Summary
In flowering plants, immotile sperm cells develop within the pollen grain and are delivered to female gametes by a pollen tube [1, 2]. Upon arrival at the female gametophyte, the pollen tube stops growing and releases sperm cells for successful fertilization [3]. Several female signaling components essential for pollen tube reception have been identified [4–11]); however, male components remain unknown. We show that the expression of three closely related MYB transcription factors is induced in pollen tubes by growth in the pistil. Pollen tubes lacking these three transcriptional regulators fail to stop growing in synergids, specialized cells flanking the egg cell that attract pollen tubes [12–16] and degenerate upon pollen tube arrival [17, 18]. myb triple mutant pollen tubes also fail to release their sperm cargo. We define a suite of pollen tube-expressed genes regulated by these critical MYBs and identify transporters, carbohydrate active enzymes, and small peptides as candidate molecular mediators of pollen-female interactions necessary for flowering plant reproduction. Our data indicate that de novo transcription in the pollen tube nucleus during growth in the pistil leads to pollen tube differentiation required for release of sperm cells.
Results and Discussion
MYB97, 101, and 120 are localized to the pollen tube nucleus
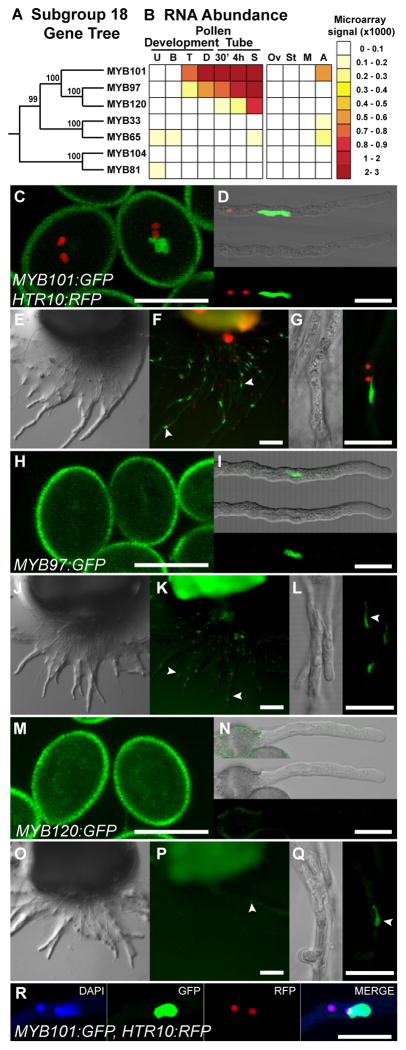
Microarray comparisons of the transcriptomes of pollen tubes grown in vitro with those grown through pistil tissue showed the pollen tube transcriptome is dramatically remodeled in response to the pistil and that multiple transcription factors are induced [19]. We proposed that these transcription factors control expression of genes required for pollen tube - pistil interactions [19]. We focused our attention on MYB120 because its expression was higher in pollen tubes grown through pistil tissue relative to pollen tubes grown in vitro [19]. MYB120 is a member of a subgroup of the >130 Arabidopsis R2-R3 MYBs called S18 [20]. We integrated the phylogeny of the Arabidopsis S18 MYB family with the expression pattern of these genes during pollen development and pollen tube growth and found that MYB97, MYB101, and MYB120 formed a closely related, pollen tube-expressed sub-clade (Figure 1A, B).
Figure 1. MYB97, MYB101, and MYB120 localize to the growing pollen tube nucleus.
(A) Phylogeny of the S18 group of MYBs (bootstrap values at branches). (B) Expression heatmap of S18 MYBs. Pollen development: U, Unicellular; B, Bicellular, T, Tricellular [29]; D, Dry Pollen. Pollen tube: 30′, 30′ in vitro grown pollen; 4h - 4 hour in vitro grown pollen; S, Semi in vivo grown pollen [19]. Ov, Ovary; St, Stigma [32], M, maximum sporophytic expression from 7 tissues; A, Anther [31]. (C, H, M) Hydrated pollen grains of plants hemizygous for MYB:GFP reporter constructs. HTR10:RFP homozygous plants were transformed with MYB101:GFP, qrt1-2 plants were transformed with MYB97:GFP or MYB120:GFP. (D, I, N) Pollen tubes grown for 6 hours in vitro. (E, J, O) DIC and (F, K, P) Epifluorescence images of semi-in vivo grown MYB:GFP pollen. (G, L, Q) Confocal micrographs of semi-in vivo grown MYB:GFP pollen tubes. (R) DAPI staining of MYB101:GFP, HTR10:RFP pollen tubes grown in vitro.
MYB97:GFP, MYB101:GFP, and MYB120:GFP fusion proteins, expressed from their native promoters in transgenic plants accumulated in the pollen tube nucleus during pollen tube growth through the pistil (Figure 1C – 1R). MYB101:GFP was detected in the vegetative nucleus of mature pollen grains and pollen tubes (Figures 1C – 1G and 1R). MYB97:GFP was detected only in the vegetative nucleus of pollen tubes grown in vitro or through pistil tissue (Figures 1H–1L); the MYB120:GFP signal was the weakest and only detected in the vegetative nucleus of pollen tubes that had grown through pistil tissue (Figures 1M–1Q). These patterns of protein accumulation in the pollen tube nucleus reflect mRNA accumulation patterns and are consistent with the hypothesis that this group of MYBS regulates pollen tube gene expression in response to growth through the pistil.
myb triple mutant pollen tubes reach ovules, but fail to burst and do not release sperm
We identified T-DNA insertions [21, 22] in MYB97, MYB101, and MYB120 that disrupted mRNA accumulation (Figure S1A, B). Analysis of seed production in wild type, single, and multiple mutants showed that only plants lacking expression of MYB97, MYB101 and MYB120 (myb97-1, myb101-4, myb120-3; myb triple mutant hereafter) had reduced seed set and this reduction is pollen specific (Figure S1C–F). Expression of either MYB97:GFP, MYB101:GFP, MYB120:GFP, or a MYB120 genomic clone rescued the myb triple mutant seed set defect (Figure S2A), indicating the seed set defect is the result of loss of MYB function and that GFP fusion constructs (Figure 1) are functional. myb triple mutant pollen development is normal and pollen tube growth is comparable to wild type either in vitro or in a semi-in vivo assay (Figure S1H–K); however myb triple mutant pollen tubes appeared to overgrow in wild-type ovules (Figure S1G). These results point to a role for these three MYBs in regulating interactions between the pollen tube and the ovule or female gametophyte.
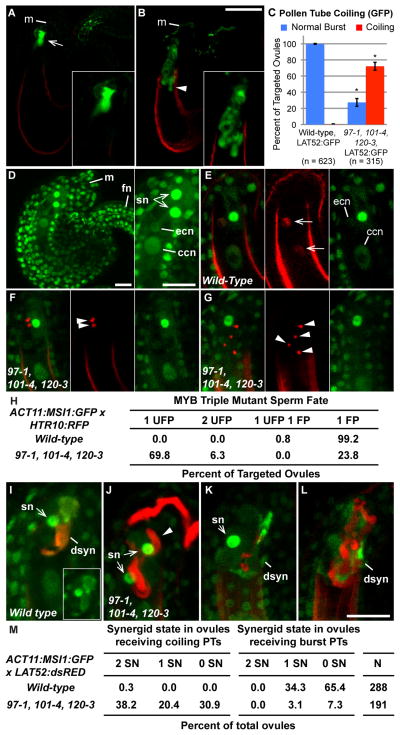
To quantitatively asses pollen tube - ovule interactions using confocal microscopy, we produced myb triple mutants that express green fluorescent protein (GFP) in the pollen tube cytoplasm (LAT52:GFP, [23] and crossed this pollen to male sterile1 (ms1) pistils. When ovules were targeted by wild-type pollen tubes expressing GFP, a diffuse green fluorescence pattern, indicative of pollen tube burst, was observed in one of the two synergids (Figure 2A). However, approximately 70% of ovules targeted by myb triple mutant pollen tubes contained intact (unburst) coiling pollen tubes and lacked the diffuse green fluorescence pattern observed following pollen tube burst (Figures 2B, C).
Figure 2. MYB97, MYB101, and MYB120 are required for pollen tube burst, sperm release and synergid degeneration.
ms1 pistils were pollinated with wild-type LAT52:GFP or myb triple mutant LAT52:GFP pollen 12 hours prior to dissection and imaging by confocal microscopy. (A) Wild-type GFP+ pollen tubes target the micropyle (m) and burst in the female gametophyte yielding a diffuse green signal (arrow). (B) myb triple mutant GFP+ target ovules, overgrow (arrowhead) and fail to burst, with a contained GFP signal inside the pollen tube (inset). Scale bars, 50μm. (C) Quantitative analysis of pollen tube burst by confocal microscopy. The percentage of ovules receiving a pollen tube with a normal burst or a coiling phenotype is shown as a percentage (+/− sd). P-value <0.005, student’s t-test. (D) Wild-type ACT11:MSI1:GFP ovule with position of micropyle (m) and funiculus (fn) indicated. Inset: sn, synergid nucleus; ecn, egg cell nucleus; ccn, central cell nucleus. (E–G) First panel, GFP and DsRed channels merged; middle panel, DsRed channel; last panel, GFP Channel. (E) Wild-type RFP+ sperm nuclei (arrows) undergo double fertilization. (F,G) myb triple mutant RFP+ sperm nuclei remain condensed and associated (arrowheads). (G) Multiple pairs of unfused sperm (arrowheads) were observed myb triple mutant pollinations. Scale bars, 50μm. (H) Quantitative analysis of double fertilization. 1 UFP, ovules with one pair of unfused sperm; 2 UFP, two pairs of unfused sperm; 1 UFP 1 FP, one unfused pair and one fused pair of sperm; 1 FP, one fused pair of sperm. (I–L) Wild-type ACT11:MSI1:GFP pistils were pollinated with wild-type LAT52:DsRed or myb triple mutant, LAT52:DsRed and allowed to grow for 12h. (I) Wild-type, LAT52:DsRed pollen tubes targeting wild-type, ACT11:MSI1:GFP ovules (dsn, degenerated synergid; sn, persisting synergid nucleus), Inset is GFP channel alone. (J) Coiling myb triple pollen tubes with two intact synergid nuclei, (K) one intact synergid or, (L) two degenerated synergids. Scale bars, 50μm. (M) Quantitative analysis of synergid degeneration. Percent of ovules targeted by pollen tubes with; 2 SN, two persisting synergid nuclei; 1 SN, one persisting synergid nucleus; 0 SN, no persisting synergid nuclei; N, total targeted ovules.
We used a sperm cell-specific histone variant fused to RFP (HTR10:HTR10:mRFP) to examine sperm cell release by myb triple mutant pollen tubes [24–26]. We crossed wild-type or myb triple mutant pollen expressing HTR10:HTR10:mRFP to females carrying a nuclear localized GFP reporter (ACT11:MSI1:GFP, [24] that marks female gametophyte nuclei (Figure 2D). When triple mutant pollen was used, ~70% of ovules contained sperm nuclei that were compact, close together, and did not co-localize with either female gamete nucleus (Figures 2F–H). In contrast, nearly all wild-type sperm nuclei had separated from each other and fused with female gamete nuclei (Figure 2E, H). These data suggest that gamete fusion does not occur in ovules targeted by myb triple mutants because sperm are not released from myb triple mutant pollen tubes and therefore cannot fuse with their female partners. We propose that pollen tube genes regulated by MYB97, MYB101, and MYB120 are essential for pollen tube growth arrest and sperm cell release in the female gametophyte.
We observed the presence of multiple un-fused sperm cell pairs in ovules targeted by myb triple mutant pollen tubes (Figure 2G), and multiple aniline blue-stained pollen tubes entering a single ovule (Figure S1G). This is consistent with our observation that sperm cells are not released in these ovules and the recent findings that fusion between male and female gametes is required to prevent multiple pollen tubes from targeting a single ovule [26, 27].
To analyze the relationship between entry and growth of myb triple mutant pollen tubes and synergid degeneration, we examined interactions between myb triple mutant pollen tubes expressing a red fluorescent protein (LAT52:DsRed, [28] and female gametophytes expressing ACT11:MSI1:GFP. Synergid degeneration was defined as loss of discrete nuclear ACT11:MSI1:GFP signal (Figure 2I, [24, 26]).
We consistently observed two GFP-positive synergid nuclei in untargeted ACT11:MSI1:GFP ovules (Figure 2D). However, we observed degeneration of one synergid nucleus that co-localized with a diffuse DsRed signal from the pollen tube cytoplasm when wild-type LAT52:DsRed pollen tubes were received by ACT11:MSI1:GFP ovules. The discharged wild-type pollen tube cytoplasm filled the position vacated by the degenerated synergid and formed a characteristic hook around the egg cell (Figure 2I). Importantly, we never observed synergid degeneration without pollen tube burst in crosses with wild type, supporting the hypothesis that a synergid cell degenerates soon after pollen tube arrival. In contrast, we observed a dramatic increase in the frequency of ovules (38%) containing two intact synergid nuclei in the presence of a coiling myb triple mutant pollen tube (Figure 2J, M). Interestingly, ~50% of ovules contained a coiling myb triple mutant pollen tube(s) that had triggered synergid degeneration, apparently without pollen tube burst (Figures 2K–M). It is possible that mechanical stress from pollen tube overgrowth may induce degeneration of one or both synergids (Figure 2K, L). However, a mechanical stress hypothesis for synergid degeneration is not consistent with the finding that two synergids frequently persist in the presence of a coiling pollen tube(s) (Figure 2J, M). These data suggest the possibility that signaling from the pollen tube, potentially disrupted in myb triple mutants, triggers synergid degeneration.
MYB97, MYB101, and MYB120 regulate pollen tube gene expression
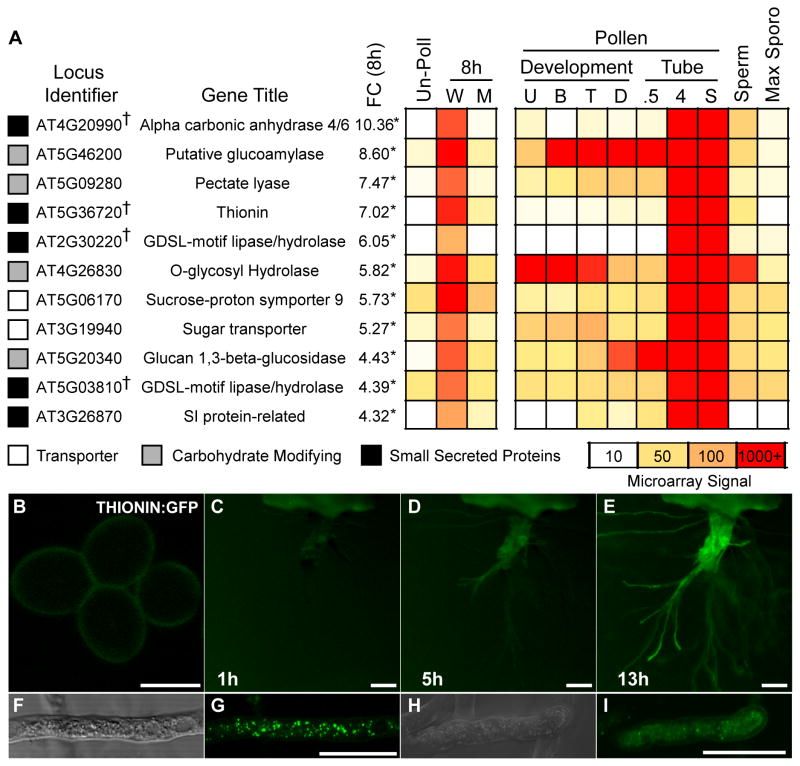
To identify the pollen tube-expressed genes regulated by MYB97, MYB101, and MYB120 as tubes grow through the pistil, we performed microarray analysis (Affymetrix ATH1, three biological replicates) on ms1 pistils pollinated with either wild-type or myb triple mutant pollen. We identified differentially expressed genes eight hours after pollination and compared these patterns with those observed in unpollinated pistils. This approach facilitates analysis of pollen tube gene expression in the context of an intact pistil. We identified 48 genes with significantly different transcript abundance in pistils pollinated with myb triple mutant compared to pistils pollinated with wild type (45 were lower in myb triple mutants [down]; 3 were higher [up]) (Table S1). Of 45 genes that were down in pistils pollinated by myb triple mutants, only three had obvious expression in unpollinated pistils (Table S1), indicating that microarray analysis of pollinated pistils detected primarily pollen tube-expressed genes that are potentially regulated by MYB97, MYB101, and MYB120 during pollen tube growth through the pistil.
We analyzed expression patterns during pollen development and tube growth for the 11 genes that were statistically different and at least four-fold lower in myb triple mutant pollinations versus wild type (Figure 3A). We compared our data with previously published pollen [19, 29], sperm cell [30], and sporophytic [31, 32] microarray data. All 11 genes have their peak expression in pollen tubes that have grown through pistil tissue (Figure 3A). Reverse-transcription followed by real time quantitative PCR (RT-qPCR) analysis indicated that MYB activity is required for mRNA accumulation in pollinated pistils for each of these 11 genes (Figure S3). We conclude these 11 genes are either direct or indirect targets of MYB activation in the pollen tube as it grows through the pistil.
Figure 3. MYB97, MYB101, and MYB120 regulate gene expression during pollen pistil interactions.
(A) Expression heat map of genes identified as differentially expressed by microarray analysis. ms1 pistils were left un-pollinated (Un-Poll), or pollinated by either wild-type (W) or myb triple mutant (M) pollen and grown for 8 hours. The genes identified (Locus Identifier) are indicated with the gene name or predicted function (Gene Title). †, Array ID identifies two. The fold change (FC) between wild-type and myb triple mutant pollinations is shown, with asterisks (*) indicating fold changes that were statistically significant (P<0.05). Previously published data from pollen grain development [29], pollen tube growth [19], sperm cells [30], and sporophytic tissues [31] are included for comparison. Pollen development: U, Unicellular; B, Bicellular; T, Tricellular; D, Dry Pollen. Pollen tube: 0.5, 30′ in vitro grown pollen; 4, 4 hour in vitro grown pollen; S, Semi in vivo grown pollen tubes; Sperm, Isolated sperm cells, M - Max sporophytic expression from 7 tissue types.
Genes regulated by pollen tube MYBs include transporters, carbohydrate active enzymes, and small proteins
The 11 potentially MYB-regulated genes identified above were enriched for transmembrane transporters (2/11), carbohydrate active enzymes (4/11), and small, secreted proteins (5/11, black squares Figure 3A). These gene categories are also enriched across all 48 differentially expressed genes (transmembrane transporters 7/48, carbohydrate active enzymes 6/48, and small secreted proteins 17/48, Table S1). One of these small secreted proteins is a cysteine-rich thionin (AT5G36720, CRP 2460[33]) that is specifically expressed in pollen tubes and requires MYB97, MYB101, and MYB120 for expression (Figures 3A, S3). Thionins have been shown to have antimicrobial properties attributed in part to their ability to form membrane pores [34–36], a function that could be involved in mechanisms required for pollen tube burst. THIONIN:GFP signal was undetectable in pollen grains (Figure 3B); however, we detected strong GFP signal in pollen tubes grown in the semi in vivo assay for at least 5 hours (Figures 3C–E). When we examined semi in vivo grown pollen tubes using confocal microscopy we observed THIONIN:GFP in puncta throughout the body of the pollen tube cytoplasm, suggesting localization in the secretory pathway (Figures 3F–I).
Conclusions
Loss of MYB97, MYB101, and MYB120 function results in lack of expression of a suite of pollen tube expressed genes (Figure 3 and Table S1) and disrupts the ability of the pollen tube to stop growing and burst once it reaches a synergid cell (Figure 2). We also found that MYB97, MYB101, and MYB120 expression is induced by growth through pistil tissue (Figure 1). These findings suggest that the ability of pollen tubes to respond to female signals required for sperm cell release is activated by growth through the pistil and controlled by this closely related group of transcription factors. MYB-regulated genes could be required for any or all of the following critical activities: pollen tube perception of female reception signals, recognition of pollen tube arrival by the female, or the ability of the pollen tube to respond to these signals and release sperm cells. It has been demonstrated that pollen tube - female gametophyte recognition fails in interspecific crosses of Rhododendron [37] or Arabidopsis [6], resulting in pollen tube coiling within the exotic ovule. Further analysis of pollen tube genes regulated by MYB97, MYB101, and MYB120 will provide insight into the determinants of species specificity in flowering plant mating and the male components of pollen tube - female gametophyte signaling required to release sperm cells.
Experimental Procedures
Plant Growth
Procedures for selection of antibiotic and/or herbicide resistant seedlings and plant growth conditions have been described previously [19, 38–40].
Genetic Analysis
Full-length MYB101 and MYB97 cDNA clones were available (NM_128805, NM_118827), but MYB120 cDNAs had not been cloned (Genbank Accession #: KC544014). ACT11:MSI1:GFP, HTR10:HTR10:mRFP, and LAT52:GFP plants were obtained from Frederic Berger (National University of Singapore, [24]. LAT52:DsRed [28] plants were obtained from Greg Copenhaver (UNC, Chapel Hill). SALK and SAIL lines (Col-0 accession) and ms1/MS1 (CS75, Landsberg ecotype) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH).
Imaging
Procedures for imaging pistils containing aniline blue-stained pollen tubes [41] or pollen tubes expressing fluorescent proteins [26] have been described.
Accessing Microarray Data
Raw data (.CEL and CHP files) from 9 microarrays reported in this study have been deposited in Gene Expression Omnibus public repository (Accession #: GSE44405).
Supplementary Material
Highlights.
Pollen tubes differentiate in response to pistil tissue to release sperm cells
Three pollen tube MYB transcription factors are required for sperm release
MYBs control pollen tube – female interactions
MYBs regulate a suite of genes potentially required for sperm release
Acknowledgments
NSF Grant IOS-1021917 funded this work. We thank Frederic Berger, Gregory Copenhaver, and Daphne Preuss for generously providing transgenic plants expressing markers. JC, KW, EC were funded by the HHMI-Brown Summer Scholars program. The Brown University Genomics Core Facility is supported by grant: 8 P30 GM10103410.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berger F, Twell D. Germline specification and function in plants. Annu Rev Plant Biol. 2011;62:461–484. doi: 10.1146/annurev-arplant-042110-103824. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci U S A. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler SA, Grossniklaus U. She’s the boss: signaling in pollen tube reception. Curr Opin Plant Biol. 2011 doi: 10.1016/j.pbi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Current Biology. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 5.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development (Cambridge, England) 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 6.Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 7.Rotman N, Gourgues M, Guitton AE, Faure JE, Berger F. A dialogue between the SIRENE pathway in synergids and the fertilization independent seed pathway in the central cell controls male gamete release during double fertilization in Arabidopsis. Mol Plant. 2008;1:659–666. doi: 10.1093/mp/ssn023. [DOI] [PubMed] [Google Scholar]
- 8.Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, Krishnan A, Alvarez-Mejia C, Vielle-Calzada JP, Lee YR, et al. Maternal Control of Male-Gamete Delivery in Arabidopsis Involves a Putative GPI-Anchored Protein Encoded by the LORELEI Gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 10.Boisson-Dernier A, Frietsch S, Kim TH, Dizon MB, Schroeder JI. The peroxin loss-of-function mutation abstinence by mutual consent disrupts male-female gametophyte recognition. Curr Biol. 2008;18:63–68. doi: 10.1016/j.cub.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 12.Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. doi: 10.1126/science.1062429. [DOI] [PubMed] [Google Scholar]
- 13.Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN. MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell. 2005;17:2981–2992. doi: 10.1105/tpc.105.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marton ML, Cordts S, Broadhvest J, Dresselhaus T. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science. 2005;307:573–576. doi: 10.1126/science.1104954. [DOI] [PubMed] [Google Scholar]
- 15.Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi H, Higashiyama T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 2012;10:e1001449. doi: 10.1371/journal.pbio.1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faure JE, Rotman N, Fortune P, Dumas C. Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J. 2002;30:481–488. doi: 10.1046/j.1365-313x.2002.01305.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 2007;144:1753–1762. doi: 10.1104/pp.107.098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 2009;5:e1000621. doi: 10.1371/journal.pgen.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 22.Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Twell D, Yamaguchi J, McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development (Cambridge, England) 1990;109:705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- 24.Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol. 2007;17:1032–1037. doi: 10.1016/j.cub.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Hamamura Y, Saito C, Awai C, Kurihara D, Miyawaki A, Nakagawa T, Kanaoka MM, Sasaki N, Nakano A, Berger F, et al. Live-Cell Imaging Reveals the Dynamics of Two Sperm Cells during Double Fertilization in Arabidopsis thaliana. Curr Biol. 2011;21:497–502. doi: 10.1016/j.cub.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Beale KM, Leydon AR, Johnson MA. Gamete Fusion Is Required to Block Multiple Pollen Tubes from Entering an Arabidopsis Ovule. Curr Biol. 2012 doi: 10.1016/j.cub.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol. 2012;22:1084–1089. doi: 10.1016/j.cub.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 28.Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, Copenhaver GP. Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proc Natl Acad Sci U S A. 2007;104:3913–3918. doi: 10.1073/pnas.0608936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijo JA, Becker JD. Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol. 2008;148:1168–1181. doi: 10.1104/pp.108.125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 32.Swanson R, Clark T, Preuss D. Expression profiling of Arabidopsis stigma tissue identifies stigma-specific genes. Sexual Plant Reproduction. 2005;18:163–171. [Google Scholar]
- 33.Silverstein KA, Moskal WA, Jr, Wu HC, Underwood BA, Graham MA, Town CD, VandenBosch KA. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 2007;51:262–280. doi: 10.1111/j.1365-313X.2007.03136.x. [DOI] [PubMed] [Google Scholar]
- 34.Stec B. Plant thionins--the structural perspective. Cell Mol Life Sci. 2006;63:1370–1385. doi: 10.1007/s00018-005-5574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florack DE, Stiekema WJ. Thionins: properties, possible biological roles and mechanisms of action. Plant Mol Biol. 1994;26:25–37. doi: 10.1007/BF00039517. [DOI] [PubMed] [Google Scholar]
- 36.Oard SV. Deciphering a mechanism of membrane permeabilization by alpha-hordothionin peptide. Biochim Biophys Acta. 2011;1808:1737–1745. doi: 10.1016/j.bbamem.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Williams EG, Kaul V, Rouse JL, Palser BF. Overgrowth of Pollen Tubes in Embryo Sacs of Rhododendron Following Interspecific Pollinations. Australian Journal of Botany. 1986;34:413–423. [Google Scholar]
- 38.Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D. Arabidopsis hapless mutations define essential gametophytic functions. Genetics. 2004;168:971–982. doi: 10.1534/genetics.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2(GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development (Cambridge, England) 2006;133:4761–4769. doi: 10.1242/dev.02683. [DOI] [PubMed] [Google Scholar]
- 40.Wong JL, Leydon AR, Johnson MA. HAP2(GCS1)-dependent gamete fusion requires a positively charged carboxy-terminal domain. PLoS Genet. 2010;6:e1000882. doi: 10.1371/journal.pgen.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.