Abstract
Background:
Pain relief remains the most fundamental and consequential aspect of surgery for patients throughout perioperative period. Dexmedetomidine has created an interest in α2-adrenoceptor agonists in the management of pain and hence the aim of this study was to evaluate the effectiveness of the drug in hilly population of North India.
Materials and Methods:
Patients, irrespective of gender, were randomly allocated to two groups, control and test, each having 30 patients. Test group received an infusion of dexmedetomidine at a rate of 0.5 μg/kg/h 30 min before induction and 0.6 μg/kg/h after inducing anesthesia. Control patients received a volume-matched infusion of normal saline as placebo. Approximately 2 min before induction, analgesia was provided in the form of pentazocine, 0.5 mg/kg in control and 0.3 mg/kg in the test group. Induction was performed by 2 mg/kg thiopentone sodium supplemented with intravenous boluses of 25 mg thiopentone sodium every 15 s until loss of eyelid reflex (determined every 15 s). Induction dose of thiopentone sodium and total pentazocine dose were recorded. Recovery was assessed on the clinical recovery score (CRS) scale.
Results:
Infusion of dexmedetomidine decreased the induction dose of thiopentone approximately by 33% and of pentazocine dose by approximately 39% in patients undergoing laparoscopic cholecystectomy. Moreover, incidence of pain was also decreased significantly. Improved CRS from 4.33 to 6.87 was noticed immediately post-operatively in dexmedetomidine group of patients.
Conclusion:
Infusion of dexmedetomidine during the laparoscopic cholecystectomy decreases the requirement of thiopentone sodium and pentazocine and leads to early recovery of patients.
Keywords: Clinical recovery score, dexmedetomidine, pain, pentazocine, thiopentone sodium
Introduction
Dexmedetomidine is the pharmacologically active D-enantiomer of medetomidine,[1,2] a substance used for sedation and analgesia in domestic animals for years.[1,3] The well-documented beneficial effects of α2-adrenoceptor agonists include anxiolysis, analgesia, sedation and sympatholysis; thus, rendering these compounds especially suitable for anesthesia and the perioperative period. Compared with clonidine, dexmedetomidine is approximately 8 times more specific for α2-adrenoceptors with an α2:α1 selectivity ratio of 1600:1.[1,3] In contrast to clonidine, dexmedetomidine possesses full agonist properties and more predictable pharmacokinetic properties.[2,3] The mechanism of action of dexmedetomidine differs from clonidine as it possess selective α2-adrenoceptor agonism, especially for the 2A subtype of this receptor, which causes it to be a much more effective sedative and analgesic agent than clonidine.[4,5]
Dexmedetomidine has created a new interest in the use of α2-adrenoceptor agonists for pain, which remains the most salient aspect of surgery for patients throughout perioperative period.[6,7] The primary role of these agents is to achieve sedation while maintaining arousability and cooperation, analgesia and maintenance of hemodynamic and respiratory stability. In this study, we have assessed the role of dexmedetomidine in reducing the dose of thiopentone sodium, pentazocine and effect of the drug on recovery in patients undergoing laparoscopic cholecystectomy.
Materials and Methods
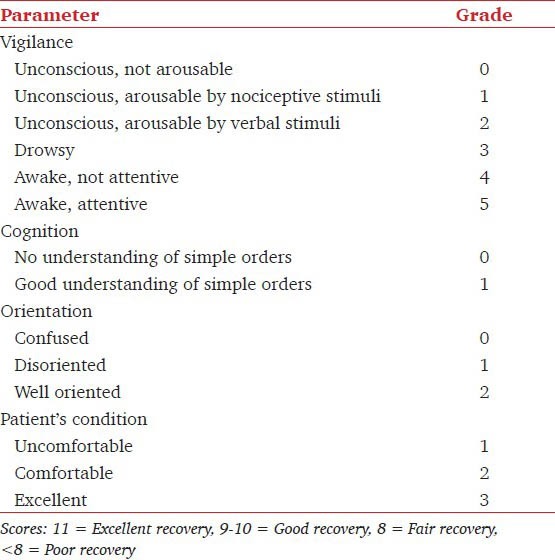
A total of 60 patients for laparoscopic cholecystectomy under general anesthesia were included in this single blinded randomized parallel-group study, which was cleared by the Institute Ethical Committee. A total of 30 patients were randomly allocated to each control and test (dexmedetomidine) groups, satisfying the minimum criteria of sample size based on Power and Cohen's d values. Patients with anemia, long-term medications or any medication within 1 week before surgery, history of any chronic disease, cardiac problem, history of drug abuse, consumption of more than 30 g alcohol/day, use of β-blockers and abnormal pre-operative electrolyte concentrations were excluded from the study. A day before surgery all patients were injected intravenously 40 mg esomeprazole at 22.00 h after achieving an intravenous (IV) access. Infusion of dexmedetomidine was initially started at a rate of 0.5 μg/kg/h, 30 min before induction. Dose was then increased to 0.6 μg/kg/h to produce moderate to deep sedation.[8] Patients in the control group received a volume-matched infusion of normal saline as a placebo. Prior to institution of dexmedetomidine infusion, monitoring was instituted in the form of electrocardiogram, oxygen saturation, blood pressure and heart rate, using a Datex Ohmeda monitor. Anesthesia was induced 30 min after starting the drug infusion. Approximately 2 min before induction of anesthesia, 0.5 mg/kg (control group) or 0.3 mg/kg (test group) pentazocine and 0.2 mg glycopyrrolate were administered intravenously. Anesthesia was induced with 2 mg/kg thiopentone sodium supplemented with 25 mg intravenous boluses every 15 s until loss of eyelid reflex (determined every 15 s). Induction dose of thiopentone sodium was recorded. Succinylcholine hydrochloride (1.5 mg/ kg) was administered to facilitate the endotracheal intubation. Vitals parameters were recorded every minute after induction and intubation. Immediately after intubation, administration of isoflurane was started along with oxygen and nitrous oxide. End-inspiratory concentration was adjusted at the predetermined value according to the “up-down” method described by Dixon and Mood.[9] Patients received 1.2% end-inspiratory isoflurane (approximately 1 minimum alveolar concentration [MAC]) of isoflurane[10] in the control group and 0.6% end-inspiratory isoflurane (approximately 0.5 MAC) of isoflurane in the dexmedetomidine group.[10] These initial isoflurane concentrations were chosen based on previous studies in which similar dexmedetomidine doses decreased isoflurane requirements for anesthetic maintenance by 25-90%.[11,12] The predetermined end inspiratory concentration of isoflurane was maintained for at least 15 min to allow adequate time for alveolar and brain isoflurane partial pressures to equilibrate.[13] Patients were ventilated using a non-rebreathing system with oxygen in air (0.5 FiO) and tidal volume approximately 10 ml/kg to maintain end-tidal carbon dioxide between 35 and 40 mmHg (4.7-5.3 kPa). Muscle relaxation was achieved and maintained with 0.8 mg/kg rocuronium, which was administered only after patient recovered from succinylcholine induced muscle relaxation. The depth of anesthesia was assessed with the clinical parameters. The end-inspiratory concentration was standardized in steps of 0.1% by assessing the depth of anesthesia. Additional boluses of pentazocine (0.1 mg/kg) were administered on signs of intraoperative pain such as tachycardia, rise in blood pressure and lacrimation. Administration of isoflurane was discontinued at the time of facial closure. On skin closure, neuromuscular block was reversed with a combination of 2.5 mg neostigmine and 0.4 mg glycopyrrolate given intravenously. As the dose range of neostigmine varies from 30 to 80 μg/kg, we chose a fixed dose of 2.5 mg (44.6 μg/kg for mean weight of 56 kg) of the drug to avoid increased probability of bradycardia because of synergy between dexmedetomidine and higher doses of neostigmine. The inducing dose of thiopentone and the total amount of intraoperative pentazocine required for anesthesia was measured. Patients were assessed by clinical recovery score (CRS) test,[14] a modified version of earlier recovery score tests.[15,16] The CRS assessed the parameters described in Table 1. The vitals were recorded in the recovery room for any side-effects. Patients were given an extra dose of pentazocine on complaint of immediate post-operative pain. The results mean and standard deviation (SD) were analyzed for statistical significance by Mann-Whitney and Chi-square tests with IBM SPSS 16 statistical pack. As the number of males in each group were low, the comparison of observations was initially checked with Student's t-test and then with Mann-Whitney test.
Table 1.
Parameters for assessment of clinical recovery score
Results
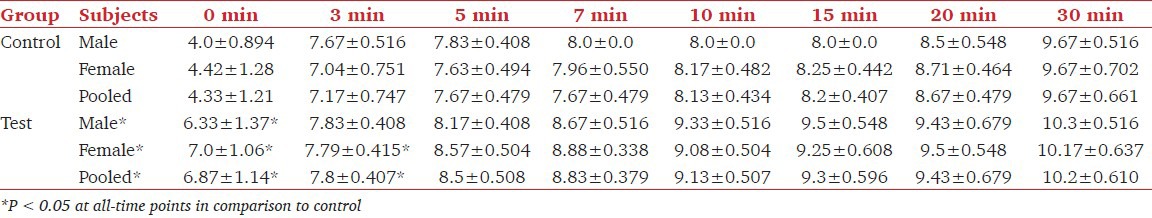
Table 2 shows the values of age range, age with SD, weight range, weights in mean plus SD and the value of hemoglobin in the control group of patients treated with dexmedetomidine for laparoscopic cholecystectomy. The mean ± SD age values in male (6), female (24) and pooled in the control group did not differ significantly from respective values in the dexmedetomidine treated group. Similarly, weight and hemoglobin values did not differ in the two groups. Effect of dexmedetomidine on thiopentone and pentazocine requirements and on the incidence of pain in patients undergoing cholecystectomy is shown in Table 3. It was found that requirement of thiopentone in males, females and in all patients decreased significantly from 3.69 ± 0.23 mg/kg, 3.92 ± 0.324 mg/kg and 3.88 ± 0.318 mg/kg to 2.76 ± 0.467 mg/kg, 2.55 ± 0.184 mg/kg and 2.59 ± 0.267 mg/kg respectively in male, female and pooled patients. Similarly, the requirement of pentazocine also decreased significantly from 30 ± 5.37 mg, 29.3 ± 4.08 mg and 29.4 ± 4.27 mg to 23.5 ± 6.12 mg, 16.5 ± 1.77 mg and 17.9 ± 4.13 mg respectively. Further, it was found that out of 30 patients in the control group, 9 patients (3 males and 6 females) had complained of post-surgical pain. Whereas in the test group of 30 patients, 4 (13.3%) reported the postsurgical pain. The decrease in incidence of pain due to dexmedetomidine was found to be significant at P < 0.001 level. Table 4 shows the effect of dexmedetomidine on CRS (on CRS scale) of the patients immediately after surgery and after 3, 5, 7, 10, 20 and 30 min of surgery in control and dexmedetomidine treated patients. These values increased with the increase of time in the two groups. Dexmedetomidine treatment improved the recovery score at all times starting from 0 to 30 min. CRS values in the pooled group were 6.87 ± 1.14, 7.8 ± 0.407, 8.5 ± 0.508, 8.83 ± 0.379, 9.13 ± 0.507, 9.3 ± 0.596, 9.42 ± 0.717 and 10.2 ± 0.610 at 0, 3, 5, 7, 10, 15, 20 and 30 min respectively. In this group, values of CRS even at 10 min in males, females and pooled patients were comparable with the values at 30 min in the control group.
Table 2.
Demographic patterns in the control group and in patients who in addition received dexmedetomidine
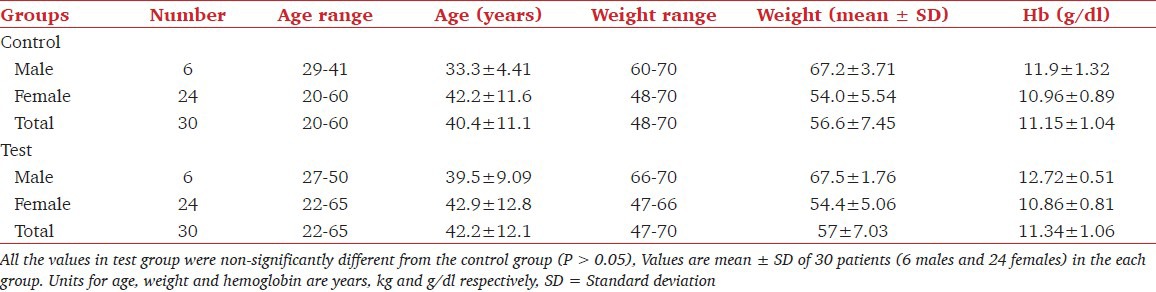
Table 3.
Effect of dexmedetomidine on thiopentone sodium and pentazocine requirement and post-operative side-effects (pain) occurrence in patients undergoing laparoscopic cholecystectomy
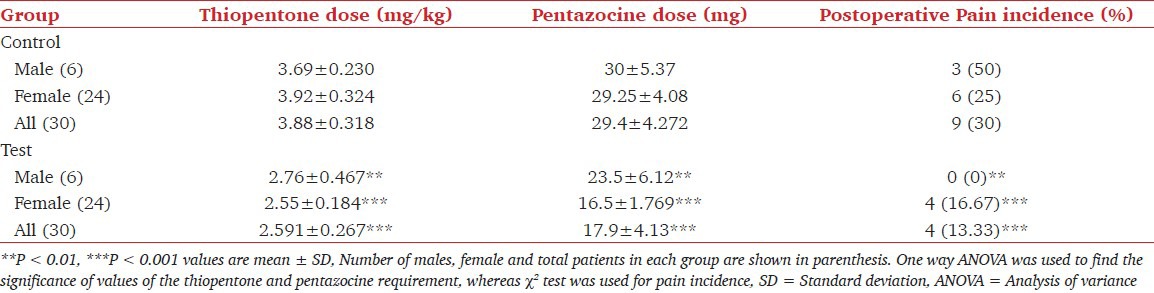
Table 4.
Clinical recovery score during first 30 min
Discussion
Dexmedetomidine, an imidazole compound, is the pharmacological active dextroisomer of medetomidine that displays specific and selective α2-agonism. Activation of α-receptors in the brain and spinal cord decrease sympathetic outflow causing sedation, analgesia, hypotension and bradycardia.[5] In our study Infusion of dexmedetomidine at the rate of 0.5 μg/kg/h, 30 min before induction and 0.6 μg/kg/h thereafter until the end of surgery caused significant sedation, decreased the induction dose of sodium thiopentone sodium by approximately 33% and pentazocine dose by approximately 39%. However, further studies are required to quantify if still lower induction doses of these drugs were sufficient. The effects of α-2 agonists in decreasing the MAC for volatile anesthetics and opioids have been previously reported[17] and possibly is mediated through both pre- and post-synaptic α-receptor activation in the central nervous system.[18,19] Reports available in the literature have reported use of dexmedetomidine as an adjunct to general anesthesia. When administered as a premedication at a dose range of 0.33-0.67 μg/kg given 15 min before surgery, it appears to be efficacious while minimizing the cardiovascular side-effects of hypotension and bradycardia. Within this dosage range, dexmedetomidine reduces thiopentone sodium requirements by around 30% for short procedures and reduces the requirements of volatile anesthetics by around 25%; thus theoretically proving helpful in reducing theater pollution.[20] Similar effects have also been noticed in patients undergoing minor gynecologic surgery.[20] In another study, it was found that when propofol, another induction agent, was used, dexmedetomidine decreased the propofol concentration necessary for sedation by approximately 60-80%.[21] Furthermore, opioid requirement was found to be decreased following 0.4 μg/kg dexmedetomidine.[12] Plasma noradrenaline concentration was markedly reduced in patients receiving dexmedetomidine. This decrement in neuronal noradrenaline release may explain in part the reduction in thiopentone sodium requirements.[20] In our study, we found an improved CRS both in males and females. The purpose of doing separate analysis for male and females was to assess if the effects were different because of possible variations in pharmacological aspects of the drug due to polymorphism.[22] However as the number of male subjects in control and test groups were only six, a larger sample size would have elucidated statistically significant findings. Overall CRS improved from 4.33 to 6.87 immediately post-operatively in dexmedetomidine treated group of pooled patients. Moreover, the incidence of pain also decreased significantly. Besides that as our study did not evaluate the effect of Body mass index, future studies can be planned to evaluate responses in obese patients where early recovery and decreased postoperative pain is helpful in reducing respiratory complications. The only constraint is the high cost of medication, which can divert the physicians for a cheaper option such high dose opioids and halogenated anesthetics all which have metabolic and environmental hazards.
In conclusion, an infusion of dexmedetomidine at the rate of 0.5 μg/kg/h 30 min before induction and 0.6 μg/kg/h thereafter until the end of surgery decreases dose requirements of thiopentone sodium, pentazocine, decreased post-operative pain and leads to better recovery of patients undergoing laproscopic cholecystectomy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 2.Iirola T, Aantaa R, Laitio R, Kentala E, Lahtinen M, Wighton A, et al. Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine in critically ill patients. Crit Care. 2011;15:R257. doi: 10.1186/cc10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Wong ES, Man RY, Vanhoutte PM, Ng KF. Dexmedetomidine induces both relaxations and contractions, via different {alpha}2-adrenoceptor subtypes, in the isolated mesenteric artery and aorta of the rat. J Pharmacol Exp Ther. 2010;335:659–64. doi: 10.1124/jpet.110.170688. [DOI] [PubMed] [Google Scholar]
- 5.Fairbanks CA, Kitto KF, Nguyen HO, Stone LS, Wilcox GL. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology. 2009;110:638–47. doi: 10.1097/ALN.0b013e318195b51d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz J, Tonner PH. Alpha2-adrenoceptor agonists in anaesthesia: A new paradigm. Curr Opin Anaesthesiol. 2000;13:437–42. doi: 10.1097/00001503-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Johnston KD, Rai MR. Conscious sedation for awake fibreoptic intubation: A review of the literature. Can J Anaesth. 2013;60:584–99. doi: 10.1007/s12630-013-9915-9. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ, Mood AM. A method for obtaining and analyzing sensitivity data. J Am Stat Assoc. 1948;43:109–26. [Google Scholar]
- 10.Matsuura T, Oda Y, Tanaka K, Mori T, Nishikawa K, Asada A. Advance of age decreases the minimum alveolar concentrations of isoflurane and sevoflurane for maintaining bispectral index below 50. Br J Anaesth. 2009;102:331–5. doi: 10.1093/bja/aen382. [DOI] [PubMed] [Google Scholar]
- 11.Erkola O, Korttila K, Aho M, Haasio J, Aantaa R, Kallio A. Comparison of intramuscular dexmedetomidine and midazolam premedication for elective abdominal hysterectomy. Anesth Analg. 1994;79:646–53. doi: 10.1213/00000539-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Aho MS, Erkola OA, Scheinin H, Lehtinen AM, Korttila KT. Effect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligation. Anesth Analg. 1991;73:112–8. doi: 10.1213/00000539-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gaumann DM, Mustaki JP, Tassonyi E. MAC-awake of isoflurane, enflurane and halothane evaluated by slow and fast alveolar washout. Br J Anaesth. 1992;68:81–4. doi: 10.1093/bja/68.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Ohri A, Sharma DR, Thakur JR, Santoshi ID. Halothane induced sedation: Antagonism with aminophylline. J Anaesthesiol Clin Pharmacol. 1998;14:255–62. [Google Scholar]
- 15.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 16.Quinn CL, Weaver JM, Beck M. Evaluation of a clinical recovery score after general anesthesia. Anesth Prog. 1993;40:67–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–8. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 19.Menda F, Köner O, Sayin M, Türe H, Imer P, Aykaç B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth. 2010;13:16–21. doi: 10.4103/0971-9784.58829. [DOI] [PubMed] [Google Scholar]
- 20.Aantaa R, Kanto J, Scheinin M, Kallio A, Scheinin H. Dexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgery. Anesthesiology. 1990;73:230–5. doi: 10.1097/00000542-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Verdejo-García A, Fagundo BA, Cuenca A, Rodriguez J, Cuyás E, Langohr K, et al. COMT val158met and 5-HTTLPR Genetic polymorphisms moderate executive control in cannabis users. Neuropsychopharmacology. 2013;38:1598–606. doi: 10.1038/npp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]


