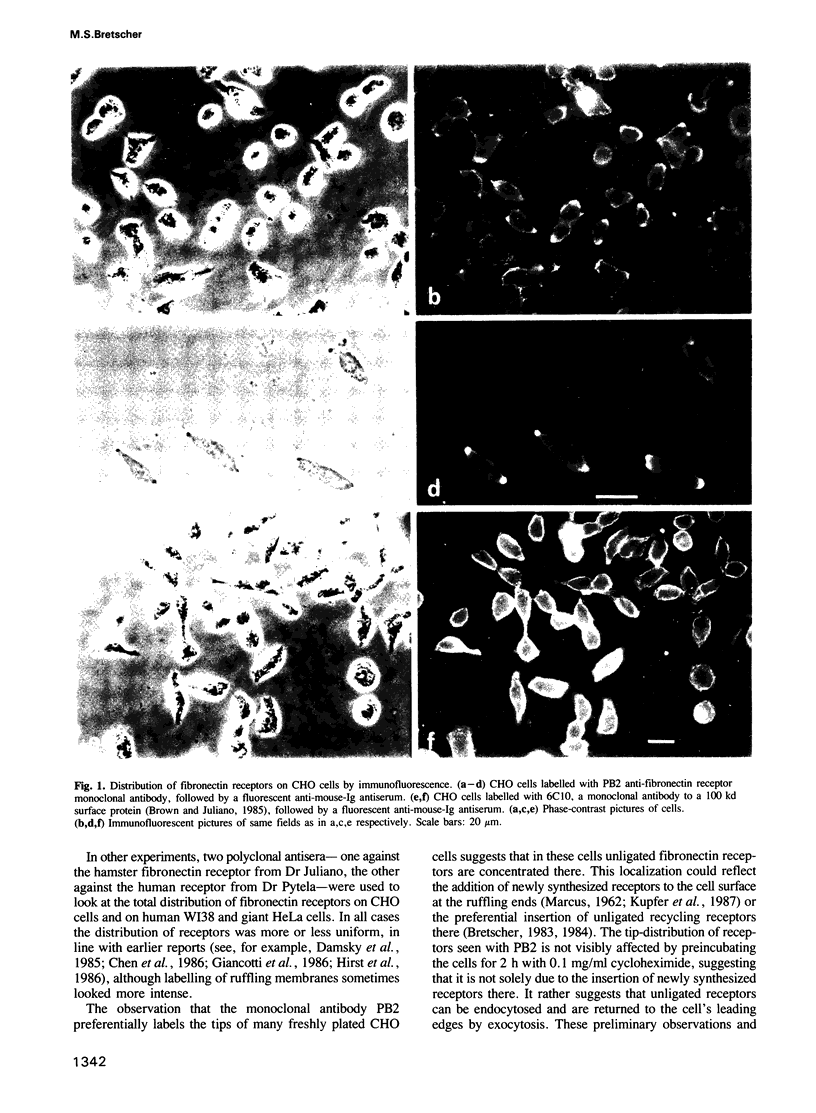
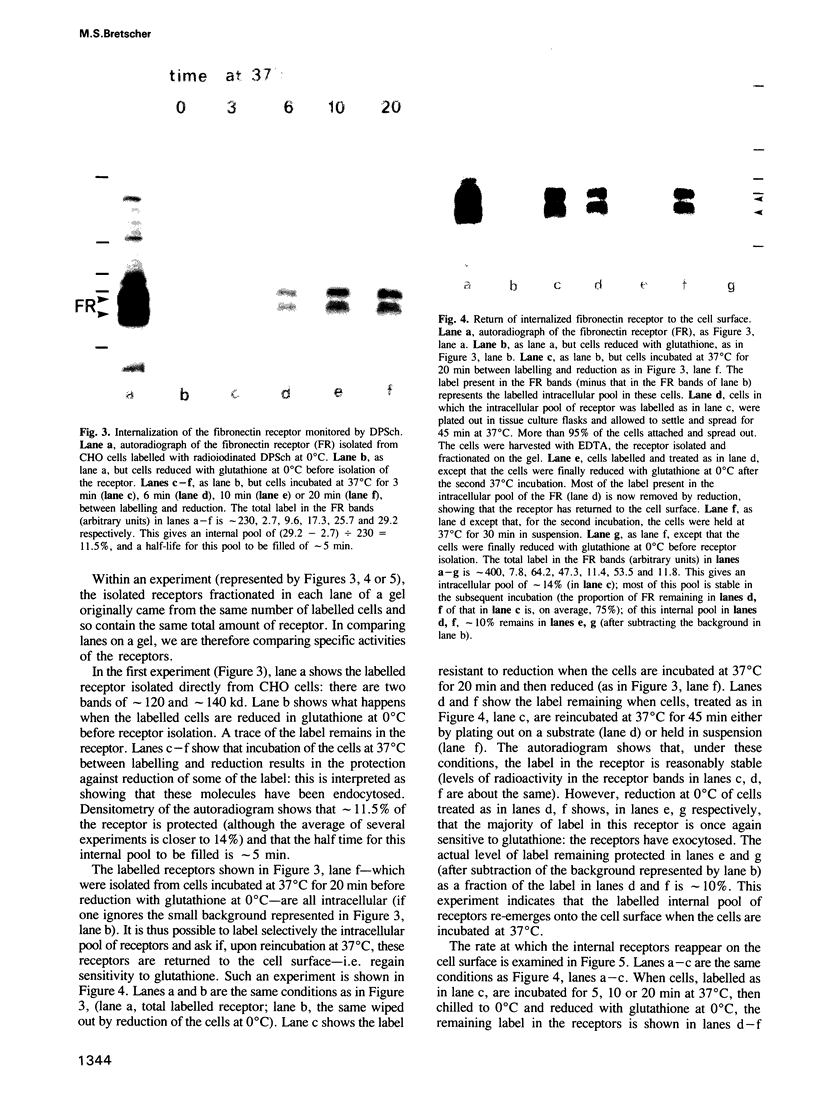
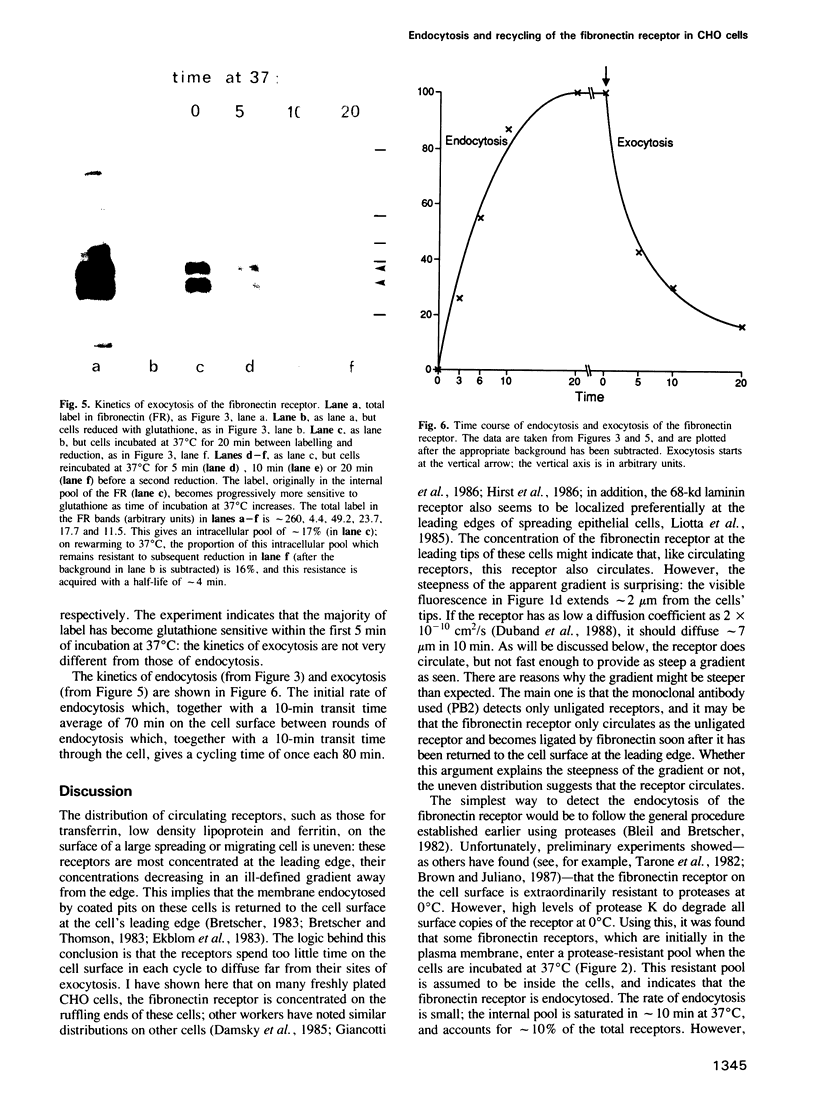
Abstract
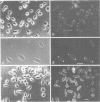
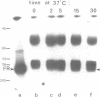
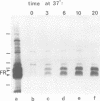
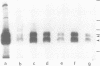
An anti-fibronectin receptor monoclonal antibody preferentially labels the leading edges of freshly plated CHO fibroblasts, suggesting that this receptor circulates through the endocytic cycle. Using a new labelling reagent, I show that this receptor is indeed endocytosed at 37 degrees C and then returned to the cell surface. These findings imply that fibronectin receptors are recirculated to the leading edge of a motile cell by the endocytic cycle, and establish that the processes of endocytosis/exocytosis and cell locomotion are intimately linked.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986 Feb;102(2):442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Bretcher M. S. Fibroblasts on the move. J Cell Biol. 1988 Feb;106(2):235–237. doi: 10.1083/jcb.106.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Directed lipid flow in cell membranes. Nature. 1976 Mar 4;260(5546):21–23. doi: 10.1038/260021a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Distribution of receptors for transferrin and low density lipoprotein on the surface of giant HeLa cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):454–458. doi: 10.1073/pnas.80.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis: relation to capping and cell locomotion. Science. 1984 May 18;224(4650):681–686. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Lutter R. A new method for detecting endocytosed proteins. EMBO J. 1988 Dec 20;7(13):4087–4092. doi: 10.1002/j.1460-2075.1988.tb03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N. Distribution of ferritin receptors and coated pits on giant HeLa cells. EMBO J. 1983;2(4):599–603. doi: 10.1002/j.1460-2075.1983.tb01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Alterations in neural crest migration by a monoclonal antibody that affects cell adhesion. J Cell Biol. 1985 Aug;101(2):610–617. doi: 10.1083/jcb.101.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Association between fibronectin receptor and the substratum: spare receptors for cell adhesion. Exp Cell Res. 1987 Aug;171(2):376–388. doi: 10.1016/0014-4827(87)90170-4. [DOI] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Expression and function of a putative cell surface receptor for fibronectin in hamster and human cell lines. J Cell Biol. 1986 Oct;103(4):1595–1603. doi: 10.1083/jcb.103.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Wang J., Hasegawa T., Yamada S. S., Yamada K. M. Regulation of fibronectin receptor distribution by transformation, exogenous fibronectin, and synthetic peptides. J Cell Biol. 1986 Nov;103(5):1649–1661. doi: 10.1083/jcb.103.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J. L., Nuckolls G. H., Ishihara A., Hasegawa T., Yamada K. M., Thiery J. P., Jacobson K. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988 Oct;107(4):1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Thesleff I., Lehto V. P., Virtanen I. Distribution of the transferrin receptor in normal human fibroblasts and fibrosarcoma cells. Int J Cancer. 1983 Jan 15;31(1):111–117. doi: 10.1002/ijc.2910310118. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Comoglio P. M., Tarone G. A 135,000 molecular weight plasma membrane glycoprotein involved in fibronectin-mediated cell adhesion. Immunofluorescence localization in normal and RSV-transformed fibroblasts. Exp Cell Res. 1986 Mar;163(1):47–62. doi: 10.1016/0014-4827(86)90557-4. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G., Knudsen K., Damsky C., Comoglio P. M. Cleavage of a 135 kD cell surface glycoprotein correlates with loss of fibroblast adhesion to fibronectin. Exp Cell Res. 1985 Jan;156(1):182–190. doi: 10.1016/0014-4827(85)90272-1. [DOI] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F., Buck C. A. A monoclonal antibody identifies a glycoprotein complex involved in cell-substratum adhesion. Exp Cell Res. 1985 Mar;157(1):218–226. doi: 10.1016/0014-4827(85)90164-8. [DOI] [PubMed] [Google Scholar]
- Kolega J., Shure M. S., Chen W. T., Young N. D. Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci. 1982 Apr;54:23–34. doi: 10.1242/jcs.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Kronebusch P. J., Rose J. K., Singer S. J. A critical role for the polarization of membrane recycling in cell motility. Cell Motil Cytoskeleton. 1987;8(2):182–189. doi: 10.1002/cm.970080210. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Horan Hand P., Rao C. N., Bryant G., Barsky S. H., Schlom J. Monoclonal antibodies to the human laminin receptor recognize structurally distinct sites. Exp Cell Res. 1985 Jan;156(1):117–126. doi: 10.1016/0014-4827(85)90266-6. [DOI] [PubMed] [Google Scholar]
- MARCUS P. I. Dynamics of surface modification in myxovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1962;27:351–365. doi: 10.1101/sqb.1962.027.001.033. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., De Boisfleury Chevance A. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982 Dec;95(3):960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H., Read M., Robinson H. Isolation of temperature sensitive mammalian cells by selective detachment. J Cell Physiol. 1973 Dec;82(3):325–331. doi: 10.1002/jcp.1040820302. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Tarone G., Galetto G., Prat M., Comoglio P. M. Cell surface molecules and fibronectin-mediated cell adhesion: effect of proteolytic digestion of membrane proteins. J Cell Biol. 1982 Jul;94(1):179–186. doi: 10.1083/jcb.94.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]