Abstract
Opiate abuse has been postulated to be a cofactor in the immunopathogenesis of acquired immunodeficiency syndrome (AIDS). This study evaluated whether methadone, a drug widely prescribed for the treatment of drug abusers with opioid dependence, affects human immunodeficiency virus (HIV) infection of human immune cells. When added to human fetal microglia and blood monocyte–derived macrophage cultures, methadone significantly enhanced HIV infection of these cells. This enhancement was associated with the up-regulation of expression of CCR5, a primary coreceptor for macrophage-tropic HIV entry into macrophages. Most importantly, the addition of methadone to the cultures of latently infected peripheral blood mononuclear cells from HIV-infected patients enhanced viral activation and replication. Although the in vivo relevance of these findings remains to be determined, the data underscore the necessity of further studies to define the role of opioids, including methadone, in the immunopathogenesis of HIV infection and AIDS.
Clinical and epidemiologic evidence derived from early pre-AIDS studies supports a strong rationale for the notion that opiates may be a factor in the immunopathogenesis of AIDS [1]. In vitro studies demonstrated that morphine activates and enhances human immunodeficiency virus (HIV) replication in human immune cells that express opiate receptors [2]. In addition, HIV-infected injection drug users have substantial pre-AIDS morbidity and mortality [3]. The cessation of injection drug use has been positively correlated with a decrease in the rate of progression to AIDS [4–6]. These data strongly indicate that, in addition to more frequent exposure of the drug user to HIV via contaminated needles, substance abuse–mediated malfunction of the human immune system also promotes HIV infection of the immune cells.
The effect of methadone, a drug widely used for the treatment of drug users with opiate dependence, on immune system cells is largely unknown. In vitro and in vivo studies indicate that methadone has suppressive effects on functions of NK cells [7], human T lymphocytes [8], and mononuclear phagocytes [9]. Peterson et al. [10] demonstrated that the generating capacity of peripheral blood mononuclear cells (PBMC) from patients receiving methadone is significantly lower than that of healthy donor PBMC. Methadone, at micromolar concentrations, suppresses the chemokine-mediated migration of neutrophils and monocytes [11]. These studies raise concern regarding the possible consequences of methadone-mediated immunologic changes that could affect HIV infection of human immune cells. We hypothesize that methadone, due to its immunosuppressive effects on T lymphocytes and mononuclear phagocytes, the primary target cells for HIV, may promote HIV infection of these cells.
Materials and Methods
Microglia isolation
Fresh human fetal brain tissues (at 14–17 weeks of gestation) were obtained from the Anatomic Gift Foundation. Human fetal brain microglia were isolated and then cultured as described elsewhere [12], with modification. In brief, fetal brain tissue was dissected free of leptomeninges and large blood vessels and was finely minced with sterile surgical scalpels. The minced tissue was then digested for 30 min at 37°C in Dulbecco’s MEM (DMEM) containing 0.25% trypsin, DNase (50 mμ/mL), and G418 (50 μg/mL). The digest was filtered through a 100-μm nylon mesh. The cells were washed in serum-containing medium and were seeded at 4 × 108 cells/T75 tissue flask in 10% DMEM with 5% giant cell tumor supernatant. After 3–4 weeks in vitro, the cultures were shaken in an orbital shaker for 16 h at 150 rpm (orbit diameter, 1.9 cm) at 37°C. The cells floating in the supernatant were collected and were reseeded into 96-well plates (2×5 104 cells/well). The purity of microglia obtained by this procedure was >.96%, as analyzed by staining with anti-CD45 antibody.
Monocyte isolation
Peripheral blood was obtained from healthy adult donors without history of drug abuse. Heparinized blood samples were identified as HIV antibody negative by anonymous ELISA testing (Coulter Immunology). Heparinized blood was separated by centrifugation over lymphocyte separation medium (Organon Teknika) for 45 min. The mononuclear layer was collected and was incubated in a 2% gelatin-coated flask for 45 min at 37°C, and nonadherent cells were removed. Adherent monocytes were detached with 10 mM EDTA. After the initial purification, >97% of the cells were found to be monocytes, as determined by nonspecific esterase staining and flow cytometry by use of a monoclonal antibody (MAb) against CD14. Freshly isolated monocytes were plated in 48-well culture plates at a density of 5 × 105 cells/well in 10% DMEM. Monocyte-derived macrophages (MDMs) are defined as monocytes cultured in vitro for 7–10 days.
Isolation and culture of PBMC from HIV-infected patients
PBMC were obtained from 6 HIV-infected patients, aged 22–48 years. All patients had >400 CD4 cells/mm3 and undetectable HIV mRNA levels (Roche Amplicor HIV Monitor assay; limit of quantification of 400 copies/mL) and were receiving highly active antiretroviral therapy at the time of phlebotomy. None had a history of drug abuse. PBMC were isolated by standard Ficoll-Paque (Pharmacia) density gradient centrifugation. Isolated PBMC then were subjected to CD8 T cell depletion by using MACS CD8 microbeads in accord with the manufacturer’s instructions (Miltenyi Biotec). CD8-depleted PBMC then were stimulated with phytohemagglutinin (PHA) for 72 h and were washed once with RPMI 1640 before seeding into 48-well culture plates (106 cells/well).
HIV strains
HIV Bal and JR-FL strains were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. HIV Bal is a macrophage (M)–tropic strain isolated from human infant lung tissue. HIV JR-FL is an M-tropic strain isolated from brain tissue of an AIDS patient with encephalopathy.
Reverse-transcriptase (RT) assay
HIV RT activity was determined on the basis of the technique of Willey et al., with modification [13]. In brief, supernatants were collected every 4 days for RT activity testing. In total, 10 μL of collected culture supernatants was added to a “cocktail” containing poly A, oligo dT (Pharmacia), MgCl2, and 32P dTTP (Amersham), which was incubated for 20 h at 37°C. We then spotted 30 μL of the cocktail onto DE81 paper, which was dried and washed 5 times with saline–sodium citrate buffer and once with 95% ethanol. Radioactivity was counted in a liquid scintillation counter (Packard Instrument).
Flow cytometry
To determine CCR5 expression by indirect immunofluorescence, 2 × 105 MDMs were suspended in 100 μL of 1 × PBS and were incubated with CCR5 antibody (2D7) for 30 min at 4°C. The cells then were washed twice with PBS and were incubated with fluorescein-conjugated goat anti-mouse antibody for 30 min at 4°C. After another wash with PBS, the cells were fixed with 1% paraformaldehyde in PBS. Fluorescein-conjugated control antibody was isotype-matched IgG2b. Fluorescence was analyzed on an EPICS-Elite flow cytometer (Beckman-Coulter Electronics).
Methadone or morphine treatment and HIV infection
We incubated 7-day-cultured human fetal microglia or MDMs, with or without methadone (10−10 M for microglia; 10−12–10−8 M for MDM) and/or naltrexone (10−8 M), for 2 h before HIV infection. For combination treatment of cells with methadone and naltrexone, naltrexone was added to the culture 30 min before methadone was added. The cells were infected with equal amounts of cell-free HIV strains JR-FL or Bal, on the basis of p24 protein content (20 ng/106 cells) for 2 h at 37°C, in the presence or absence of the reagents described above. The cells then were washed 3 times with DMEM to remove unabsorbed virus, and fresh medium containing methadone and/or naltrexone was added to cell cultures. The final wash was tested for viral RT activity and was found to be free of residual inoculum. Untreated cells served as controls. The cells were treated with the reagents described every 4 days after infection. Supernatants were collected (days 8, 12, and 16 for microglia; day 8 for MDMs) for HIV RT activity assay. In some experiments, we included morphine treatment of MDMs, for comparison with the effects of methadone on HIV infection of MDMs.
Methadone treatment of latently infected PBMC
CD8 T lymphocyte–depleted PBMC from HIV-infected patients were stimulated with PHA for 72 h and then were seeded into 48-well plates at 106 cells/well in 0.5 μL of culture medium containing anti-CD3 antibody (2 mg/mL; R&D Systems) and interleukin-2 (50 U/mL; Boehringer Mannheim). The cultures then were incubated at 37°C in the presence or absence of methadone or morphine at 10−10M. Supernatants were collected from the cell cultures at day 6 to determine HIV RT activity. The reagents described above were readded whenever the medium was changed.
Statistical analysis
When appropriate, data were expressed as mean ± SD. For comparison of the means of 2 groups, statistical significance was assessed by Student’s t test. Significance was defined as P < .05.
Results
Effect of methadone on HIV infection of human fetal microglia and MDMs
We first determined whether methadone affected HIV infection of human fetal microglia and blood MDMs. Microglia cultured for 7 days in 96-well plates were challenged with HIV M-tropic strain JR-FL in the presence or absence of methadone (10−10 M) and/or naltrexone (10−8 M) for 2 h at 37°C. Culture supernatants were collected on postinfection days 8, 12, and 16 for RT assays. Methadone significantly enhanced (2.1-, 1.6-, and 1.7-fold on postinfection days 8, 12, and 16, respectively) HIV JR-FL replication in the fetal microglia (figure 1A). When 7-day-cultured MDMs treated with methadone or morphine at different concentrations (10−12–10−8 M) for 2 h were challenged with HIV Bal, both morphine and methadone significantly increased the viral replication in MDMs (figure 1B). Naltrexone (10−8 M) abrogated the effect of methadone or morphine on the viral replication in microglia and MDMs (figure 1), whereas naltrexone alone had little effect on HIV replication (figure 1).
Figure 1.
Effect of methadone (Me) or morphine (Mo) on human immunodeficiency virus (HIV) infection of microglia (A) and monocyte-derived macrophages (MDMs) (B). MDMs and microglia (cultured for 7 days) were treated with Me and/or naltrexone (Nalt) at indicated concentrations for 2 h and then were infected with HIV strain JR-FL (microglia) or Bal (MDMs) in the presence of Me or Mo. Controls were cultures with neither Me nor Nalt. Cultures were refed with fresh medium containing indicated reagents every 4 days. Culture supernatants were collected for HIV reverse-transcriptase (RT) assay at indicated time points. Results, representative of 3 experiments, are mean ± SD (*P < .05, Me vs. control). Plus (+) and minus (−) signs (A) indicate presence or absence.
Effect of methadone on CCR5 expression
We examined the effect of methadone on the expression of CCR5 receptor, a primary coreceptor for M-tropic HIV entry into macrophages. Macrophages cultured for 7–10 days were incubated in the presence or absence of methadone at 10−10 M for 24 h before cell harvest for flow cytometry using a MAb (2D7; R&D Systems) against CCR5. As shown in figure 2, methadone significantly up-regulated CCR5 expression on methadone-treated MDMs.
Figure 2.

Effect of methadone on CCR5 expression in 7-day-cultured monocyte-derived macrophages (MDMs). MDMs were incubated with or without methadone at 10−10 M for 24 h, and expression of CCR5 on MDMs was analyzed by flow cytometry. Shaded histogram, control staining with isotope-matched antibody (IgG2b); open histogram, CCR5 expression with monoclonal antibody 2D7. Results, representative of 4 experiments, are shown as percentage of CCR5-positive cells.
Effect of methadone or morphine on HIV activation in latently infected PBMC
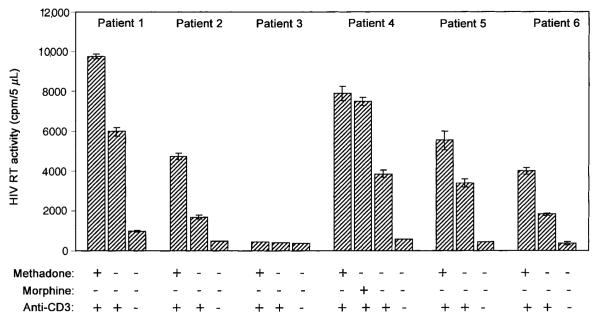
After we demonstrated that methadone could augment HIV replication in human fetal microglia and blood MDMs in vitro, we considered the possibility that methadone or morphine treatment might enhance HIV replication in latently infected PBMC. Thus, we determined the effect of methadone or morphine on HIV activation and replication in latently infected PBMC of HIV-infected patients. As shown in figure 3, methadone enhanced HIV activation and replication in latently infected PBMC of 5 of 6 HIV-infected persons. Morphine, when added to the cultures, also up-regulated HIV replication in the latently infected PBMC from patient 4 (figure 3).
Figure 3.
Effect of methadone on human immunodeficiency virus (HIV) replication in peripheral blood mononuclear cells (PBMC) from 6 HIV-infected patients. CD8 T cell–depleted PBMC were stimulated with 1% phytohemagglutinin (vol/vol) for 72 h. Cells then were incubated with or without methadone (10−10 M) and/or anti-CD3 (2 μg/mL), as indicated above. In patient 4, morphine (10−10 M) was also tested in an HIV induction experiment. Cultures were refed with fresh medium containing the indicated reagents every 3 days. Day 6 culture supernatants were collected for HIV reverse-transcriptase (RT) assay. Data are mean ± SD of HIV RT activity in triplicate cultures. Plus (+) and minus (−) signs indicate presence or absence.
Discussion
Methadone is the most common maintenance pharmacotherapy for drug users with opiate dependence. As an opioid agonist, methadone may share the direct and indirect immuno-regulatory effects of other opioids and thus affect susceptibility to, and the natural history of, HIV infection. In this study, we analyzed the effect of methadone on HIV infection of macrophages, a primary target for HIV. Our data support the hypothesis that methadone up-regulates HIV infection. First, methadone, at nanomolar concentrations or lower, significantly enhanced HIV infection of MDMs (figure 1), and, second, methadone enhanced activation and replication of HIV in latently infected PBMC isolated from persons infected with HIV (figure 3). The latter finding may have an important implication for the role of methadone in regulation of HIV replication in vivo. The effects of methadone on HIV infection of MDMs were mediated through the opioid receptors, since naltrexone blocked these effects.
Although the precise mechanism(s) whereby methadone potentiates HIV infection of MDMs is not known, our data support 2 coexisting potential mechanisms. First, methadone may inhibit production of endogenous β-chemokines (data not shown) by MDMs and enhanced expression of CCR5 receptor on MDMs (figure 2). This is supported by recent findings that showed that morphine induces gene expression of CCR5 in a human T lymphoid cell line [14]. Because β-chemokines interfere with HIV infection of MDMs by competing for CCR5 receptor, a primary coreceptor for HIV entry into macrophages, methadone-mediated up-regulation of CCR5 receptor and down-regulation of β-chemokine production may be responsible for its effect on HIV M-tropic infection of MDMs. A second possibility is that methadone activates the HIV long-terminal repeat (LTR) promoter, as demonstrated by increased LTR-driven chloramphenicol acetyltransferase activity (data not shown). This effect of methadone on HIV LTR may be the basis for methadone-induced activation of HIV in latently infected PBMC (figure 3).
Because methadone is used to treat drug abusers with opiate dependence, many of whom are infected with HIV, it is essential to understand the immunologic consequences of methadone treatment and its role in the immunopathogenesis of HIV disease. Carballo-Dieguez et al. [15] found that methadone treatment was associated with a lower CD4 cell percentage and lower CD4/CD8 cell ratio in subjects with or without HIV infection. Our data support the hypothesis that methadone, like other opiates, may act as a cofactor in the immunopathogenesis of HIV infection. Although our findings, along with those of other studies on immunosuppressive activities of opioids (including methadone), raise concerns regarding the possible consequences of methadone-mediated up-regulation of HIV infection of immune cells in vivo, no compelling clinical evidence supports our conclusion. Furthermore, Novick et al. [16] demonstrated that significant abnormalities of cellular immunity in parenteral heroin abusers can be normalized by successful long-term methadone treatment. Thus, further studies to define the role of opioids, including methadone, in the immunopathogenesis of HIV infection and AIDS are essential.
Acknowledgments
We thank Li Song and Shuang Sun for technical assistance.
Financial support: National Institutes of Health (DA-12815 to W.-Z.H.; MH-49981 to S.D.D.).
Footnotes
Presented in part: 8th annual conference on NeuroImmune Circuits and Infectious Disease of the Society on NeuroImmune Pharmacology, Atlanta, March 2001 (poster 8).
Informed consent was obtained from all subjects in accordance with institutional review board guidelines.
References
- 1.Donahoe RM. Neuroimmunomodulation by opiates: relationship to HIV infection and AIDS. Adv Neuroimmunol. 1993;3:31–46. [PubMed] [Google Scholar]
- 2.Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–73. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Selwyn PA, Alcabes P, Hartel D, et al. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N Engl J Med. 1992;327:1697–703. doi: 10.1056/NEJM199212103272401. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 1993;328:671. erratum: [Google Scholar]
- 4.Alcabes P, Friedland G. Injection drug use and human immunodeficiency virus infection. Clin Infect Dis. 1995;20:1467–79. doi: 10.1093/clinids/20.6.1467. [DOI] [PubMed] [Google Scholar]
- 5.Ronald PJ, Robertson JR, Elton RA. Continued drug use and other cofactors for progression to AIDS among injecting drug users. AIDS. 1994;8:339–43. doi: 10.1097/00002030-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Specter S. Drugs of abuse and infectious diseases. J Fla Med Assoc. 1994;81:485–7. [PubMed] [Google Scholar]
- 7.Klimas NG, Blaney NT, Morgan RO, et al. Immune function and anti–HTLV-I/II status in anti–HIV-1–negative intravenous drug users receiving methadone. Am J Med. 1991;90:163–70. [PubMed] [Google Scholar]
- 8.Singh VK, Jakubovic A, Thomas DA. Suppressive effects of methadone on human blood lymphocytes. Immunol Lett. 1980;2:177–80. [Google Scholar]
- 9.Tubaro E, Avico U, Santiangeli C, et al. Morphine and methadone impact on human phagocytic physiology. Int J Immunopharmacol. 1985;7:865–74. doi: 10.1016/0192-0561(85)90049-9. [DOI] [PubMed] [Google Scholar]
- 10.Peterson PK, Gekker G, Brummitt C, et al. Suppression of human peripheral blood mononuclear cell function by methadone and morphine. J Infect Dis. 1989;159:480–7. doi: 10.1093/infdis/159.3.480. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y, Chuang LF, Lam KM, et al. Inhibition of chemokine-induced chemotaxis of monkey leukocytes by mu-opioid receptor agonists. In Vivo. 1999;13:389–96. [PubMed] [Google Scholar]
- 12.Hassan NF, Campbell DE, Rifat S, Douglas SD. Isolation and characterization of human fetal brain–derived microglia in in vitro culture. Neuroscience. 1991;41:149–58. doi: 10.1016/0306-4522(91)90205-3. [DOI] [PubMed] [Google Scholar]
- 13.Willey RL, Smith DH, Lasky LA, et al. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–47. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEMx174 lymphocytes. J Biol Chem. 2000;275:31305–10. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- 15.Carballo-Dieguez A, Sahs J, Goetz R, el Sadr W, Sorell S, Gorman J. The effect of methadone on immunological parameters among HIV-positive and HIV-negative drug users. Am J Drug Alcohol Abuse. 1994;20:317–29. doi: 10.3109/00952999409106017. [DOI] [PubMed] [Google Scholar]
- 16.Novick DM, Ochshorn M, Ghali V, et al. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term metha-done maintenance patients. J Pharmacol Exp Ther. 1989;250:606–10. [PubMed] [Google Scholar]