Abstract
Background
Experimental studies suggest that metabolic myocardial support by intravenous (IV) glucose, insulin, and potassium (GIK) reduces ischemia-induced arrhythmias, cardiac arrest, mortality, progression from unstable angina pectoris (UAP) to acute myocardial infarction (AMI), and MI size. However, trials of hospital administration of IV GIK to patients with ST elevation MI (STEMI) have generally not shown favorable effects, possibly due to the GIK intervention taking place many hours after ischemic symptom onset. A trial of GIK used in the very first hours of ischemia has been needed, consistent with the timing of benefit seen in experimental studies.
Objective
The Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care (IMMEDIATE) Trial tested whether, if given very early, GIK could have the impact seen in experimental studies. Accordingly, distinct from prior trials, IMMEDIATE tested the impact of GIK 1) in patients with acute coronary syndromes (ACS), rather than only AMI or STEMI, and 2) administered in prehospital emergency medical service (EMS) settings, rather than later, in hospitals, following emergency department evaluation.
Design
IMMEDIATE was an EMS-based randomized placebo-controlled clinical effectiveness trial conducted in 13 cities across the US which enrolled 911 participants. Eligible were patients age 30 or older for whom a paramedic performed a 12-lead electrocardiogram (ECG)to evaluate chest pain or other symptoms suggestive of ACS for whom electrocardiograph-based ACI-TIPI (acute cardiac ischemia time-insensitive predictive instrument) indicated a > 75% probability of ACS, and/or the TPI (thrombolytic predictive instrument) indicated presence of a STEMI, or if local criteria for STEMI notification of receiving hospitals were met. Prehospital IV GIK or placebo was started immediately.
Pre-specified were the primary endpoint of progression of ACS to infarction, and as major secondary endpoints, the composite of cardiac arrest or in-hospital mortality; 30-day mortality; and the composite of cardiac arrest, 30-day mortality or hospitalization for heart failure (HF). Analyses were planned on an intent-to-treat basis, on a modified intent-to-treat group who were confirmed in emergency departments to have ACS, and for participants presenting with STEMI.
Conclusion
The IMMEDIATE Trial tested whether GIK, when administered as early as possible in the course of ACS by paramedics using ACI-TIPI and TPI decision support, would reduce progression to AMI, mortality, cardiac arrest, and HF. It also tested whether it would provide clinical and pathophysiological information on GIK’s biological mechanisms.
BACKGROUND
The Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care(IMMEDIATE) Trial was a National Institutes of Health (NIH) National Heart Lung and Blood Institute (NHLBI) sponsored double-blind randomized controlled clinical effectiveness trial to evaluate the impact of intravenous (IV) glucose, insulin, and potassium (“GIK”) for acute coronary syndromes (ACS). The Trial tested whether myocardial metabolic support by GIK, administered as early as possible to patients with ACS, would reduce progression of unstable angina pectoris (UAP) to acute myocardial infarction (AMI), cardiac arrest, mortality, heart failure (HF), and other endpoints related to potential myocardial salvage and the biological mechanisms related to such effects.
Prior experimental and clinical studies have shown that GIK metabolically protects the myocardium against ischemic injury and related metabolic perturbations and slows the rate of ischemic cell death.1–8 Research also suggests that for patients with electrocardiogram (ECG) ST segment elevation MI (STEMI), GIK may lengthen the time-window for benefit from coronary reperfusion, thereby enhancing the effect of reperfusion in limiting infarction.9 These benefits are clearly related to the time that GIK is administered in the course of cardiac ischemia, with effectiveness increasing with early administration.10 However, although GIK has been tested for AMI in hospital settings, it had not previously been tested clinically for all ACS, i.e., prior to manifest infarction. Nor had it been tested when used as early as possible, in initial prehospital emergency medical service (EMS) care. Such an approach would avoid delays in treatment while getting to the hospital as well as further delays at the hospital. To address these issues, this trial focused on immediate GIK treatment at the earliest possible time in the treatment of ACS, in EMS settings.
RATIONALE FOR STUDY APPROACH AND DESIGN
Target Patients and Treatment Model
The IMMEDIATE Trial treatment model is based on the fact that in actual clinical practice, the decision to treat immediately with GIK must be made using presenting features of ACS rather than a confirmed AMI diagnosis; ischemic myocardium will be most salvageable early in the ischemic process, prior to progressing to infarction, usually within six hours. An agent that protects ischemic myocardium from progressing to necrosis could prevent or mitigate infarction in those early hours and thereby preserve left ventricular (LV) function.
The importance of very early treatment is also underscored by the fact that 50% of deaths from ACS/AMI occur in the first hour, frequently attributed to ischemia-related ventricular fibrillation (VF) progressing to cardiac arrest. Ischemia related arrhythmias and cardiac arrest are considered related to increasing levels of cellular free fatty acids (FFAs) and their derivatives that accumulate during ischemia. Arrhythmias and cardiac arrest are thought to be due to FFA detergent-like properties associated with increased sarcolemmal and mitochondrial membrane damage, increased intracellular calcium, primary arrhythmias, and accelerated functional deterioration.1,11 Because GIK decreases both circulating FFA levels and myocardial FFA uptake, it could reduce susceptibility to ischemic VF and cardiac arrest, with impact dependent on how early in ischemia it is instituted.
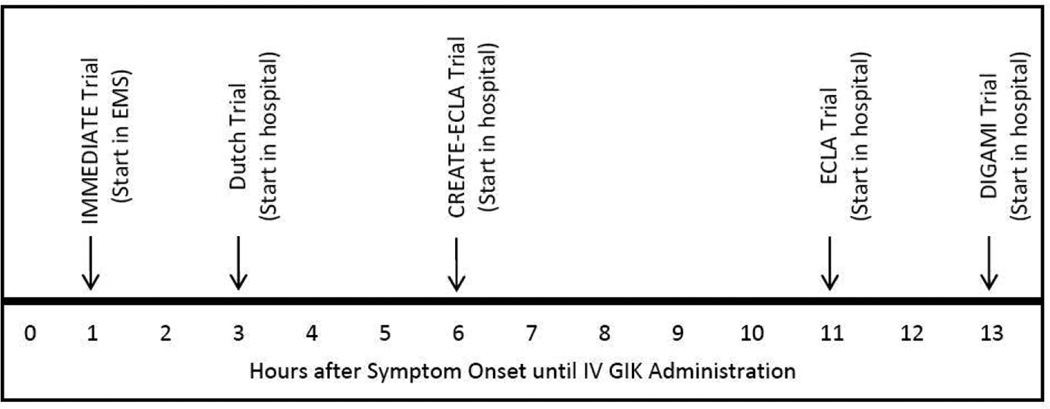
In prior trials of GIK, the interval from symptom onset to GIK initiation was the total time the patient waited before calling for medical attention, the time elapsed before the ambulance arrived (or the patient arrived at a hospital), the time taken to diagnose ACS, and the time required to start IV GIK. Additionally, prior trials waited until MI was evident, and in recent trials, the time until starting GIK had varied from 2 hours 8 to 11 hours;6 in earlier trials, up to several days (Figure 1).12,13 Given that cardiac arrest and death most commonly occur in the first hour, and that the optimal opportunity for myocardial salvage also occurs early, these trials were not optimally designed to test GIK’s impact on these outcomes.
Figure 1.
Time from symptom onset to use of GIK in major trials
Additionally, if GIK administration is to prolong the time-dependent potential benefit from coronary reperfusion for STEMI, then it must be started as soon as possible. Such a GIK effect will be related to the time spent in transport to a cardiac center where reperfusion therapy can be performed and the promptness of treatment, once there, which in some circumstances could be a considerable period. This emphasized the need to test GIK in the range of “real-world” settings with the broad inclusion characteristic of effectiveness trials.
Clinically, to accrue these time-dependent benefits, target patients must be those with suspected and high likelihood ACS, not those with already established AMI. When a physician or paramedic first encounters a patient with ACS, often it is not possible to distinguish UAP from AMI–and to wait for the diagnosis of AMI to become clear would be to miss the most important period for benefit from GIK. Moreover, GIK is likely to be beneficial throughout the evolution of ACS, not just upon establishment of AMI. Indeed, early GIK treatment of ACS may abort the evolution of UAP to AMI. Rogers et al14 found that among patients with ischemic chest pain randomized to GIK or control, AMI was confirmed in 64% (61/95) of those treated with GIK, vs. 77% (73/95) of controls, a 17% relative reduction in progression to AMI (P=0.06).
Thus, for general clinical use, administering GIK very early requires very rapid and accurate identification of ACS in EMS and emergency department (ED) settings. To do this, in the IMMEDIATE Trial, an approach was developed for paramedic decision support by the electrocardiograph-based ACI-TIPI (acute cardiac ischemia time-insensitive predictive instrument) and TPI (thrombolytic predictive instrument).15–17 The ACI-TIPI calculates a 0–100% prediction of acute coronary syndrome for a given individual and has been shown to aid clinicians’ assessment and triage of patients presenting with chest pain and other symptoms suggestive of acute ischemia.16,17 The TPI has been shown to improve recognition and treatment of STEMI, especially for patients who are less likely to be recognized for use of reperfusion therapy, such as women and those with non-anterior STEMI, and when consultation with off-site physicians is required, such as in very small EDs or EMS.15,17 The ACI-TIPI and TPI are available in conventional electrocardiographs’ software so that their predictions are printed on the patient’s ECG to supplement clinicians’ decision-making. In the wide variety of IMMEDIATE Trial sites, we showed that the use of these predictive instruments, based on an ACI-TIPI threshold of >75% probability of ACS and/or detection of a STEMI by the TPI, improved recognition of ACS and STEMI to the level needed for the Trial.17
GIK Formula
Based on dose-response studies of glucose and insulin aimed to maximize myocardial glucose uptake and decrease arterial FFA levels and myocardial FFA uptake, and data showing improved cardiac function, decreased ventricular arrhythmias, and a trend toward a decreased mortality risk,4 the IMMEDIATE Trial used the Rackley GIK formula: 30% glucose (300 gm/L), 50 units of regular insulin per liter, and 80 mEq of KCl/L, given IV at 1.5 ml/kg/hour, approximately 100 ml/hour for a 70 kg patient.
In studies of the Rackley formula, during GIK treatment for AMI, despite the significant volume infusion, pulmonary capillary pressure decreased and cardiac output and ejection fraction increased.4 This presumably resulted from improved systolic and/or diastolic function, consistent with experiments showing glucose and insulin treatment can improve both systolic and diastolic dysfunction during ischemia and reperfusion. Nonetheless, for the IMMEDIATE Trial, concern about the volume load during AMI, especially in the setting of HF, led to two modifications in the use of the Rackley regimen. First was exclusion of the approximately 5% of patients with ACS (10% of AMI) manifesting significant pulmonary congestion and/or cardiogenic shock (Killip classes 3 and 4). These patients have the highest mortality risk and thus might have the greatest potential to benefit from GIK, but they also may be at significant risk for worsening of their pulmonary congestion by GIK infusion, which would be challenging to manage in the EMS setting. Moreover, patients with HF receiving placebo would receive an infused volume without the potential benefit of GIK, an unjustifiable risk. Second was the use of 12 rather than the 48 hours of infusion that Rackley and others had used. This too was related to concerns about the volume load leading to HF, especially for placebo arm participants, who would receive no potential benefit, and to make the logistics as unobtrusive as possible and consistent with the emphasis on testing very early use of GIK. Also, although prior trials had demonstrated the safety of GIK infusion, given that adverse effects are related to duration of administration, their occurrence could be mitigated by shorter administration.
METHODS
Study Hypotheses
Based on the factors outlined above, the original primary study hypothesis was that GIK would reduce all-cause mortality; the two primary study endpoints were survival at 30 days and at one year. Major secondary hypotheses were that GIK would reduce the pre- and in-hospital incidence of cardiac arrest or mortality, that it would reduce development of HF; and that it would reduce progression of UAP to AMI. The study design then included both EMS and ED patients. However, at initiation, based on the recommendation of the NHLBI Protocol Review Committee, with which the investigators agreed, the decision was made to enroll only EMS patients, to fully focus on immediate use of GIK as early as possible in prehospital settings. However, eliminating patients who would have been enrolled in EDs reduced study candidates by over 50%. Because enrollment in the prehospital setting was difficult and required much more resources than were available to the Trial, enrollment of the 15,450 participants needed to provide acceptably high statistical power to detect the likely impact on the mortality endpoints was no longer realistic. To adapt to this, in conjunction with the NHLBI and its IMMEDIATE Trial Data Safety and Monitoring Board (DSMB), the Trial was temporarily suspended and the study hypotheses re-ordered to adapt to the projected 880 participants that were eventually enrolled with the new EMS-based approach. The new primary endpoint became progression to completed infarction, for which there would be sufficient statistical power, and accordingly, the original primary mortality hypothesis joined the other major secondary hypotheses. The revised list of hypotheses, testing GIK vs. placebo was:
Primary Hypothesis
Immediate GIK will reduce progression of UAP to AMI, as evidenced by fewer patients having biomarker and ECG evidence of AMI.
Major Secondary Hypotheses
Immediate GIK treatment will lead to better survival at a) 30 days; and b) one year.
Immediate GIK will reduce the pre/in-hospital incidence of cardiac arrest, VF or ventricular tachycardia requiring defibrillation or cardioversion, or mortality.
Immediate GIK treatment will reduce patients’ propensity for developing HF, reflected by lower rates of a) hospitalization for HF or mortality within 30 days; and b) hospitalization for HF or mortality within one year.
Other Secondary Hypotheses
The impact of GIK on mortality, cardiac arrest, and development of HF will be modified by time duration from ischemic symptom onset until the initiation of GIK.
For patients with ACS presenting with ECG ST segment elevation, immediate GIK will reduce the composite endpoint of pre- or in-hospital cardiac arrest, hospitalization for HF, or mortality.
For patients receiving coronary reperfusion treatment for STEMI, immediate GIK treatment will modify a) the overall impact of reperfusion therapy on the composite endpoint of pre/in-hospital cardiac arrest, hospitalization for HF, or mortality; and b) the influence of time duration from initial presentation (EMS or ED) until reperfusion.
In a sample of patients with ACS to study biological mechanisms, immediate GIK use will reduce FFA levels and increase the proportion of FFA comprised by n-3 polyunsaturated fatty acids (n-3 PUFA).
In the biological mechanism sample, immediate GIK use will be associated with physiologic indicators reflecting a lower propensity for HF at 30 days including a) smaller infarct size by sestamibi perfusion imaging; b) better preserved LV function by gated SPECT imaging; and c) lower brain natriuretic peptide (BNP) levels.
Enrollment and Intervention
During the enrollment period, at participating EMS systems, all patients for whom a 12-lead ECG was obtained for the evaluation of chest pain or other symptoms suggestive of ACS in the prehospital setting had a screening checklist completed by their paramedic, to determine eligibility for enrollment. To be candidates for the IMMEDIATE Trial (www.clinicaltrials.gov Identifier NCT00091507), patients needed to be age 30 or older and to be having ACS as determined by the EMS paramedic, based on symptoms and presenting EMS 12-lead ECG. Paramedic identification of potential study participants was aided by electrocardiograph-based ACI-TIPI and TPI decision support, as detailed elsewhere,17 and for most of the Trial, this included use of a ACI-TIPI threshold of 75% or greater predicted probability of having ACS and/or the detection of STEMI by the TPI.17 (EMS defibrillator-electrocardiographs were provided with ACI-TIPI and TPI capabilities by their manufacturers, Medtronic Physio-Control, Phillips Healthcare, and Zoll Medical.) Patients were excluded if they had end stage renal failure requiring dialysis, were hemodynamically unstable (systolic blood pressure <100), were determined to be unable to give consent due to impaired reasoning, altered mental status or dementia, or had clinically significant HF (more than basilar rales or Killip Class 3 or 4 AMI).
To obtain informed consent, we followed the Exception from Informed Consent Requirements for Emergency Research per the Code of Federal Regulations 21 CFR 50.24,18 including community consultation, institutional review board (IRB) approval, and full written consent once stabilized in the hospital.19 For the ancillary biological mechanism study, additional written consent was obtained for blood tests during the first 12 hours and to have 30-day assessment by LV imaging scan and a blood test for BNP level.
Randomization
The IMMEDIATE Data Coordinating Center (DCC) generated and provided a randomization table to the manufacturer of the study drug; the randomization block size was 4. Intervention and placebo drug infusion packets, labeled and shipped from the manufacturer to the study centers for distribution to the paramedic units, were identical in appearance and contained no treatment-identifying information. The treatment could only be unblinded by comparing the patient identifier, pre-printed on the packet, to the randomization schedule, which was held confidentially and locked and stored at the DCC, and not available to any participants or personnel involved in the conduct of the study. Thus, double-blinded randomization occurred when the intravenous study drug was started. This approach, based on the blinded study drug packet used, was controlled and not subject to investigator inclusion bias and, importantly, avoiding delaying EMS care or interfering with paramedics’ work.
Clinical and Laboratory Evaluation
During the 12-hour study drug infusion, glucose and potassium levels were tested at three times: 1) upon ED arrival; 2) at six hours after the start of the study drug infusion; and 3) once the infusion was completed or stopped prematurely. Treatment of abnormal test results or changes in fluid status was according to standard practice.
Participants in the biological mechanism cohort, a subset of enrolled patients, had FFA levels and fractionation drawn shortly after hospital arrival, at six hours, and at 12 hours. Those who proved to have ACS returned at 30 days for sestamibi LV imaging and a BNP blood test.
Core Laboratories included the LV Core Laboratory at Tufts Medical Center that interpreted imaging studies; Omega Quant in Sioux Falls, SD, that analyzed the FFA samples; and the core lab of Tufts Clinical and Translational Science Institute that ran the BNP, insulin levels, and other analytes.
Assignment of Diagnosis
To allow for real-time monitoring during enrollment, based on prehospital, ED, and 24-hour ECGs, biomarker test results, information on ED presentation and hospitalization, results of cardiac catheterization, and other tests, site investigators assigned diagnoses from four main categories, AMI by Killip Class, UAP by Canadian Class, non-ACS cardiac disease, and non-cardiac disease, based on the systems used in our prior ACS trials.15,16,20 For study endpoints, independently, the Clinical Events Committee were provided the same documents and conducted a formal adjudication process to determine the confirmed diagnosis of AMI, and made the additional determination as to whether a participant had an aborted MI. In assigning diagnoses, reviewers were blinded to the study group, glucose and potassium tests, and to whether the study drug was stopped early.
Study Participant Follow-Up
At 30 days and 1 year after study entry, all-cause mortality and hospitalization for HF since the study admission are assessed. One-year follow-up will be completed in summer of 2012.For all re-hospitalizations during the follow-up period, source documents are provided for review by the Clinical Events Committee, to determine if the hospitalization was related to HF.
Study Organization, Administration and Funding
The IMMEDIATE Trial Coordinating Center (CC) was located in the Center for Cardiovascular Health Services Research (CCHSR) at Tufts Medical Center, Boston, as was the DCC, with participation of Harvard Clinical Research Institute, Boston, where was located an independent statistician who generated periodic interim safety reports to the DSMB. The DSMB was appointed by NHLBI and provided oversight during enrollment to ensure safe and ethical study conduct. The Scientific Advisory Committee was made up of leaders in emergency medicine, cardiology, and cardiac physiology with particular research and clinical expertise in treatment of ACS and the use of GIK.
The 13 study sites ranged widely by location, city size, type of EMS system, and sociodemographic features, as shown in Table I.
Table I.
Site Characteristics
| Site Name | Type of EMS System |
Population Community Served |
Number of Paramedics |
Number of ALS Vehicles |
Number of Receiving Hospitals |
|---|---|---|---|---|---|
| Albuquerque, NM | Private, Fire | 1,166,096 | 156 | 64 | 4 |
| Anchorage, AK | Fire | 258,455 | 50 | 10 | 2 |
| Bellingham, WA | Fire | 166,814 | 50 | 6 | 1 |
| Brockton, MA | Private, Fire | 110,000 | 50 | 14 | 2 |
| Concord, MA | Hospital | 63,306 | 28 | 2 | 2 |
| Dallas, TX | Fire | 1,200,000 | 1100 | 60 | 13 |
| El Paso, TX | Fire | 676,365 | 126 | 25 | 6 |
| Hershey, PA | Hospital | 268,100 | 34 | 8 | 1 |
| Macon, GA | Hospital | 293,447 | 76 | 19 | 1 |
| Milwaukee, WI | Fire | 604,477 | 350 | 20 | 5 |
| New Haven, CT | Private, Fire | 408,288 | 225 | 59 | 2 |
| Sioux Falls, SD | Private | 158,424 | 27 | 8 | 3 |
| St. Paul, MN | Fire, Hospital | 458,456 | 180 | 35 | 2 |
Primary Trial Analysis
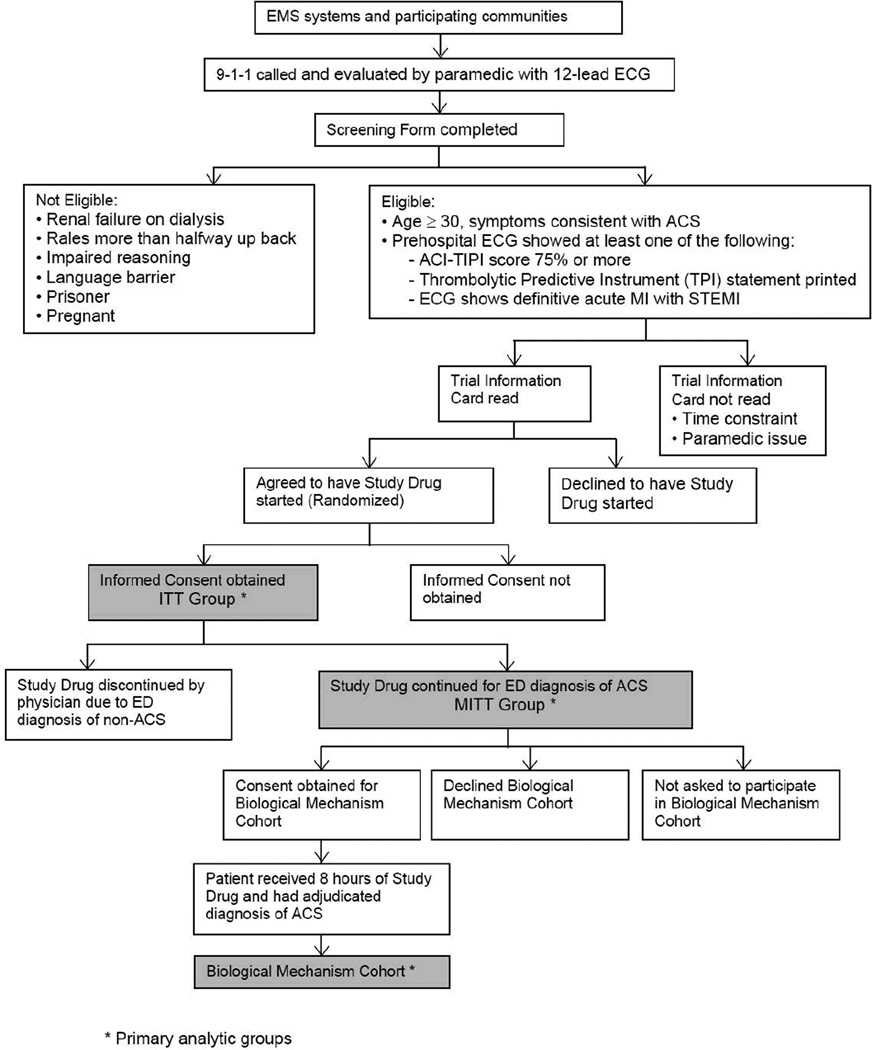
The flow of enrollment and the analytic groups are shown in the enrollment plan, in Figure 2. Of the hypotheses listed above, the last two were assessed on the subset of patients shown as the “biological mechanism cohort.”
Figure 2.
IMMEDIATE Trial Enrollment Plan
Sample Size
Power analysis indicated that at a two-sided 0.05 level of significance, an evaluable sample of 800 subjects (400 in each of GIK and placebo) would provide 90% power to detect a relative 20.4% reduction in the primary endpoint rate of adjudicated AMI for GIK versus placebo from 55.7% to 44.3% (or a difference of 11.4%).At least 880 subjects would need to be randomized to account for an anticipated attrition and patients withdrawing consent; the trial ultimately randomized 911 and enrolled 871 participants. One-hundred-and-forty-three participants with ACS were enrolled into the biological mechanism cohort.
Statistical Methodology
The primary analyses were on an intent-to-treat (ITT) cohort including all randomized participants who provided informed consent; patients were analyzed according to treatment received. Analyses also were conducted on a modified-intent-to-treat (MITT) cohort, constituting all those in the ITT cohort who were considered by the physician at the receiving ED to be having ACS and therefore were continued on the study drug. The MITT cohort most closely matches the way that GIK would be used in practice: initiated in the EMS setting and continued in the ED only for patients whom the ED physician considered to be having ACS. An additional analytic “efficacy” cohort was those who were confirmed to be having ACS (AMI or UAP) in the initial 24 hours as determined by site investigator review and who received their full randomized treatment course or who died during treatment infusion. A subsample of MITT patients who received treatment for at least eight hours were eligible for enrollment into the biological mechanism cohort; patients enrolled in this cohort received additional testing to provide FFA, BNP, and SPECT scan information needed to address the biological mechanism hypotheses.
Treatment comparisons on the primary endpoint were carried out using logistic regression adjusting for the 13 study sites at a two-sided 0.05 level of significance. Assessment of treatment-by-center interaction on the primary endpoint was carried out at the 0.10 level of significance. These analyses were carried out on the ITT (primary), MITT, and efficacy analysis cohorts. Due to the potential loss of randomization in the MITT and evaluable analysis sets, treatment comparisons were also adjusted for quintiles of a propensity score for treatment allocation. For adjusted analyses, forced logistic regression models were used for propensity scores that predicted treatment group (GIK/placebo) that includedparticipants’ age, sex, ACI-TIPI score, systolic blood pressure, chief complaint, current home medications, and medical history of MI, coronary bypass surgery, HF, coronary angioplasty, diabetes, hypertension, and stroke as independent variables.
For the biological mechanism cohort, mean FFA levels were compared across time periods using a generalized estimating equation (GEE) analysis of variance for which the total FFA level was the dependent variable, and treatment (GIK vs. control) and time from start of randomized drug infusion were the independent variables. The GEE model also included a subject factor to adjust for the design where each subject had multiple measurements (i.e., the GEE approach which accounted for the within-subject correlation). Also, on the biological mechanism cohort, 30-day mean infarct size, LV ejection fraction, and BNP were compared between the GIK and control study groups, using analysis of variance or rank analysis of variance, depending on the distribution, adjusting for study center.
Other secondary hypotheses involving binary or continuous outcomes were analyzed in similar way to the above. Hypotheses involving time-to-event outcomes were assessed using Cox proportional hazards regression adjusting for propensity quartile. All secondary hypotheses were carried out on a two-sided 0.05 level of significance.
SUMMARY
The IMMEDIATE Trial was a placebo-controlled, double-blinded, randomized NIH supported trial of the use of GIK for ACS, i.e., threatened or established AMI. It was an “effectiveness trial”rather than an “efficacy trial,” and thus inclusion and treatment was done as it would be in widespread usual practice. The IV GIK was administered by paramedics in the field using the 12-lead electrocardiographs with decision support by the ACI-TIPI and TPI that automatically print on ECGs a patient’s probability of ACS and/or the presence of STEMI.17 The inclusion criteria were broad: patients age 30 years or older calling 9-1-1 with a clinical picture suggesting ACS/AMI based on paramedic judgment, with a prehospital ECG with at least one of the following: 1) ACI-TIPI probability of ACS of 75% or more; 2) detection by TPI of STEMI; or 3) definitive STEMI deemed by local standards for which the receiving hospital catheterization laboratory would be notified based on local community EMS procedures.
The primary endpoint was progression to AMI confirmed by biomarkers and ECG; major secondary endpoints included 30-day mortality, one-year mortality, the composite of in-hospital mortality or cardiac arrest, the composite of 30-day mortality or hospitalization for HF, and the composite of in-hospital cardiac arrest, 30-day mortality or hospitalization for HF. Biological mechanism cohort endpoints included infarct size by sestamibi perfusion imaging at 30 days, LV ejection fraction by sestamibi SPECT scanning at 30 days, BNP at 30 days, and metabolic parameters. If impact is seen on these endpoints, we believe emergency cardiac practice will be altered – and because it appears to work in a broad array of usual-care settings, the IMMEDIATE Trial approach could be adopted widely. However, even prior to pending results for these endpoints, the Trial has already demonstrated a model of very early prehospital paramedic recognition and treatment of patients with ACS/AMI/STEMI, which provides an opportunity for other important EMS-based interventions in the first hours.17
In sum, the IMMEDIATE Trial tested GIK myocardial metabolic support in a way analogous to how it has worked in experimental research, in patients with ACS, i.e., with threatening or established AMI, at the time they are most at risk, in the very first hours, which is most often in the EMS setting. We consider this an example of translation of experimental lab findings into clinical care, and in this case, into wide use in the community, an important goal of NIH supported research.
Acknowledgments
Funding and Acknowledgement
Funding support for the IMMEDIATE Trial was provided by the National Heart, Lung and Blood Institute (grants: U01HL07782, U01HL077826, U01HL077823). Eli Lilly and Company donated insulin, and Physio-Control and Zoll Medical donated ACI-TIPI and TPI software for the electrocardiographs. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript and its final contents. The investigators sincerely thank Dr. Thomas Killip for his support and guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERECNES
- 1.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 2.Oliver MF. Sudden unexpected cardiac death. Eur Heart J. 2002;23:1797–1798. doi: 10.1053/euhj.2002.3373. [DOI] [PubMed] [Google Scholar]
- 3.Fath-Ordoubadi F, Beatt KJ. Glucose-Insulin-Potassium (GIK) therapy for treatment of acute myocardial infarction: An overview of randomized placebo controlled trials. Circulation. 1997;96:1152–1156. doi: 10.1161/01.cir.96.4.1152. [DOI] [PubMed] [Google Scholar]
- 4.Rackley CE, Russell RO, Rogers WJ, Mantle JA, McDaniel HG, Papapietro SE. Clinical experience with glucose-insulin-potassium therapy in acute myocardial infarction. Am Heart J. 1981;102:1038–1049. doi: 10.1016/0002-8703(81)90488-9. [DOI] [PubMed] [Google Scholar]
- 5.Malmberg K for the DIGAMI study group. Prospective randomized study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. BMJ. 1997;314:1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz R, Paolasso EC, Piegas LS, et al. on behalf of the ECLA (Estudios Cardiologicos Latinoamerica) Collaborative Group. Metabolic Modulation of Acute Myocardial Infarction. The ECLA Glucose-Insulin-Potassium Pilot Trial. Circulation. 1998;98:2227–2234. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- 7.Apstein CS. Glucose-insulin-potassium for acute myocardial infarction. Remarkable results from a new, prospective randomized trial. Circulation. 1998;98:2223–2226. doi: 10.1161/01.cir.98.21.2223. [DOI] [PubMed] [Google Scholar]
- 8.Van der Horst I, Zijlstra F, van’t Hof A, et al. on behalf of Zwolle Infarct Study Group. Glucose-insulin-potassium infusion in patients treated with primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2003;42:784–791. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 9.Apstein CS, Opie LH. Glucose-insulin-potassium (GIK) for acute myocardial infarction: A negative study with a positive value. Cardiovasc. Drugs and Therapy. 1999;13:185–189. doi: 10.1023/a:1007757407246. [DOI] [PubMed] [Google Scholar]
- 10.Opie LH, Bruyneel K, Owen P. Effects of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation. 1975;52:49–57. doi: 10.1161/01.cir.52.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Kurien V, Yates P, Oliver M. Free fatty acids, heparin, and arrhythmias during experimental myocardial infarction. Lancet. 1969;2:185–187. doi: 10.1016/s0140-6736(69)91424-x. [DOI] [PubMed] [Google Scholar]
- 12.Malmberg K, Ryden l, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 13.CREATE-ECLA Trial Group Investigators. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction, The CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 14.Rogers WJ, McDaniel HG, Mantle JA, Rackley CE. Prospective randomized trial of glucose-insulin-potassium in acute myocardial infarction: effects on hemodynamics, short- and long-term survival. Am J Cardiol. 1983;1:628. doi: 10.1016/0002-9149(79)90081-x. [DOI] [PubMed] [Google Scholar]
- 15.Selker HP, Beshansky JR, Griffith JL for the TPI Trial Investigators. Use of the electrocardiograph-based thrombolytic predictive instrument to assist thrombolytic and reperfusion therapy for acute myocardial infarction. Ann Intern Med. 2002;137:87–95. doi: 10.7326/0003-4819-137-2-200207160-00006. [DOI] [PubMed] [Google Scholar]
- 16.Selker HP, Beshansky JR, Griffith JL, et al. The use of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) to assist emergency department triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia: a multicenter controlled clinical trial. Ann Intern Med. 1998;129:845–855. doi: 10.7326/0003-4819-129-11_part_1-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Selker HP, Beshansky JR, Ruthazer R, et al. Emergency Medical Service Predictive Instrument–Aided Diagnosis and Treatment of Acute Coronary Syndromes and ST-segment Elevation Myocardial Infarction in the IMMEDIATE Trial. Prehosp Emerg Care. 2011;15:139–148. doi: 10.3109/10903127.2010.545478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Guidance for institutional review boards, clinical investigators, and sponsor; exception from informed consent requirements for emergency research. Rockville, MD: 2011. Mar, [Google Scholar]
- 19.Beshansky JR, Sheehan PR, Hadar N, Klima K, Selker H. National implementation of exception form informed consent requirements for emergency research (21CFR50.24) process and 1-year experience in the IMMEDIATE trial. Clinical Trials. 2010;7:428. [Google Scholar]
- 20.Udelson JE, Beshansky JR, Ballin DS, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA. 2002;288:2693–2700. doi: 10.1001/jama.288.21.2693. [DOI] [PubMed] [Google Scholar]