Abstract
Chemokines are important mediators in immune responses and inflammatory processes of neuroimmunologic and infectious diseases. Although chemokines are expressed predominantly by cells of the immune system, neurons also express chemokines and chemokine receptors. We report herein that human neuronal cells (NT2-N) produce macrophage inflammatory protein-1α and -1β (MIP-1α and MIP-1β), which could be enhanced by interleukin (IL)-1β at both mRNA and protein levels. The addition of supernatants from human peripheral blood monocyte-derived macrophage (MDM) cultures induced MIP-1β mRNA expression in NT2-N cells. Anti-IL-1β antibody removed most, but not all, of the MDM culture supernatant-induced MIP-1β mRNA expression in NT2-N cells, suggesting that IL-1β in the MDM culture supernatants is a major factor in the induction of MIP-1β expression. Investigation of the mechanism(s) responsible for IL-1β-induced MIP-1α and -1β expression demonstrated that IL-1β activated nuclear factor kappa B (NF-κB) promoter-directed luciferase activity in NT2-N cells. Caffeic acid phenethyl ester, a potent and specific inhibitor of activation of NF-κB, not only blocked IL-1β-induced activation of the NF-κB promoter but also decreased IL-1β-induced MIP-1α and -1β expression in NT2-N cells. These data suggest that NF-κB is at least partially involved in the IL-1β-mediated action on MIP-1α and -1β in NT2-N cells. IL-1β-mediated up-regulation of β-chemokine expression may have important implications in the immuno-pathogenesis of inflammatory diseases in the CNS.
Keywords: β-chemokines, interleukin-1β, macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, nuclear factor-κB, NT2-N
There is a unique bidirectional connection between the immune and the nervous systems (Benveniste 1992; Hopkins and Rothwell 1995; De Simoni and Imeri 1998; Maier et al. 1998). Cytokines have a dynamic role in the communication between the immune system and the CNS. A number of cytokines have been implicated in this interaction (Benveniste 1992; Hopkins and Rothwell 1995; De Simoni and Imeri 1998; Maier et al. 1998). Interleukin (IL)-1β, a pro-inflammatory cytokine, is involved in the early response during the inflammatory process. IL-1β is produced by a large variety of cells, including immune cells and neuronal cells. IL-1β has a key role in the induction of the complex immune response to antigens, malignant cells, inflammatory stimuli and tissue injury (Dinarello 1988; Fibbe et al. 1989). It stimulates multiple cells to act as immune or inflammatory response effector cells. The human CNS contains neuronal pathways and receptors for IL-1 that are important in mediating the acute-phase response (Breder et al. 1988). During peripheral inflammatory processes, increased IL-1β levels in the CNS are involved in the induction of fever, sickness behaviors and neuroendocrine signaling (Dantzer et al. 1991; Rothwell 1991; Rothwell and Hopkins 1995).
The primary function of chemokines is recruitment of leukocytes to inflammatory sites. Leukocyte infiltration into the CNS is a key major pathogenic event in the inflammatory processes of neuroimmunologic and neuroinfectious diseases. Although chemokines are produced predominantly by cells of the immune system, there have been a number of reports showing the presence of some chemokines and their receptors in the CNS (Halks-Miller et al. 1997; Bajetto et al. 1999a; Hesselgesser and Horuk 1999; Klein et al. 1999; Coughlan et al. 2000; Boutet et al. 2001). Macrophage inflammatory protein (MIP)-1α and MIP-1β have potentially important roles in the development of inflammatory responses during infection by recruiting mononuclear cells to sites of inflammation, modulating cytokine production and mediating fever. MIP-1α and -1β also are the natural ligands for the chemokine receptor CCR5, a primary co-receptor for human immunodeficiency virus entry into macrophages (Capobianchi et al. 1998). Thus, the regulation of β-chemokine expression in the CNS may have an important implication in the pathogenesis of neuroinfectious diseases including neuro-acquired immune deficiency syndrome (neuroAIDS).
The role of IL-1β in the modulation of expression of β-chemokines such as MIP-1− and -1β in human neuronal cells is unknown. Coughlan et al. (2000) recently showed that human NT2-N cells express multiple chemokine receptors and produce macrophage chemoattractant protein-1. These findings have provided a basis for the investigation of factors that regulate the expression of chemokines and chemokine receptors in neuronal cells. As the interaction between IL-1β and β-chemokines in neuronal cells may have important implications in CNS inflammatory diseases, we investigated whether IL-1β modulates MIP-1α and -1β expression in human neuronal NT2-N cells. In addition, because microglia and macrophages in the CNS might be responsible for production of IL-1β and other cytokines, we studied whether monokine-enriched supernatants from macrophage cultures regulate β-chemokine expression in NT2-N cells.
Materials and methods
Reagents
Recombinant human IL-1β, IL-3, IL-4, IL-6, granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor-α (TNF-α) were purchased from R & D Systems (Minneapolis, MN, USA). Rabbit anti-IL-1β neutralizing antibody (IL-1βAb) and lipopolysaccharide (LPS) were purchased from Sigma (St Louis, MO, USA). Caffeic acid phenethyl ester (CAPE) was purchased from Calbiochem–Novabiochem Corp. (San Diego, CA, USA).
NT2-N cell culture
Human teratocarcinoma (Ntera2/c1.D1, NT2) cells were cultured and differentiated into neurons as described previously (Andrews 1984). Cells were plated at a density of 2.3 × 106 per T75 flask and fed twice weekly with Dulbecco’s modified Eagle’s medium (DMEM) containing high glucose (Gibco, Grand Island, NY, USA) and 10% fetal bovine serum (Hyclone, Iogan, UT, USA), 100 IU/mL penicillin, 100 μg/mL streptomycin (Gibco) and 10 μM retinoic acid (Sigma, St Louis, MO, USA) for up to 6 weeks. The cells were then divided 1 : 4 and grown for an additional 48 h in identical medium without retinoic acid. Neuronal cells growing above a monolayer of non-neuronal cells were dislodged with trypsin and plated in six- or 12-well plates (Falcon; Becton Dickinson, Lincon Park, NJ, USA) at 106 cells/well for this study.
Monocyte isolation and preparation of macrophage culture supernatant
Peripheral blood was obtained from healthy adult donors with no known history of drug abuse. Heparinized blood samples were identified as human immunodeficiency virus antibody negative by anonymous testing by ELISA (Coulter Immunology, Hialeah, FL, USA). Informed consent was obtained, and the Institutional Review Board approved this study. Monocytes were purified as described previously (Hassan et al. 1986). In brief, heparinized blood was separated by centrifugation over Lymphocyte Separation Medium (Organon Teknika Corporation, Durham, NC, USA) at 400–500 g for 45 min. The mononuclear cell layer was collected and incubated with DMEM (Life Technologies, Grand Island, NY, USA) in a 2% gelatin-coated flask for 45 min at 37 °C, followed by the removal of the non-adherent cells with DMEM. Adherent monocytes were detached with 10 mm EDTA. Following the initial purification, more than 97% of the cells were monocytes, as determined by nonspecific esterase staining and flow cytometry analysis using a monoclonal antibody against CD14 (Leu-M3). Freshly isolated monocytes were plated in 48-well culture plates at a density of 5 × 105 cells/well in DMEM containing 10% fetal bovine serum. Monocyte-derived macrophages (MDMs) refer to monocytes cultured in vitro for 7 days. Monocyte and MDM viability was monitored by trypan blue exclusion and maintenance of cell adherence. Monokine-enriched supernatants were generated from MDM cultures that were stimulated with lipopolysaccharide (LPS; 10 ng/mL) for 24 h. Cell-free supernatants were collected, aliquoted, and stored at − 70 °C before use.
ELISA for MIP-1α and -1β
β-chemokine ELISA kits for MIP-1α and -1β were purchased from Endogen, Inc. (Cambridge, MA, USA) and the assay was performed according to the protocol provided by the manufacturer. In brief, culture supernatants (50 μL) were added to antibody-coated wells and incubated for 1 h at room temperature (20–24 °C). The plate was washed with the buffer solution provided and incubated with 100 μL biotinylated antibody reagent for 1 h at room temperature. The plate was washed again, treated with 100 μL of prepared streptavidin–horseradish peroxidase solution, and incubated for 30 min at room temperature. After an additional wash, 100 μL 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution was added to each well, and color was allowed to develop at room temperature for 30 min. The reaction was stopped by the addition of 100 μL stop solution to each well. The plate was read on a microplate reader (ELX800; Bio-Tek Instruments Inc., Winooski, VT, USA).
Nuclear factor-κB (NF-κB) promoter activation assay
The plasmid that contains the NF-κB promoter linked to a luciferase gene (pNF-κB-luc) was generated by Dr D. Petrak (Petrak et al. 1994). Two copies of the mouse κ light chain enhancer (Pierce et al. 1988) were cloned into pBLCAT3 vector (Luckow and Schutz 1987). The construct was then modified by replacing the CAT reporter with the luciferase gene obtained from pGEM-luc (Petrak et al. 1994). Plasmid DNA was prepared by miniprep techniques, according to the manufacturer’s instruction (Wizard plus minipreps; Promega, Madison, MI, USA) and then used in transfection experiments.
NT2-N cells were cultured in DMEM medium containing 5% fetal calf serum. For each transfection experiment, the cells were seeded in a 12-well tissue culture plate at a density of 106 cells/well. The cells were transfected with the pNF-κB-luc using Fugene 6 Transfection Reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA) at a ratio of 6 μL Fugene 6 to 1 μg plasmid. Twenty-four hours after the transient transfection, the cells were treated with IL-1β (4 ng/mL) and/or IL-1βAb or CAPE for 12 h. IL-1β and IL-1βAb were co-incubated for 2 h at 37 °C before use in the same experiment. When IL-1β and CAPE were used in the same experiment, CAPE was added to the cell cultures for 2 h before the addition of IL-1β. At the termination of the experiments, cells were harvested and washed twice with phosphate-buffered saline by centrifugation at 3300 g for 3 min at room temperature. The cell pellets were lysed in 1 × Reporter Lysis Buffer (Promega) followed by a cycle of freezing and thawing in dry ice. Cell-free lysates were obtained by centrifugation at 10 000 g for 30 s at room temperature.
The effects of IL-1β on the activation of the NF-κB promoter in these transient transfected cells were determined by the NF-κB promoter-directed luciferase activity. Luciferase activity in cell lysate (25 μL/sample) was quantified using a luciferase assay system (Promega) and a luminometer. The results were presented as relative light units.
RNA extraction and reverse transcription
Total cellular RNA was isolated from NT2-N cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA). In brief, total RNA was extracted by a single-step, guanidium thiocyanate–phenol–chloroform extraction. After centrifugation at 13 000 g for 15 min at 4 °C, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were washed once in 75% ethanol and resuspended in 30 μL Rnase-free water. Total cellular RNA (1 μg) was subjected to reverse transcription using the reverse transcription system from Promega, with specific primers (antisense) for MIP-1α and -1β genes for 1 h at 42 °C. The reaction was terminated by incubating the reaction mixture at 99 °C for 5 min and then kept at 4 °C. The resulting cDNA was ready to serve as a template for PCR amplification.
PCR analysis
PCR amplification of MIP-1− and -1β cDNA was performed in a GeneAmp PCR System 2400 (Perkin Elmer-Cetus, Norwalk, CT, USA). The PCR reaction mixture contained 0.2 mm dNTPs, 20 pm of each of two primers, and 1.5 units of AmpliTaq Gold in 1 × reaction buffer (Perkin Elmer-Cetus). Each of the PCR amplifications included heat activation of AmpliTaq Gold for 9 min at 94 °C, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, and further elongation at 72 °C for 7 min. The specific oligonucleotide primers were follows: MIP-1α gene primers: 5′-GCTGACTACTTTGAGACGAGC-3′ (sense) and 5′-CCAGTCCATAGAAGAGGTAGC-3′ (antisense); MIP-1β gene primers: 5′-CCAAACCAAAAGAAGCAAGC-3′ (sense) and 5′-AGAAACAGTGACAGTGGACC-3′ (antisense); β-actin gene primers: 5′-ATGTGGCACCACACCTTCTACAATGAGCTGCG-3′ (sense) and 5′-CGTCATACTCCT GCTTGCTGATCCACATCTGC-3′ (anti-sense). The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA). PCR-amplified products were electrophoresed on ethidium bromide-stained 3% NuSieve 3 : 1 agarose gel (FMC BioProducts, Pockland, ME, USA).
Real-time PCR
Real-time PCR was performed with ABI Prism 7700 Sequence Detection System (Perkin Elmer). For MIP-1α amplification, the primers listed above and the brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA, USA) were used to quantify MIP-1α mRNA according to the manufacturer’s instructions. For MIP-1β gene amplification, the reaction mixture contained 0.25 mm dNTPs, AmpliTaq Gold (1.5 U), 5 mm MgCl2, 50 pm of each of the two primers [5′-GCTGCTCAGAGACAGGAAGTCTT-3′ (sense), 5′-ACAGGAACTGCGGAGAGGAGT-3′ (antisense)], 20 pm of the molecular beacon probe (5′-GCGAGCCCCGGATGCTTCTCCATG AGACACAGCTCGC-3′) labeled with 6-carboxyfluorescein (FAM) (a fluorophore) at the 5′ end and 4-(4′-dimethylaminophenylaso) benzoic acid (DABCYL) (a quencher) at the 3′ end. The cycle conditions were set as follows: 95 °C for 10 min followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min. A known amount of plasmid encoded with MIP-1β cDNA was used as a standard control. All controls and samples were run in triplicate in the same plate. Glyceraldehyde-3-phosphate dehydrogenase mRNA levels of the samples in the same plate were analyzed by real-time PCR to normalize the mRNA contents among the samples tested.
Treatment of NT2-N cells with IL-1β or macrophage culture supernatants
In experiments for MIP-1α and -1β induction, NT2-N cells were cultured in the presence or absence of cytokines at different time points as shown in the figure legends. MDM culture supernatants were diluted with the cell culture medium (1 : 5, v/v) before addition to NT2-N cultures. Cell-free supernatants were collected and stored at − 70 °C for β-chemokine ELISA assay. IL-1βAb and CAPE were also used in these experiments to block the effect of IL-1β on the production of MIP-1− and -1β in NT2-N cells. When IL-1β and IL-1βAb were used in the same experiment, they were co-incubated for 2 h at 37 °C before addition to the cell cultures. In experiments that examined the effect of CAPE on IL-1β, the cells were treated with CAPE (25 μg/mL) for 2 h before the addition of IL-1β (4 ng/mL). Total cellular RNA was then extracted and subjected to real-time RT–PCR to determine MIP-1α and -1β mRNA expression.
Statistical analysis
Variables were tested in triplicate, and experiments were repeated at least twice. Variability between experiments was < 15%. One-way anova was used to test for differences in means, and a post-hoc t-test was used for comparisons. Differences were considered significant at p < 0.05.
Results
IL-1β induces MIP-1α and -1β expression
We first determined whether IL-1β modulated mRNA expression of MIP-1α and -1β in NT2-N cells. When MIP-1α and -1β mRNA transcripts were analyzed by standard RT–PCR, increased mRNA expression for these chemokines was observed in IL-1β-treated NT2-N cells in a concentration-dependent fashion (Fig. 1a). In order to quantitate the effects of IL-1β on MIP-1α and -1β mRNA expression in NT2-N cells, we performed real-time PCR assays. IL-1β significantly enhanced MIP-1α and -1β gene expression (Figs 1b and c). When human NT2-N cells were treated with IL-1β (4 ng/mL) and analyzed at different time points, we observed a consistent increase over the course of incubation with IL-1β by real-time PCR (Fig. 2). The stimulatory effect of IL-1β on MIP-1α and -1β expression was abrogated by IL-1βAb (Fig. 3).
Fig. 1.
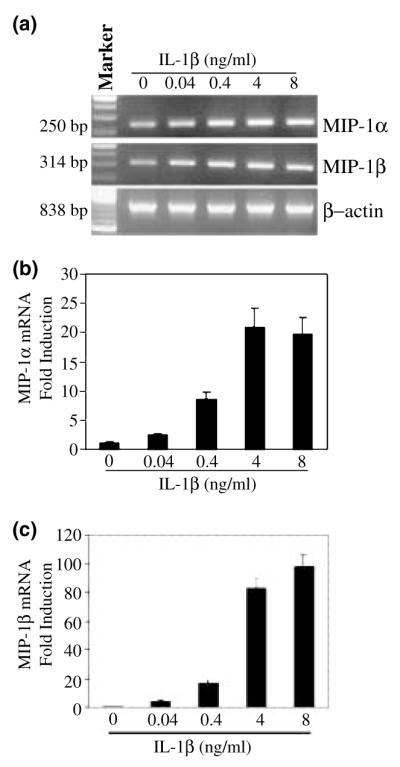
Effect of IL-1β on MIP-1α and β expression in NT2-N cells. (a) NT2-N cells were incubated with IL-1β at the indicated concentrations for 6 h, and total RNA was isolated and subjected to RT–PCR and electrophoresis for MIP-1α and -1β mRNA. Expression of MIP-1α (b) and MIP-1β (c) mRNA in NT2-N cells was quantified by real-time RT–PCR. Data are mean ± SD of triplicate cultures, representative of three independent experiments.
Fig. 2.
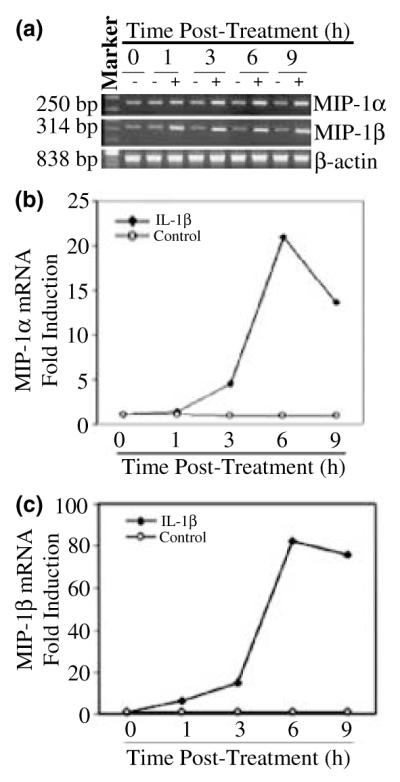
Time course of IL-1β effect on MIP-1α and -1β mRNA expression in NT2-N cells. NT2-N cells were incubated with (+) or without (−) IL-1β (4 ng/mL) and expression was measured at the indicated times after treatment. (a) Total RNA was isolated and subjected to RT–PCR and electrophoresis for MIP-1α and -1β mRNA. Expression of MIP-1α (b) and MIP-1β (c) mRNA in NT2-N cells was also quantified by real-time RT–PCR. Data are means of triplicate cultures, representative of three independent experiments.
Fig. 3.
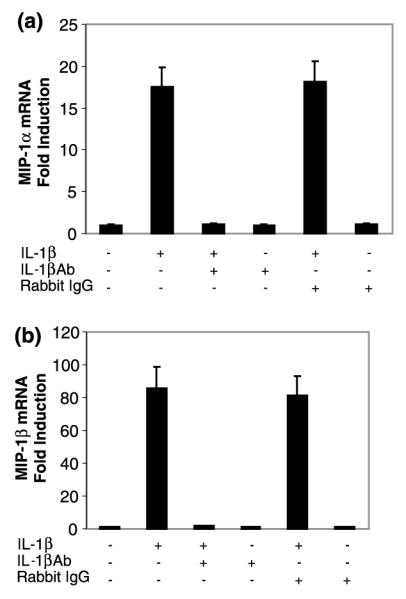
Effect of IL-1βAb on IL-1β-induced MIP-1α and -1β mRNA expression in NT2-N cells. NT2-N cells were incubated with (+) or without (−) IL-1β (4 ng/mL) and/or IL-1βAb (10 μL) or normal rabbit IgG (10 μL) for 6 h. Total cellular RNA was isolated and subjected to real-time RT–PCR to quantify MIP-1α (a) and -1β (b) mRNA. Data are mean ± SD of triplicate cultures, representative of three independent experiments.
We also examined whether IL-1β enhanced MIP-1α and -1β protein production in NT2-N cells. IL-1β significantly enhanced MIP-1α and -1β production in NT2-N cells in a dose-dependent fashion (Fig. 4).
Fig. 4.
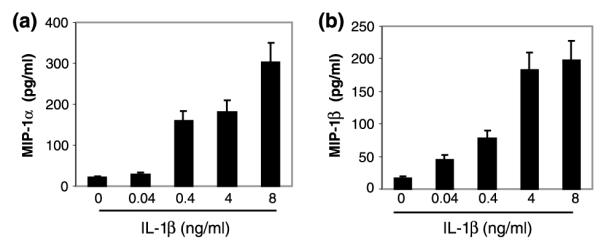
Effect of IL-1β on MIP-1α and -1β production. NT2-N cells were incubated with or without IL-1β for 24 h at the concentrations indicated. MIP-1α (a) and -1β (b) produced by NT2-N cells in the supernatants were determined by ELISA. Data are mean ± SD of triplicate cultures, representative of two independent experiments.
MDM culture supernatant-mediated increase in MIP-1β expression in NT2-N cells
Biological effects of an individual cytokine or chemokine are often modified by the presence of other cytokines and chemokines in a microenvironment in vivo. Thus, it is important to determine whether IL-1β in the presence of other cytokines (analogous to the microenvironment) modulates MIP-1α and -1β expression in NT2-N cells. As activated macrophages produce significant quantities of monokines, including IL-1β, IL-6 and TNF-α, we analyzed the effect of monokine-enriched supernatants from LPS-stimulated MDM cultures on MIP-1β expression in NT2-N cells. Monokine-enriched supernatants enhanced MIP-1β mRNA expression in NT2-N cells (Fig. 5). In order to determine whether the IL-1β present in LPS-stimulated MDM culture supernatants is responsible for β-chemokine induction in NT2-N cells, MDM culture supernatants were incubated with IL-1βAb before addition to NT2-N cell cultures. The addition of IL-1βAb to the cultures partially blocked the MDM supernatant-induced MIP-1β expression, but IL-1βAb itself had no effect on MIP-1β gene expression (Fig. 5).
Fig. 5.
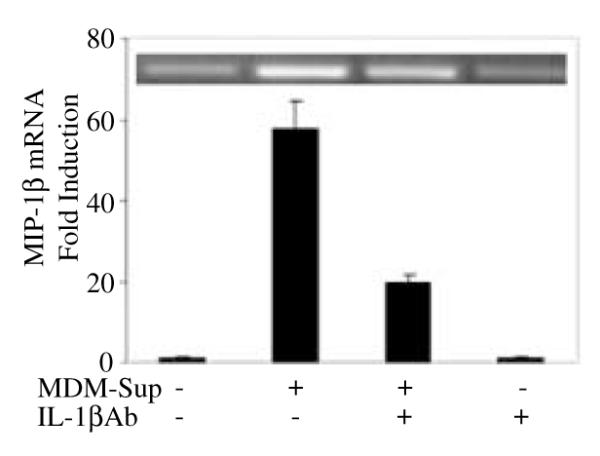
Effect of MDM culture supernatants (MDM-Sup) on MIP-1β mRNA expression in human NT2-N cells. NT2-N cells were incubated with or without LPS-stimulated MDM supernatants (1 : 5 dilution) and/or IL-1βAb for 6 h. Total cellular RNA was isolated and subjected to RT–PCR (insert) and real-time RT–PCR to quantify MIP-1β mRNA. Data are mean ± SD of triplicate cultures, representative of three independent experiments.
Effect of other cytokines on MIP-1α and -1β expression in NT2-N
We also examined the effects of other cytokines on MIP-1α and -1β expression in NT2-N cells. Among the cytokines tested, TNF-α stimulated MIP-1α and -1β expression, whereas GM-CSF down-regulated MIP-1α and -1β expression in NT2-N cells (Fig. 6). As we used LPS to stimulate MDM cultures, it was necessary to determine whether LPS could induce MIP-1α and -1β expression in NT2-N cells. However, LPS did not affect MIP-1α and -1β gene expression in these cells (Fig. 6).
Fig. 6.
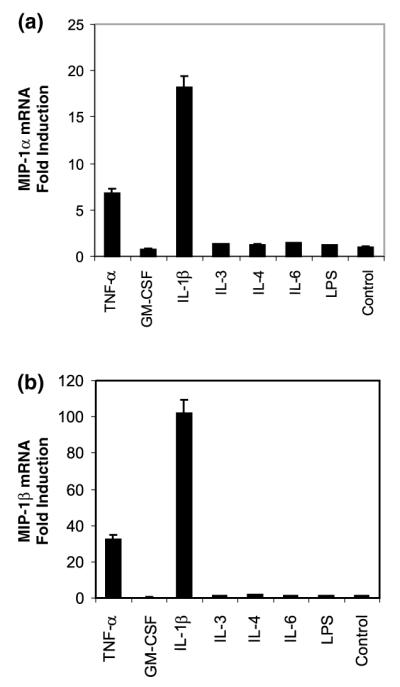
Effect of cytokines on MIP-1α and -1β mRNA expression in human NT2-N cells. NT2-N cells were incubated with medium alone (Control), TNF-a (10 ng/mL), GM-CSF (10 ng/mL), IL-1β (4 ng/mL), IL-3 (10 ng/mL), IL-4 (10 ng/mL), IL-6 (10 ng/mL) or LPS (10 ng/mL) for 6 h. Total cellular RNA was isolated and subjected to real-time RT–PCR using specific primers for MIP-1α (a) and -1β (b) Data are mean ± SD of triplicate cultures, representative of two independent experiments.
NF-κB is involved in IL-1β-induced β-chemokine expression
As NF-κB, a key transcriptional factor, plays a crucial role in the control of cytokine expression and NF-κB binding sites are also found on β-chemokine genes (Rezzonico et al. 2001), we hypothesized that NF-κB activation is a prerequisite for IL-1β-mediated enhancement of MIP-1α and -1β expression in NT2-N. In order to test this hypothesis, we first examined whether IL-1β has the ability to activate the NF-κB promoter. We transfected NT2-N cells with a plasmid containing the NF-κB promoter (pNF-κB-luc) and then incubated the cells in the presence or absence of IL-1β and/or IL-1βAb. IL-1β activated NF-κB promoter-directed luciferase activity, and IL-1βAb completely blocked the effect of IL-1β on NF-κB promoter activation in NT2-N cells (Fig. 7).
Fig. 7.
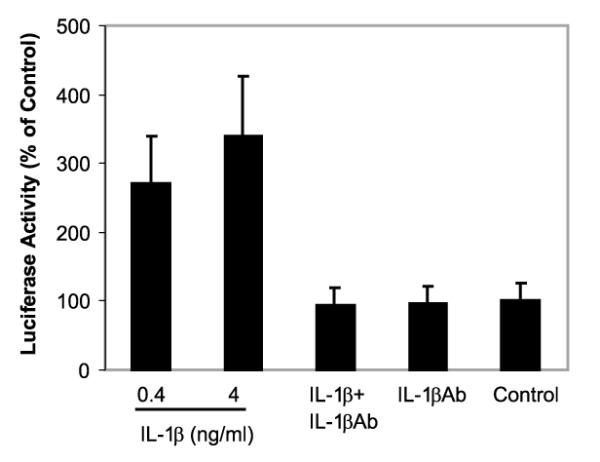
Effect of IL-1β on the activation of the NF-κB promoter in NT2-N cells. NT2-N cells were transfected with plasmid containing the NF-κB promoter (pNF-κB-luc). The cells were then incubated with or without IL-1β and/or IL-1βAb for 12 h. The effect of IL-1β and/or IL-1βAb on the activation of the NF-κB promoter in transfected cells was determined by NF-κB promoter-directed luciferase activity. Luciferase activity in the cell-free lysates was quantified using a luciferase assay system and a luminometer. Data are mean ± SD of triplicate cultures, representative of three independent experiments.
CAPE, a potent and specific NF-κB inhibitor, also abrogated the IL-1β-induced NF-κB promoter activation in NT2-N cells (Fig. 8a). In order to determine whether NF-κB plays a direct role in up-regulation of MIP-1α and -1β by IL-1β, we then studied whether CAPE could block the action of IL-1β when added to NT2-N cell cultures. Preincubation of NT2-N cells with CAPE partially blocked IL-1β-induced expression of MIP-1α and -1β (Fig. 8b). These results suggest that the activation of NF-κB is one of the pathways involved in the up-regulation of MIP-1α and -1β expression by IL-1β in NT2-N cells.
Fig. 8.
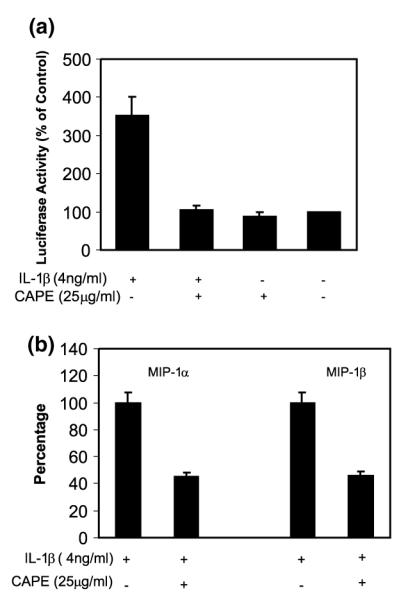
Effect of NF-κB inhibitor CAPE on IL-1β-induced activation of the NF-κB promoter (a) and MIP-1α and -1β expression (b) in NT2-N. (a) NT2-N cells were transfected with plasmid containing the NF-κB promoter (pNF-κB-luc). The cells were then incubated with or without IL-1β and/or CAPE for 12 h. Data are mean ± SD of triplicate cultures, representative of three independent experiments. (b) NT2-N cells were preincubated with or without CAPE (25 μg/mL) for 2 h. The cells were then treated with IL-1β (4 ng/mL) for 6 h. Total cellular RNA was extracted and subjected to real-time RT–PCR to detect MIP-1α and -1β mRNA expression. Data are mean ± SD of triplicate cultures, representative of two independent experiments.
Discussion
The pro-inflammatory cytokine IL-1β plays a key role in the induction of the early and complex immune response to inflammatory stimuli, and tissue injury (Dinarello 1988; Fibbe et al. 1989; Copray et al. 2001). IL-1β is also produced in the CNS (Rothwell and Luheshi 2000; Copray et al. 2001). Increased levels of IL-1β in the CNS have been observed in neurodegenerative diseases (Allan and Rothwell 2001). We therefore investigated the role of IL-1β in regulation of β-chemokines in human neuronal cells. The role of chemokines and their receptors in CNS diseases has become a major focus of studies of immunopathogenesis (Zhang et al. 2000). The CNS-released chemokines may contribute to neuroinflammatory disorders, as they are capable of recruiting leukocytes across the blood–brain barrier. Aberrant expression of some of these chemokines is implicated in CNS diseases. Over-expression of MIP-1 is associated with acute onset of inflammatory lesions in autoimmune encephalomyelitis (Karpus and Kennedy 1997). Immunohistochemical staining demonstrated a diffuse expression of both MIP-1β and regulated upon activation, normal T cell expressed and secreted (RANTES) in necrotic tissue after brain trauma, and MIP-1β on reactive astrocytes near the lesion site (Ghirnikar et al. 1996). Increased levels of MIP-1α occurred in cerebrospinal fluid of patients with multiple sclerosis (Miyagishi et al. 1995). In rodent models of autoimmune encephalomyelitis, increased expression of β-chemokines was observed (Miyagishi et al. 1997; Sun et al. 1997). Thus, it is critical to study factors that regulate β-chemokine expression in the CNS.
In the present communication, we have demonstrated that human NT2-N cells express the b-chemokines MIP-1α and -1β, which is in consistent with recent studies showing that glial and neuronal cells express multiple functional chemokine receptors and chemokines in the CNS (Bajetto et al. 1999b; Coughlan et al. 2000). Chemokines are produced upon activation by a wide spectrum of cell types, predominately by immune cells including T cells, microglia and macrophages. It is critical to identify factors that regulate chemokine expression in the CNS. We hypothesized that IL-1β, an important inflammatory cytokine in the early immune response, may activate NT2-N cells to produce β-chemokines. Our data showing that IL-1β significantly enhances MIP-1α and -1β expression support this hypothesis. Because biological effects of an individual cytokine or chemokine are often modulated by the presence of other cytokines and chemokines in a microenvironment in the CNS, it is important to investigate the effects of multiple cytokines or cytokine-containing supernatants from microglia or macrophage cultures on the function of neuronal cells. Owing to the limited sources of human brain samples and difficulty of isolation of highly purified microglia, we examined whether cytokine-enriched supernatants from LPS-stimulated human blood MDM cultures modulated MIP-1β expression in NT2-N cells. Our data demonstrated that IL-1β present in the supernatants from MDM cultures plays a major role in the up-regulation of MIP-1β, indicating that the effect of IL-1β on MIP-1β expression remains in the presence of other cytokines. This MDM culture supernatant-mediated induction of MIP-1β is not due to the addition of LPS to MDM cultures, because LPS did not directly affect MIP-1α and -1β expression when added to NT2-N cell cultures, (Fig. 6).
Cytokines and chemokines interacting with specific receptors on both immune and CNS cells are involved in neuroimmunoregulation. IL-1β is one of these important mediators that interact with both the immune and nervous system. The intracellular signaling pathway by which IL-1β enhances β-chemokine expression in neuronal cells remains unknown. The synthesis of many cytokines and other inflammatory gene products is under control of the transcription activator NF-κB (Baeuerle and Henkel 1994). There is increasing evidence to suggest that NF-κB is also an inducible transcription factor present in the brain (Kaltschmidt et al. 1993, 1995). The functional NF-κB binding site is located in the promoter of MIP-1α and -1β genes (Rezzonico et al. 2001). We therefore examined whether IL-1β, through the activation of NF-κB, induces MIP-1α and -1β expression in human neuronal cells. We demonstrated that IL-1β has the ability to activate the NF-κB promoter in NT2-N cells. To further test the linkage of NF-κB activation and MIP-1α and -1β expression, we employed CAPE, a potent and specific inhibitor of activation of NF-κB (Natarajan et al. 1996), in IL-1β stimulation experiments. When added to NF-κB-transfected NT2-N cells, CAPE abrogated IL-1β-induced NF-κB promoter activation (Fig. 8a). In addition, it decreased IL-1β-induced MIP-1α and -1β expression in NT2-N cells (Fig. 8b). These data strongly indicate that NF-κB indeed plays a role in IL-1β-mediated up-regulation of MIP-1α and -1β in NT2-N cells. Our results support other studies by showing that IL-1β activates NF-κB transcription in other cell systems (Uehara et al. 1999; Wang et al. 1999; Garcia et al. 2000; Jedrzkiewicz et al. 2000; Miyamoto et al. 2000; Chikaraishi et al. 2001; John et al. 2001; Rovin et al. 2001).
Chemokines have been found in tissues in several pathologic conditions characterized by distinct leukocytic infiltrates, including multiple sclerosis and neuroAIDS. Levels of MIP-1α and -1β are raised in patients with multiple sclerosis and neuroAIDS. Thus, factors that regulate chemokine expression in the CNS have important implications in neuroimmunologic and neuroinfectious diseases. Up-regulation of production of MIP-1α and -1β by IL-1β in neuronal cells may constitute a critical mechanism for continuous recruitment of inflammatory cells to neurons and maintenance of chronic inflammation. IL-1β-induced β-chemokine expression may be one of the mechanisms responsible for the pathogenesis of the inflammatory process related to neuronal disorders in the CNS. Further understanding of the molecular mechanisms that control production of chemokines may lay a foundation for development of new anti-inflammatory treatments for inflammatory neuronal diseases including multiple sclerosis and AIDS dementia.
Acknowledgements
This investigation was supported by grants from the National Institutes of Health (DA12815 to WZH, MH49981 and AA13547 to SDD, and NS25044 and NS08075 to DEP). This study was also supported by the National Multiple Sclerosis Society.
Abbreviations used
- CAPE
caffeic acid phenethyl ester
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL
interleukin
- IL-1βAb
anti-IL-1β neutralizing antibody
- LPS
lipopolysaccharide
- MDM
monocyte-derived macrophage
- MIP-1α
macrophage inflammatory protein-1α
- MIP-1β
macrophage inflammatory protein-1β
- neuroAIDS
neuro-acquired immune deficiency syndrome
- NF-κB
nuclear factor-κB
- TNF-α
tumor necrosis factor-α
References
- Allan SM, Rothwell NJ. Cytokines and acute neurode-generation. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Costa A, Schettini G. Expression of chemokine receptors in the rat brain. Ann. N. Y. Acad. Sci. 1999a;876:201–209. doi: 10.1111/j.1749-6632.1999.tb07640.x. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J. Neurochem. 1999b;73:2348–2357. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am. J. Physiol. 1992;263:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Boutet A, Salim H, Leclerc P, Tardieu M. Cellular expression of functional chemokine receptor CCR5 and CXCR4 in human embryonic neurons. Neurosci. Lett. 2001;311:105–108. doi: 10.1016/s0304-3940(01)02149-8. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Capobianchi MR, Abbate I, Antonelli G, Turriziani O, Dolei A, Dianzani F. Inhibition of HIV type 1 BaL replication by MIP-1alpha, MIP-1beta, and RANTES in macrophages. AIDS Res. Hum. Retroviruses. 1998;14:233–240. doi: 10.1089/aid.1998.14.233. [DOI] [PubMed] [Google Scholar]
- Chikaraishi A, Hirahashi J, Takase O, Marumo T, Hishikawa K, Hayashi M, Saruta T. Tranilast inhibits interleukin-1beta-induced monocyte chemoattractant protein-1 expression in rat mesangial cells. Eur. J. Pharmacol. 2001;427:151–158. doi: 10.1016/s0014-2999(01)01215-8. [DOI] [PubMed] [Google Scholar]
- Copray JC, Mantingh I, Brouwer N, Biber K, Kust BM, Liem RS, Huitinga I, Tilders FJ, Van Dam AM, Boddeke HW. Expression of interleukin-1 beta in rat dorsal root ganglia. J. Neuroimmunol. 2001;118:203–211. doi: 10.1016/s0165-5728(01)00324-1. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Kelley KW. Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Res. 1991;557:115–120. doi: 10.1016/0006-8993(91)90123-d. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Imeri L. Cytokine–neurotransmitter interactions in the brain. Biol. Signals Recept. 1998;7:33–44. [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Ann. N. Y. Acad. Sci. 1988;546:122–132. doi: 10.1111/j.1749-6632.1988.tb21627.x. [DOI] [PubMed] [Google Scholar]
- Fibbe WE, Schaafsma MR, Falkenburg JH, Willemze R. The biological activities of interleukin-1. Blut. 1989;59:147–156. doi: 10.1007/BF00320059. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Xia Y, Chen S, Wang Y, Ye RD, Harrison JK, Bacon KB, Zerwes HG, Feng L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J. Leukoc. Biol. 2000;67:577–584. doi: 10.1002/jlb.67.4.577. [DOI] [PubMed] [Google Scholar]
- Ghirnikar RS, Lee YL, He TR, Eng LF. Chemokine expression in rat stab wound brain injury. J. Neurosci. Res. 1996;46:727–733. doi: 10.1002/(SICI)1097-4547(19961215)46:6<727::AID-JNR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Halks-Miller M, Hesselgesser J, Miko IJ, Horuk R. Chemokine receptors in developing human brain. Meth. Enzymol. 1997;288:27–38. doi: 10.1016/s0076-6879(97)88005-6. [DOI] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J. Immunol. Meth. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Horuk R. Chemokine and chemokine receptor expression in the central nervous system. J. Neurovirol. 1999;5:13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Jedrzkiewicz S, Nakamura H, Silverman ES, Luster AD, Mansharamani N, K. H, Tamura G, Lilly CM. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1058–L1065. doi: 10.1152/ajplung.2000.279.6.L1058. [DOI] [PubMed] [Google Scholar]
- John GR, Simpson JE, Woodroofe MN, Lee SC, Brosnan CF. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J. Neurosci. 2001;21:4134–4142. doi: 10.1523/JNEUROSCI.21-12-04134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech. Dev. 1993;43:135–147. doi: 10.1016/0925-4773(93)90031-r. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc. Natl Acad. Sci. USA. 1995;92:9618–9622. doi: 10.1073/pnas.92.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus WJ, Kennedy KJ. MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J. Leukoc. Biol. 1997;62:681–687. [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J. Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucl. Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann. N. Y. Acad. Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Miyagishi R, Kikuchi S, Fukazawa T, Tashiro K. Macrophage inflammatory protein-1 alpha in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological diseases. J. Neurol. Sci. 1995;129:223–227. doi: 10.1016/0022-510x(95)00004-l. [DOI] [PubMed] [Google Scholar]
- Miyagishi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K. Identification of cell types producing RANTES, MIP-1 alpha and MIP-1 beta in rat experimental autoimmune encephalomyelitis by in situ hybridization. J. Neuroimmunol. 1997;77:17–26. doi: 10.1016/s0165-5728(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto NG, Medberry PS, Hesselgesser J, Boehlk S, Nelson PJ, Krensky AM, Perez HD. Interleukin-1beta induction of the chemokine RANTES promoter in the human astrocytoma line CH235 requires both constitutive and inducible transcription factors. J. Neuroimmunol. 2000;105:78–90. doi: 10.1016/s0165-5728(00)00195-8. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl Acad. Sci. USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak D, Memon SA, Birrer MJ, Ashwell JD, Zacharchuk CM. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J. Immunol. 1994;153:2046–2051. [PubMed] [Google Scholar]
- Pierce JW, Lenardo M, Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc. Natl Acad. Sci. USA. 1988;85:1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood. 2001;97:2932–2940. doi: 10.1182/blood.v97.10.2932. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol. Sci. 1991;12:430–436. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Lu L, Cosio A. Cyclopentenone prostaglandins inhibit cytokine-induced NF-kappaB activation and chemokine production by human mesangial cells. J. Am. Soc. Nephrol. 2001;12:1659–1667. doi: 10.1681/ASN.V1281659. [DOI] [PubMed] [Google Scholar]
- Sun D, Hu X, Liu X, Whitaker JN, Walker WS. Expression of chemokine genes in rat glial cells: the effect of myelin basic protein-reactive encephalitogenic T cells. J. Neurosci. Res. 1997;48:192–200. [PubMed] [Google Scholar]
- Uehara T, Matsuno J, Kaneko M, Nishiya T, Fujimuro M, Yokosawa H, Nomura Y. Transient nuclear factor kappaB (NF-kappaB) activation stimulated by interleukin-1beta may be partly dependent on proteasome activity, but not phosphorylation and ubiquitination of the IkappaBalpha molecule, in C6 glioma cells. Regulation of NF-kappaB linked to chemokine production. J. Biol. Chem. 1999;274:15875–15882. doi: 10.1074/jbc.274.22.15875. [DOI] [PubMed] [Google Scholar]
- Wang XC, Jobin C, Allen JB, Roberts WL, Jaffe GJ. Suppression of NF-kappaB-dependent proinflammatory gene expression in human RPE cells by a proteasome inhibitor. Invest. Ophthalmol. Vis. Sci. 1999;40:477–486. [PubMed] [Google Scholar]
- Zhang GX, Baker CM, Kolson DL, Rostami AM. Chemokines and chemokine receptors in the pathogenesis of multiple sclerosis. Mult. Scler. 2000;6:3–13. doi: 10.1177/135245850000600103. [DOI] [PubMed] [Google Scholar]


