Sir,
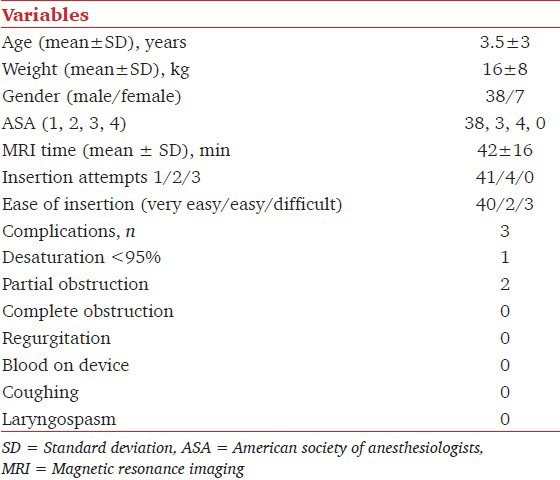
Magnetic resonance imaging (MRI) is a noninvasive, radiation-free procedure increasingly used on children for diagnostic purposes. Although various nonpharmacological approaches have been described in literature, pediatric MRI often requires sedation or general anesthesia.[1] The i-gel™ (Intersurgical, Wokingham, UK) is a new disposable, second generation supraglottic airway device, that does not require inflation of the cuff. There are few published studies evaluating the pediatric i-gel™, and none in MRI setting.[2] We conducted this retrospective observational study to evaluate the efficacy and safety during clinical use of the pediatric i-gel™ in the MRI suite (at the “G. B. Morgagni-L. Pierantoni” Hospital, Forlì, Italy.) Institutional Review Board approval was sought (1st January 2012) but considered unnecessary in view of the observational nature of the study. We reviewed the prospective perioperative electronic medical records available in our hospital system. Through this computerized database, preoperative, intraoperative, and postoperative data regarding airway management are recorded routinely by anesthetists for each patient undergoing airway management for surgical or nonsurgical procedures. Within each intraoperative record, basic demographic data are recorded including age, weight, sex, American Society of Anesthesiologists (ASA) score, and MRI time. The number of insertion attempts and ease of insertion (very easy, easy, and difficult) and any airway-related complication are also recorded. From this database, a search query was performed to obtain all relevant data for this study. Inclusion criteria were pediatric (less than 18 year) patients who underwent general anesthesia with a planned i-gel™ from March 1, 2012 to December 31, 2012. Exclusion criteria were instances in which the laryngoscopy was performed before i-gel™ placement, and when the i-gel™ removal was related to a change in surgical plan. In our hospital, the use of the i-gel™ is included as part of the standard local hospital consent procedure. I-gel™ sizes 1 (2-5 kg), 1.5 (5-12 kg), 2 (10-25 kg), and 2.5 (25-35 kg) were available [Figure 1]. The type of anesthesia used was at the discretion of the anesthetist. The i-gel™ was inserted in accordance with the manufacturer's instructions. In the study period, 45 medical records were reviewed. I-gel™ size ranged from 1 to 2.5. Table 1 summarizes the characteristics of the study population and the results. In our experience, i-gel™ has proved to be an airway device which is easy to position, with a good rate of successful first attempt insertion and safe in view of the reduced number of complications recorded. The laryngeal mask airways (LMAs) are well-established in pediatric anesthetic practice and their use in the MRI suite has been described. I-gel™ does not cause artefacts[3] and its successful use in children undergoing MRI has been previously described.[4] The three reported complications were all related to the selection of the correct size. In all three cases, in fact, the airway obstruction was resolved simply by changing to the smaller size. Recently, Agnoletti, et al.,[5] provided an explanation for airway obstruction following i-gel™ insertion, suggesting a change to the next size down in case of otherwise inexplicable airway obstruction. In conclusion, i-gel™ is a useful device for MRI in children. It offers many advantages such as ease of insertion, availability of all pediatric sizes, no artefacts, and a low rate of complications.
Figure 1.
Pediatric i-gel, size 1–2.5
Table 1.
Demographic data of the patients and complications
References
- 1.Arlachov Y, Ganatra RH. Sedation/anaesthesia in paediatric radiology. Br J Radiol. 2012;85:e1018–31. doi: 10.1259/bjr/28871143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JR, Kim MS, Kim JT, Byon HJ, Park YH, Kim HS, et al. A randomised trial comparing the i-gel (TM) with the LMA Classic (TM) in children. Anaesthesia. 2012;67:606–11. doi: 10.1111/j.1365-2044.2012.07072.x. [DOI] [PubMed] [Google Scholar]
- 3.Schieble T, Patel A, Davidson M. Laryngeal mask airway (LMA) artefact resulting in MRI misdiagnosis. Pediatr Radiol. 2008;38:328–30. doi: 10.1007/s00247-007-0671-2. [DOI] [PubMed] [Google Scholar]
- 4.Taxak S, Bhardwaj M, Gopinath A. The i-gel(™) - A promising airway device for magnetic resonance imaging suite. J Anaesthesiol Clin Pharmacol. 2012;28:263–4. doi: 10.4103/0970-9185.94917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnoletti V, Piraccini E, Corso RM, Cittadini A, Maitan S, Della Rocca G, et al. Tracheal compression caused by oversized i-gel in children. Minerva Anestesiol. 2013;79:107–8. [PubMed] [Google Scholar]


