart, Lung and Vessels. 2014; 6(1): 52-59.
References
- Taal M W, Chertow G M, Marsden P A, Skorecki K, Yu A S L, Brenner B M. 9th ed., W.B. Saunders, 2011. Taal: Brenner and Rector's The Kidney. [Google Scholar]
- Della Rocca G, Costa M G, Bruno K, Coccia C, Pompei L, Di Marco P. et al. Pediatric renal transplantation: anesthesia and perioperative complications. Pediatr Surg Int. 2001;17:175–179. doi: 10.1007/s003830000486. [DOI] [PubMed] [Google Scholar]
- Kabutan K, Mishima M, Takehisa S, Morimoto N, Taniquchi M. Postoperative pancreatitis after total hip replacement under general anesthesia with sevoflurane in a patient with chronic renal failure on hemodialysis. Masui. 2000;49:309–311. [PubMed] [Google Scholar]
- Maruyama K, Agata H, Ono K, Hiroki K, Fujihara T. Slow induction with sevoflurane was associated with complete atrioventricular block in a child with hypertension, renal dysfunction, and impaired cardiac conduction. Paediatr Anesth. 1998;8:73–78. doi: 10.1046/j.1460-9592.1998.00673.x. [DOI] [PubMed] [Google Scholar]
- Figueroa W, Alankar S, Pai N, Dave M. Subxiphoid pericardial window for pericardial effusion in end-stage renal disease. Am J Kidney Dis. 1996;27:664–667. doi: 10.1016/s0272-6386(96)90100-6. [DOI] [PubMed] [Google Scholar]
- Horibe M, Kuroda M, Tajima M, Kawanishi H, Yamane S. Intra-operative management during laparotomy for patients under hemodialysis. Masui. 1996;45:1135–1139. [PubMed] [Google Scholar]
- Adesanya A, Joshi G. Hospitalists and anesthesiologists as perioperative physicians: Are their roles complementary? Proc (Bayl Univ Med Cent) 2007:140–142. doi: 10.1080/08998280.2007.11928271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter T, Goeddel L, Boudreaux A, Hunt T, Jones K, Pittet J. The Perioperative Surgical Home: how can it make the case so everyone wins? BMC Anesthesiology. 2013;13:6–6. doi: 10.1186/1471-2253-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Simone G. Left ventricular geometry and hypotension in end-stage renal disease: a mechanical perspective. J Am Soc Nephrol. 2003;14:2421–2427. doi: 10.1097/01.asn.0000088724.66957.fc. [DOI] [PubMed] [Google Scholar]
- Sadowski R H, Allred E N, Jabs K. Sodium modeling ameliorates intradialytic and interdialytic symptoms in young hemodialysis patients. J Am Soc Nephrol. 1993;4:1192–1198. doi: 10.1681/ASN.V451192. [DOI] [PubMed] [Google Scholar]
- Oliver M J, Edwards L J, Churchill D N. Impact of sodium and ultrafiltration profiling on hemodialysis-related symptoms. J Am Soc Nephrol. 2001;12:151–156. doi: 10.1681/ASN.V121151. [DOI] [PubMed] [Google Scholar]
- Churchill D N. Sodium and water profiling in chronic uraemia. Nephrol Dial Transplant. 1996;11:38–41. doi: 10.1093/ndt/11.supp8.38. [(Suppl. 8)] [DOI] [PubMed] [Google Scholar]
- Mann H, Stiller S. Sodium modeling. Kidney Int Suppl. 2000;76:79–88. [PubMed] [Google Scholar]
- Song J H, Park G H, Lee S Y, Lee S W, Lee S W, Kim M J. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol. 2005;16:237–246. doi: 10.1681/ASN.2004070581. [DOI] [PubMed] [Google Scholar]
- Peixoto A J, Gowda N, Parikh C R, Santos S F. Long-term stability of serum sodium in hemodialysis patients. Blood Purif. 2010;29:264–267. doi: 10.1159/000274460. [DOI] [PubMed] [Google Scholar]
- Santos S F, Peixoto A J. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:522–530. doi: 10.2215/CJN.03360807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl H, Paeprer H, Unger V, Kessel M W. Hemodynamics during hemodialysis, sequential ultrafiltration and hemofiltration. J Dial. 1979;3:51–71. doi: 10.3109/08860227909064912. [DOI] [PubMed] [Google Scholar]
- Cruz D N, Mahnensmith R L, Brickel H M, Perazella M A. Midodrine and cool dialysate are effective therapies for symptomatic intradialytic hypotension. Am J Kidney Dis. 1999;33:920–926. doi: 10.1016/s0272-6386(99)70427-0. [DOI] [PubMed] [Google Scholar]
- Selby N M, Burton J O, Chesterton L J, McIntyre C W. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1:1216–1225. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- Kaye A D, Riopelle J M. 2009. Fluid and electrolye physiology. pp. 1729–1730. [In Miller RD, ed. Anesthesia, 9th ed. Philadelphia: Churchill Livingstone] [Google Scholar]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic KidneyDisease: Evaluation, Classification and Stratification. Am J Kidney Dis. 2002;39:1–266. [PubMed] [Google Scholar]
- Knaus W A, Draper E A, Wagner D P, Zimmerman J E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Prytherch D R, Whiteley M S, Higgins B, Weaver P C, Prout W G, Powell S J. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg. 1998;85:1217–1220. doi: 10.1046/j.1365-2168.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- Copeland G P, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- Chen W, Fong J W, Lind C R, Knuckey N W. P-POSSUM scoring system for mortality prediction in general neurosurgery. J Clin Neurosci. 2010;17:567–570. doi: 10.1016/j.jocn.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Pasternak L R. Preanesthesia Evaluation of the Surgical Patient. Proceedings of American Society of Anesthesiologists Refresher Course. 1996.