Abstract
Background:
i-gel™ and the ProSeal™ laryngeal mask airway (PLMA) are two supraglottic airway devices with gastric channel used for airway maintenance in anesthesia. This study was designed to evaluate the efficacy of i-gel compared with PLMA for airway maintenance in pediatric patients under general anesthesia with controlled ventilation.
Materials and Methods:
A total of 60 American Society of Anesthesiologists physical status 1 and 2 patients were included in the study and randomized to either i-gel or PLMA group. After induction of anesthesia using a standardized protocol for all the patients, one of supraglottic airway devices was inserted. Insertion parameters, ease of gastric tube insertion and fiber-optic scoring of the glottis were noted. Airway parameters such as end-tidal carbon dioxide (EtCO2), peak airway pressures and leak airway pressures were noted. Patients were observed for any complications in the first 12 h of the post-operative period.
Results:
Both groups were comparable in terms of ease of insertion, number of attempts and other insertion parameters. Ease of gastric tube insertion, EtCO2, airway pressures (peak and leak airway pressure) and fiber-optic view of the glottis were comparable in both groups. There were no clinically significant complications in the first 12 h of the post-operative period.
Conclusion:
i-gel is as effective as PLMA in pediatric patients under controlled ventilation.
Keywords: Controlled ventilation, fiber-optic view of glottis, leak airway pressure, peak airway pressure, pediatric i-gel airway, ProSeal™ laryngeal mask
Introduction
ProSeal laryngeal mask airway (PLMA), a supraglottic airway device (SAD) with a (gastric) drain tube has been widely used in pediatric and adult patients under both controlled and spontaneous ventilation.[1,2,3] The i-gel™[4,5] (Intersurgical Inc., Berkshire, UK), a single use non-inflatable SAD with a (gastric) drain tube has been introduced in clinical practice from last few years. The non-inflatable cuff of i-gel is made of a soft gel-like medical grade thermoplastic elastomer. The device has a buccal cavity stabilizer and integral bite block, which helps in alignment of the device with oropharyngeal curvature of the patient and prevents malrotation. There is an epiglottic rest with a protective ridge which prevents down folding of the epiglottis during insertion. i-gel has easier insertion with minimal risk of tissue compression[5] Pediatric i-gel is available in different sizes (1, 1.5, 2, and 2.5) according to the weight of the children.[6] We hypothesized that i-gel will be as effective as PLMA in airway maintenance in pediatric patients under controlled ventilation. Our primary objective was to compare the oropharyngeal sealing pressures (OSP) and secondary objectives were to compare the insertion parameters, positioning and any immediate complications.
Materials and Methods
After approval from institutional ethics committee, this study was conducted on 60 American Society of Anesthesiologists physical status 1 and 2 children aged 1-12 years of either sex scheduled for elective short duration (<1-2 h) pediatric surgery. Patients with expected difficult airway, history of obstructive sleep apnea, patients at risk of pulmonary aspiration of gastric contents and patients undergoing laparoscopic surgeries were excluded from the study. The patients were randomized to either i-gel (Group I) or PLMA (Group P) by opaque sealed envelope technique. Written consent was obtained from all parents of children recruited in the study. Patients were pre-medicated with midazolam orally at 0.5 mg/kg 15 min before the scheduled surgery. In the operating theater standard monitoring (pulse oximetry, non-invasive blood pressure recording, electro-cardiography and capnography) were connected to the child. Anesthesia was induced by inhalational induction with sevoflurane (up to 6%) along with 50% nitrous oxide. Intravenous (IV) line was secured when the children were in adequate plane following which fentanyl 2 μg/kg body weight was administered. After ensuring bag and mask ventilation, neuromuscular blockade was achieved with atracurium besylate 0.5 mg/kg body weight IV and patients were ventilated for 3 min with sevoflurane (4%), nitrous oxide and oxygen (50:50) to allow full jaw relaxation to take place and adequate size supraglottic airway device was inserted in “sniffing the morning air position.” Size of the device was selected according to the body weight of the patient (i-gel: 5-12 kg: 1.5 size; 10-25 kg: 2 size; 25-35 kg: 2.5 size[5] and PLMA: 5-10 kg: 1.5 size; 10-20 kg: 2 size; 20-30 kg: 2.5 size). Devices were kept ready on the machine after lubrication with a water soluble lubricant. i-gel was introduced by firmly grasping the device such that the cuff outlet was facing the chin of the patient and the device was gently guided along the hard palate until definitive resistance was felt (as per manufacturer's recommendation). Insertion of PLMA was done as per the manufacturer's recommendations, using the index finger digital method. The cuff was inflated according to size of PLMA (7 ml: 1.5 size; 10 ml: 2 size and 14 ml: 2.5 size). Both the devices were inserted by single anesthesiologist experienced in using supraglottic airway devices, according to the manufacturer's recommendation. After connecting the pediatric circle breathing circuit to the i-gel or PLMA, appropriate placement and ventilation was determined by chest wall movement, auscultation of breath sounds, a square-wave capnograph and lack of gastric insufflation. The presence of gastric insufflations was determined by epigastric auscultation. The fresh gas flow was set at 3 L/min. Maintenance of anesthesia was continued with sevoflurane, nitrous oxide and oxygen in 2:1 ratio. Bolus doses of 0.1 mg/kg atracurium were given for neuromuscular blockade maintenance and ventilation was initiated with tidal volume (VT) 10 ml/kg and respiratory rate (12-18/min) adjusted to obtain an end-tidal carbon dioxide (EtCO2) between 35 and 40 mm Hg. Ease of insertion was subjectively graded by single anesthesiologist in all cases. An “easy insertion” was defined as insertion within the pharynx without resistance in a single maneuver. A “difficult insertion” was one in which there was resistance to insertion or where more than one maneuver was required to seat the device within the pharynx. Ease of airway device insertion was graded subjectively on a scale from 1 to 3 (1-very easy, 2-easy and 3-difficult). Insertion time defined as the time between picking up the device and obtaining an effective airway with EtCO2 trace on the monitor. Three insertion attempts were allowed before a failure of insertion was recorded. If i-gel or PLMA were not able to achieve a satisfactory airway within three attempts, trachea was intubated conventionally. A satisfactory placement was noted if the expired VT was more than 8 ml/kg and there was no (gastric) drain tube leak. Peak Paw (cm H2O) was recorded when patient was put on the volume control ventilatory mode at 10 ml/kg body weight VT. Leak Paw (cm H2O) (Datex-Ohmeda S/5 anesthesia delivery system) was recorded when the patient was put on to bag mode and the adjustable pressure limiting valve fully closed with 3 L/min, the particular pressure (sealing pressure) at which audible leak (auscultation of anterior neck) through the device was noted along with equilibrium of pressure on the aneroid manometer and ventilator pressure time scalar. Leak pressure was subtracted from peak airway pressure and the difference (Law-Paw in cm H2O) was calculated. Visualization of glottis was determined by passing a fiber-optic scope (diameter, 3.7 mm: Karl-Storz, Tuttlingen, Germany) through the airway tube to a position 1 cm proximal to the end of the airway tube. The airway tube view was scored using the Brimacombe Score[7] (1-vocal cords not seen, 2-vocal cords plus anterior epiglottis seen, 3-vocal cords plus posterior epiglottis seen and 4-only vocal cords visible). Scores 3 and 4 were considered as a good view in our study. After correct insertion was confirmed, well-lubricated appropriate size (6 Fr, 8 Fr and 10 Fr sizes for 1.5, 2 and 2.5 sized airway devices respectively[8]) gastric tube was inserted through the drain tube. Correct gastric tube placement was assessed by suction of fluid or detection of injected air by epigastric-stethoscopy. Ease of gastric tube insertion was graded subjectively (1-very easy/2-easy/3-difficult/4-very difficult). After the surgery, neuromuscular blockade was antagonized with 0.05 mg/kg neostigmine and 0.01 mg/kg of glycopyrrolate. Device was removed once the child was fully awake or easily arousable. The supraglottic airway was observed for any blood staining or any other injuries. The child was followed-up the evening of surgery to elicit a history of sore throat.
For power analysis calculation, we considered as 20% difference in mean sealing pressure between the groups to be significant based on previous study[1,9] between PLMA and classic LMA (cLMA) in children (PLMA: OSP 18.72 ± 3.28 cm of H2O and cLMA 15.43 ± 2.94 cm of H2O[1]). G*Power version 3.1 (Franz Faul, Universitat Kiel, Germany) was used for power and sample size analysis. A power analysis based on 95% confidence interval, a sample size of 30 in each group with the total sample size of 60 for 80% statistical power and 5% level of significance was considered. All statistics were performed using the Statistical Package for the Social Sciences (SPSS) version 18 (SPSS Inc., Chicago, IL, USA) and Graphpad Instat 3.0 for Windows (GraphPad Software, San Diego, CA, USA). Distributions of data were determined using Kolmogorov-Smirnov analysis. Continuous measurements were expressed as mean ± standard deviation (SD) and categorical measurements as number percentage. Unpaired Student t-test for parametric data and Chi-square or Fischer's exact test was used for nominal data. Mann-Whitney test was used for fiber-optic scores. P < 0.05 was considered to be statistically significant.
Results
Patient demographic characteristics were comparable in both groups [Tables 1 and 2]. Size-2 airway devices were used in the majority of the cases [Table 2]. There was no statistically significant difference in distribution of sizes in both groups. Success rate for the first attempt insertion was 86.7% with i-gel and 93.3% with (PLMA). Ease of insertion, mean time of insertion and manipulation was not statistically significant between the groups. The ease of gastric tube insertion was comparable in both the groups with 90% grade 1 in both the groups although two cases in Group P had difficult insertion [Table 2]. One case of oropharyngeal air leakage and two cases of tube displacement [Table 2] occurred in the study, but the distribution of other events in both groups was not statistically significant. Airway and ventilator parameters studied were similar in both the groups [Table 3]. The mean peak airway pressure, mean leak airway pressure and leak-peak airway pressures were also similar in both the groups. Brimacombe Scores [Table 4] were statistically similar in both the groups with 11 cases (36.7%) in both having grade 4 view, 8 (26.7%) in Group P and 12 (40.0%) cases in Group I had grade 3 view. 10 (33.3%) cases in Group P and 7 (23.3%) cases in Group I had grade 2 view with one case (3.3%) in Group P grade 1 view.
Table 1.
Patient characteristics and type of surgery
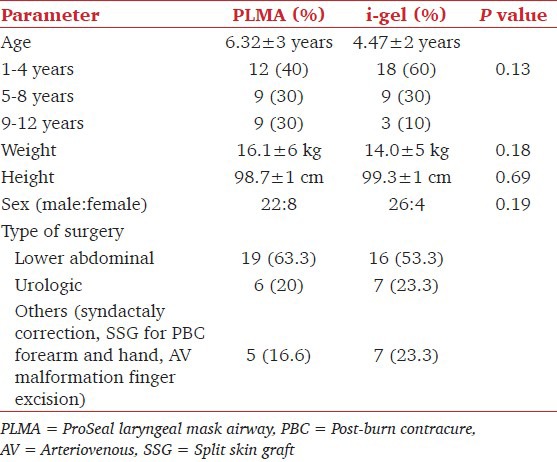
Table 2.
Insertion parameters

Table 3.
Ventilator parameters

Table 4.
Fiber-optic view of glottis

Discussion
The insertion time in our study was comparable between the two groups; however, we took lesser time as compared to the study by Gasteiger et al.[9] The number of attempts to insert the devices is comparable to study done by Goyal et al.[2] in which only size-2 supraglottic airway devices were compared. In their study, i-gel was inserted in 95% cases in first attempt, PLMA and cLMA's were inserted in 90% of cases in first attempt. The lower first attempt success rate in our study for i-gel can be attributed to use of different sizes and overlap in size selection according to body weight as recommended by the manufacturer (size 1.5: 5-12 kg and size 2: 10-25 kg). This problem with size selection was reported in previous studies too.[8,10,11] Ease of insertion of airway device and ease of insertion of gastric tube was similar in both the groups. To eliminate observer bias all these grading were subjectively done by single anesthesiologist inserting the devices. This was found to be similar with other studies.[2,12,13]
In our study, the mean oropharyngeal leak pressure was comparable between the groups. PLMA sizes (1.5, 2 and 2.5), which we used has no dorsal cuff,[11] this might also be the reason for similar leak pressures. The leak pressure of i-gel in this study was similar to study done by Theiler et al.[13] in which leak pressure of i-gel was significantly higher than the leak pressure of the ambu aura (mean ± SD: 22 ± 5 cm H2O vs. 19 ± 3, P < 0.01). These findings were also comparable with the results of the study by Goyal et al.[2] and Gasteiger et al.[9] However, in the study by Goyal et al.,[2] the difference between i-gel and both LMA groups were statistically significant. The possible explanation for this is that, in their study specific size-2 supraglottic airway devices are used with a larger sample size compared to our study. In our study, the mean sealing pressure (leak-peak airway pressure) was 10.1 ± 5 cm H2O in i-gel group and 10.3 ± 6 cm H2O in PLMA group which were statistically similar. The supraglottic airway device that can provide ventilation with low peak airway pressure and high leak pressure is supposed to have wider margin of safety for ventilation as represented in previous study by Beylacq et al.[14] The margin of safety for ventilation was similar in both the groups in our study.
Fiber-optic visualization of glottis by Brimacombe Score was statistically comparable in both the groups. The view in i-gel group of this study (76.7%) was similar to i-gel group of Lee et al.[12] in which good view was obtained in 74% of cases. There was no critical incident in both the groups except for one case of oro-pharyngeal air leakage and two cases of tube displacement in PLMA group during positioning for caudal anesthesia. Incidence of post-operative sore throat and hoarseness were similar in both the groups. A previous study done by Francksen et al.[15] reported more post-operative sore throat and dysphagia with PLMA compared to i-gel.
Though most of the devices used in both the groups were size-2, we did not specify any single specific size of both airway devices to be used in our study, which could be a limitation of our study. Moreover the study was done under controlled ventilation, though the ease of insertion would have been defined better in spontaneously breathing patients.
Hence to conclude, an i-gel is as effective as PLMA in pediatric patients under controlled ventilation in general anesthesia. Ease of insertion, number of attempts, OSP, ease of gastric tube insertion and visualization of glottis with fiber-optic scope were comparable in both groups. i-gel can thus be used as a good alternative to PLMA in pediatric patient under control ventilation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kanthed P, Sharma B, Sood J, Kumar VP. Comparison of LMA-ProSeal™ with LMA Classic™ in anaesthetised paralysed children. Indian J Anesth. 2008;52:44–8. [Google Scholar]
- 2.Goyal R, Shukla RN, Kumar G. Comparison of size 2 i-gel supraglottic airway with LMA-ProSeal™ and LMA-Classic™ in spontaneously breathing children undergoing elective surgery. Paediatr Anaesth. 2012;22:355–9. doi: 10.1111/j.1460-9592.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 3.Jagannathan N, Sohn LE, Sawardekar A, Gordon J, Langen KE, Anderson K. A randomised comparison of the LMA Supreme™ and LMAProSeal™ in children. Anaesthesia. 2012;67:632–9. doi: 10.1111/j.1365-2044.2012.07088.x. [DOI] [PubMed] [Google Scholar]
- 4.Levitan RM, Kinkle WC. Initial anatomic investigations of the i-gel airway: A novel supraglottic airway without inflatable cuff. Anaesthesia. 2005;60:1022–6. doi: 10.1111/j.1365-2044.2005.04258.x. [DOI] [PubMed] [Google Scholar]
- 5.Richez B, Saltel L, Banchereau F, Torrielli R, Cros AM. A new single use supraglottic airway device with a noninflatable cuff and an esophageal vent: An observational study of the i-gel. Anesth Analg. 2008;106:1137–9. doi: 10.1213/ane.0b013e318164f062. [DOI] [PubMed] [Google Scholar]
- 6.i-gel instructions for use (Paediatric sizes only) [Access as on 2013, June 20]. Availavle from, http://www.i-gel.com/igel-for-anaesthesia .
- 7.Brimacombe J, Berry A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position. Anesth Analg. 1993;76:457. [PubMed] [Google Scholar]
- 8.Janakiraman C, Chethan DB, Wilkes AR, Stacey MR, Goodwin N. A randomised crossover trial comparing the i-gel supraglottic airway and classic laryngeal mask airway. Anaesthesia. 2009;64:674–8. doi: 10.1111/j.1365-2044.2009.05898.x. [DOI] [PubMed] [Google Scholar]
- 9.Gasteiger L, Brimacombe J, Oswald E, Perkhofer D, Tonin A, Keller C, et al. LMA ProSeal(TM) vs. i-gel(TM) in ventilated children: A randomised, crossover study using the size 2 mask. Acta Anaesthesiol Scand. 2012;56:1321–4. doi: 10.1111/j.1399-6576.2012.02765.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldmann K, Roettger C, Wulf H. The size 1(1/2) ProSeal laryngeal mask airway in infants: A randomized, crossover investigation with the classic laryngeal mask airway. Anesth Analg. 2006;102:405–10. doi: 10.1213/01.ane.0000194300.56739.1a. [DOI] [PubMed] [Google Scholar]
- 11.Cattano D, Ferrario L, Maddukuri V, Sridhar S, Khalil Y, Hagberg CA. A randomized clinical comparison of the intersurgical i-gel and LMA unique in non-obese adults during general surgery. Minerva Anestesiol. 2011;77:292–7. [PubMed] [Google Scholar]
- 12.Lee JR, Kim MS, Kim JT, Byon HJ, Park YH, Kim HS, et al. A randomised trial comparing the i-gel (TM) with the LMA Classic (TM) in children. Anaesthesia. 2012;67:606–11. doi: 10.1111/j.1365-2044.2012.07072.x. [DOI] [PubMed] [Google Scholar]
- 13.Theiler LG, Kleine-Brueggeney M, Luepold B, Stucki F, Seiler S, Urwyler N, et al. Performance of the pediatric-sized i-gel compared with the Ambu AuraOnce laryngeal mask in anesthetized and ventilated children. Anesthesiology. 2011;115:102–10. doi: 10.1097/ALN.0b013e318219d619. [DOI] [PubMed] [Google Scholar]
- 14.Beylacq L, Bordes M, Semjen F, Cros AM. The i-gel, a single-use supraglottic airway device with a non-inflatable cuff and an esophageal vent: An observational study in children. Acta Anaesthesiol Scand. 2009;53:376–9. doi: 10.1111/j.1399-6576.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 15.Francksen H, Renner J, Hanss R, Scholz J, Doerges V, Bein B. A comparison of the i-gel with the LMA-unique in non-paralysed anaesthetised adult patients. Anaesthesia. 2009;64:1118–24. doi: 10.1111/j.1365-2044.2009.06017.x. [DOI] [PubMed] [Google Scholar]


