Abstract
Sudden unexplained death in toddlers has been associated with febrile seizures, family history of febrile seizures, and hippocampal anomalies. We investigated the mode of inheritance for febrile seizures in these families. A three-generation pedigree was obtained from families enrolled in the San Diego Sudden Unexplained Death in Childhood Research Project, involving toddlers with sudden unexplained death, febrile seizures, and family history of febrile seizures. In our six cases, death was unwitnessed and related to sleep. The interval from last witnessed febrile seizure to death ranged from 3 weeks to 6 months. Hippocampal abnormalities were identified in one of three cases with available autopsy sections. Autosomal dominant inheritance of febrile seizures was observed in three families. A fourth demonstrated autosomal dominant inheritance with incomplete penetrance or variable expressivity. In two families, the maternal and paternal sides manifested febrile seizures. In this series, the major pattern of inheritance in toddlers with sudden unexplained death and febrile seizures was autosomal dominant. Future studies should develop markers (including genetic) to identify which patients with febrile seizures are at risk for sudden unexplained death in childhood, and to provide guidance for families and physicians.
Introduction
Sudden unexplained death in childhood is defined as the sudden death of a child older than 1 year of age that remains unexplained after a thorough investigation, including reviews of the clinical history and circumstances of death, and the performance of a complete autopsy with appropriate ancillary testing [1]. Sudden unexplained death in childhood is most common in toddlers aged 1–5 years, with approximately 1.3/100,000 deaths per year [2]. In a retrospective study of 49 toddlers with sudden unexplained death in childhood [3], 24% (12/49) had a history of febrile seizures, approximately fivefold higher than the general pediatric population incidence of 2–5% [4–6]. In addition, 67% (8/12) of these children also had a family history of febrile seizures, at 2.8-fold higher than the general population incidence of 24% [5]. These observations led to the hypothesis that a pathophysiologic connection exists between febrile seizures and sudden unexplained death in childhood, suggesting that a genetic susceptibility to febrile seizures may be important in defining this subset of patients with sudden unexplained death in childhood.
Among cases of sudden unexplained death in childhood with an individual or family history or both of febrile seizures, 82% (9/11) demonstrated gross asymmetry and microscopic anomalies of the hippocampus [3]. These microscopic anomalies were similar to those observed in chronic temporal lobe epilepsy [7–12] or sudden unexpected death in epilepsy [13–15], and the mechanism may be similar, or identical, to sudden unexpected death in epilepsy [15].
Given the striking association of sudden unexplained death in childhood and febrile seizures in toddlers and their families, we sought to delineate the mode of inheritance of febrile seizures in these families. We selected cases of sudden unexplained death in childhood with an individual and family history of febrile seizures. Although this strategy limits our sample size, we think it holds the greatest potential to provide information on inheritance patterns. We report on the pedigrees of six such patients.
Study Design and Methods
Classification of sudden unexplained death in childhood
Families were identified from the registry of the San Diego Sudden Unexplained Death in Childhood Research Project, which is under the direction of H.F.K. and was approved by the Institutional Review Board at Rady Children’s Hospital. Final classifications of cases as sudden unexplained death in childhood were based on reviews by H.F.K. of: (1) a Family Survey completed by the parents; (2) hospital and clinic records; (3) forensic reports of the death scene investigation, autopsy, and ancillary testing; and (4) microscopic slides from the autopsy. Slides of the central nervous system were reviewed by pediatric neuropathologists (H.C.K. and/or M.R.G.).
Identification of families
Families with a child diagnosed with sudden unexplained death in childhood were notified about the study by the director of the Sudden Unexplained Death in Childhood Program (L.C.), which provides information and support for families. Families interested in participating contacted the study geneticist (I.A.H.), who performed the interviews by telephone. The inclusion criteria comprised: (1) the death of a toddler aged 1–5 years; (2) a history of febrile seizures in the toddler; and (3) a family history of febrile seizures.
Pedigree assessment
A three-generation pedigree was obtained, with information for each family member that included sex, age, cause of death, a history of febrile seizures, other seizures, neurologic disorders, or developmental delay, and a history of sudden infant death. Information about each deceased child’s maternal history and perinatal, postnatal, and early childhood periods was obtained from the Family Survey. A pediatric neurologist (A.P.) reviewed the neurologic history and details concerning febrile seizures. The inheritance pattern of febrile seizures was determined using standard definitions for autosomal dominant, autosomal recessive, X-linked dominant, and X-linked recessive. In pedigrees where members of the maternal and paternal sides of the family were affected, the pattern of inheritance was considered “nonclassic.”
Results
Study population
We obtained family histories from a parent of each deceased child. Interviews were performed 1–10 years (median, 4.6 years) after the event of sudden unexplained death in childhood.
Clinical summary
All deaths were related to a sleep period (Table 1). Two cases (patients 2 and 3) underwent an evaluation for febrile seizures by a neurologist, with unremarkable results. None of the deaths were witnessed, and thus the occurrence of seizure at time of death is unknown.
Table 1.
Summary of clinicopathologic information of the six patients with sudden unexplained death in childhood in this study
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Postnatal age at death | 25 months | 18 months | 55 months | 27 months | 17 months | 21 months |
| Sex | Male | Female | Female | Male | Male | Male |
| Race | Caucasian | Caucasian | Caucasian/Asian | Caucasian | Caucasian | Caucasian |
| Number of febrile seizures | 1 | 3 | 4 | 2 | 2 | 2 |
| Age onset febrile seizures | 18 months | 8.5 months | 24 months | 22 months | 15 months | 12.5 months |
| Age at subsequent febrile seizures | 24 months | 9 months, 16 months | 27 months, 31 months, 33 months | 22 months | N/A | 19 months |
| Interval between febrile seizures and death | 3 weeks | 2 months | 2 months | 6 months | 2 months | 2.5 months |
| Fever within 48 hours of death | No | No | Yes | Yes | No | Yes |
| Simple or complex febrile seizures | Simple | Simple | Simple | Simple | Simple | Simple |
| Time elapsed since most recent immunization | >2 weeks | >2 weeks | >2 weeks | >2 weeks | >2 weeks | >2 weeks |
| Birth history | ||||||
| Gestational age | 39.5 weeks | 41.5 weeks | 31.8 weeks | 39 weeks | 39 weeks | 36.3 weeks |
| Twin | No | No | Yes | No | No | Yes |
| Details at death | ||||||
| Position found | Prone | Prone | Prone | Prone | Prone | Prone |
| Percentile length | 75th–90th | 90th | 5th–10th | 50th | 50th–75th | 75th |
| Percentile weight | N/A | 90th | 50th–75th | 95th | 25th | 75th–90th |
| Percent OFC | 90th–95th | 90th–95th | 25th–50th | N/A | 50th | N/A |
| Intrathoracic petechiae | No | Yes | No | No | Yes | Yes |
| Fresh brain weight (g)/ expected for length | 1450/1155 | 1230/1110 | 1225/1145 | 1130/1150 | 1130/1062 | 1550/1275 |
| Hippocampal anomaly | N/A | N/A | Asymmetry of hippocampi because of reduced volume of left hippocamus; thin pyramidal cell layer in CA3; interstitial neurons in bilateral cingulum | No gross or microscopic anomalies | N/A | No gross or microscopic anomalies |
| Other hippocampal abnormalities | N/A | N/A | No | Hypoxic-ischemic changes | N/A | No |
| Other brain abnormalities | Acute hypoxic-ischemic injury, transtentorial herniation | Cerebral edema; ectopic neurons in cerebral white matter | Cerebral edema; small, bilateral, acute subdural hematomas | Diffuse, acute hypoxic-ischemic injury; cerebral edema | None; limited sections available | White matter gliosis; subependymal gliosis |
Abbreviations:
N/A = Not available
OFC = Head circumference
Autopsy summary
Metabolic screening produced negative results in the four cases (patients 1, 2, 4, and 6) in whom testing was undertaken (Table 1). None of the patients demonstrated postmortem evidence consistent with a terminal seizure. Three cases (patients 2, 5, and 6) manifested intrathoracic petechiae, consistent with agonal upper airway closure [16].
Neuropathology summary
The brain weight was higher than expected for height in five patients (Table 1). The acute hypoxic-ischemic changes in two patients were considered related to the terminal cardiorespiratory arrest. Macroscopic asymmetry of the hippocampus was evident in one of the three cases for whom sections were available, and was previously described [16].
Pedigree summary
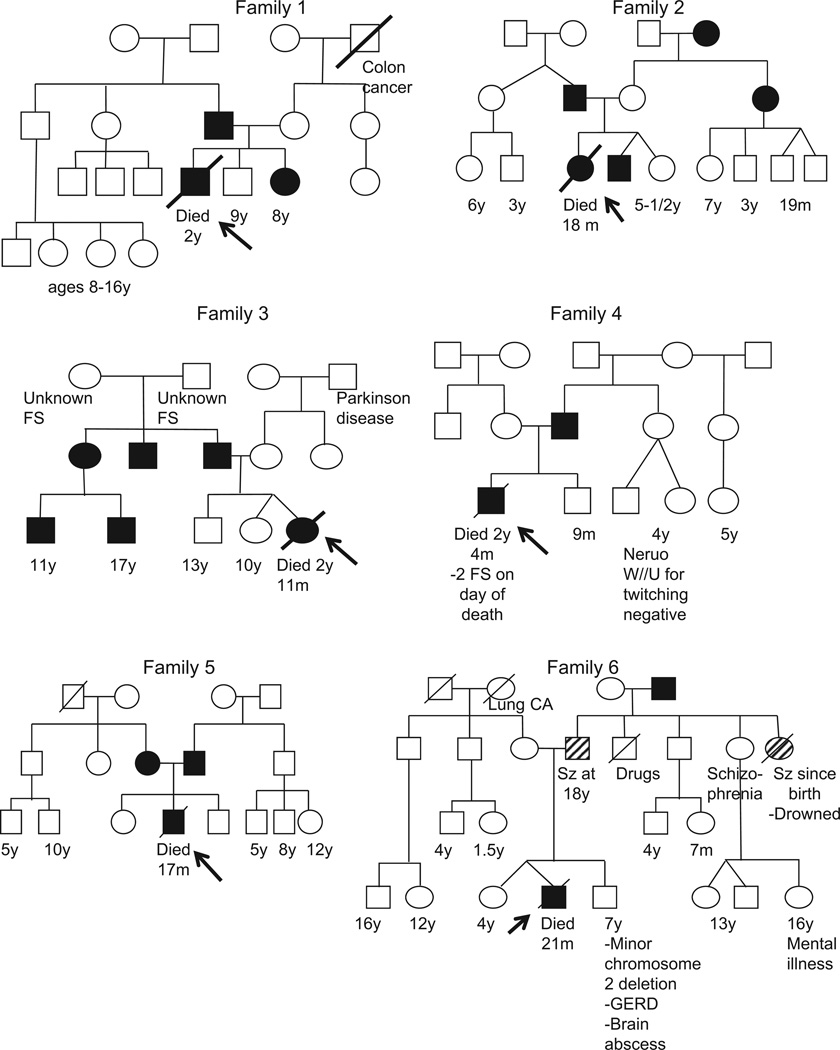
No history of consanguinity was evident (Fig 1). In five cases, at least one parent had manifested febrile seizures, and no history of other neurologic problems was reported. A classic autosomal dominant pattern of inheritance was observed in three cases (patients 1, 3, and 4), with febrile seizures in two generations and not in the grandparents, although the information on the grandparents was incomplete for patient 3. In a fourth case (patient 6), the father and a paternal aunt had manifested epilepsy but no febrile seizures; the paternal grandfather had manifested a history of febrile seizures; and a history of schizophrenia in a paternal aunt and an unspecified “mental illness” in her son were reported. The pattern of inheritance was autosomal dominant, but with either incomplete penetrance or variable expressivity. The patterns of inheritance in patients 2 and 5 were nonclassic, i.e., the maternal and paternal sides of the family were affected.
Figure 1.
Six pedigrees of families from the San Diego Sudden Unexplained Death in Childhood Research Project with a history of febrile seizures in autopsied toddlers with sudden unexplained death in childhood and a family history of febrile seizures. Circles represent females; squares represent males. Ages are given for the youngest generation only and years are indicated by “y” and months by “m.” Solid circles and squares indicate individuals with a history of febrile seizures. Circles and squares with hatched lines indicate individuals with a history of a seizure type other than febrile. A line through an individual indicates death. An arrow indicates the patient with sudden unexplained death in childhood.
Discussion
This series of pedigrees involving six toddlers with sudden unexplained death associated with an individual and family history of febrile seizures constitutes a first step in the search for the genetic basis of sudden unexplained death in childhood, to identify those at risk for sudden death in the setting of familial febrile seizures. In this series, the major pattern of inheritance for febrile seizures was autosomal dominant. The finding of two families with febrile seizures on the maternal and paternal sides is not surprising, given the high incidence of febrile seizures in the general pediatric population, and represents a challenge for future genetic analyses of this entity.
One of the three cases with hippocampal sections available exhibited maldevelopment of the hippocampus, reinforcing our previous observations that the hippocampus is abnormal in some cases of sudden unexplained death in childhood associated with febrile seizures [3,16]. However, in this report, we suggest an emphasis on a history of febrile seizures as the key feature, a departure from our former emphasis on hippocampal anomalies at autopsy as the sine qua non of sudden unexplained death in childhood [3,16]. This paradigm shift may prove more useful clinically, because the focus centers on febrile seizures and sudden death, and not on neuropathologic findings in the hippocampus that require autopsy for detection. The patient with developmental asymmetry of the hippocampus and mild sclerosis raises the possibility that genes related to hippocampal development may play a role in sudden unexplained death in childhood with hippocampal anomalies, offering an avenue for further research.
The autosomal dominant pattern of inheritance for febrile seizures in the present families is identical to that observed in genetic epilepsy with febrile seizures plus and familial febrile seizures [17,18], raising the possibility that our group with sudden unexplained death in childhood may fall into these broader categories. Genetic epilepsy with febrile seizures plus and familial febrile seizures have not been associated with sudden unexplained death at early ages, and thus our patients may represent a previously unrecognized and extreme end of the phenotypic spectrum, and may be genetically linked. The association of febrile seizures with sudden death in childhood is supported by a population-based study from Denmark [19], in which the risk of sudden death in children with febrile seizures was increased fivefold, suggesting an increased risk for sudden unexplained death in childhood. A family history of febrile seizures, hippocampal pathology, and autopsy findings were not addressed in that study.
The potential limitations of our study include the small sample of cases with sudden unexplained death in childhood, involving both an individual and family history of febrile seizures, available in the San Diego Sudden Unexplained Death in Childhood Research Project. This small sample underscores that such cases are rare in sudden unexplained death in childhood, an entity rare in itself. The small size of our cohort implies we cannot rule out that sudden unexplained death in childhood may occur in the setting of familial febrile seizures by chance, although the high prevalence of febrile seizures in the children who died and their families argues strongly against chance occurrence.
A second potential limitation of this study involves ascertainment bias. We have not analyzed the entire spectrum of families with sudden unexplained death in childhood and febrile seizures. A third potential limitation, as in all pedigree analyses, concerns the accuracy of family histories of febrile seizures [20]. Despite these limitations, the finding of autosomal dominant inheritance of febrile seizures among children with sudden unexplained death is robust, and demonstrates a consistent genetic pattern in a disorder that is extraordinarily rare and therefore difficult to study.
Conclusion
Febrile seizures may be the marker of an underlying process that leads to sudden unexplained death in childhood, and seizures may or may not be directly involved in the final lethal event. The potential pathophysiologic connection between sudden unexplained death in childhood and febrile seizures warrants urgent attention to develop means, potentially including the use of genetic markers, to determine who among the vast number of young children with febrile seizures may be at risk of sudden unexplained death, particularly in the setting of autosomal dominant familial febrile seizures, and to avoid undue alarm for the parents and physicians of those millions of children with febrile seizures who do not run an additional risk for sudden unexplained death.
Acknowledgments
The authors are grateful to the families with sudden unexplained death in childhood who participated in this study. The authors appreciate the assistance of the medical examiners involved in forensic evaluations. The authors thank Ms. Laura Hernandez and Amy Chadwick, BA for help with data acquisition. The authors appreciate the critical comments of Kyriacos Markianos, PhD and David S. Paterson, PhD during preparation of the manuscript. This study was supported by the CJ Foundation for SIDS, the National Institute of Child Health and Development (through grant P30 HD18655 to the Intellectual and Developmental Disabilities Research Center, Children’s Hospital Boston), and the National Institute of Neurological Disorders and Stroke (grant K23 NS069784 to A.P.).
References
- 1.Krous HF, Chadwick AE, Crandall L, Nadeau-Manning JM. Sudden unexpected death in childhood: A report of 50 cases. Pediatr Dev Pathol. 2005;8:307–319. doi: 10.1007/s10024-005-1155-8. [DOI] [PubMed] [Google Scholar]
- 2.United States Department of Health and Human Services, Centers for Disease Control, National Center for Health Statistics. United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), compressed mortality file (CMF) compiled from CMF 1968–1988, series 20, no. 2A 2000, CMF 1989–1998, series 20 no. 2E 2003 and CMF 1999–2002, series 20 no. 2H 2004 on CDC WONDER On-Line Database, 2008. Available at: http://wonder.cdc.gov.
- 3.Kinney H, Chadwick A, Crandall LA, et al. Sudden death, febrile seizures, and hippocampal maldevelopment in toddlers: A new entity. Pediatr Dev Pathol. 2009;12:455–463. doi: 10.2350/08-09-0542.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann RJ, Duffner PK. Treatment of children with simple febrile seizures: The AAP practice parameter. American Academy of Pediatrics. Pediatr Neurol. 2000;23:11–17. doi: 10.1016/s0887-8994(00)00148-x. [DOI] [PubMed] [Google Scholar]
- 5.Sadleir LG, Scheffer IE. Febrile seizures. Br Med J [Clin Res] 2007;334:307–311. doi: 10.1136/bmj.39087.691817.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17(Suppl. 1):S44–S52. doi: 10.1177/08830738020170010601. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993;52:433–443. doi: 10.1097/00005072-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535:195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- 9.Kasper BS, Stefan H, Buchfelder M, Paulus W. Temporal lobe microdysgenesis in epilepsy versus control brains. J Neuropathol Exp Neurol. 1999;58:22–28. doi: 10.1097/00005072-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Meencke HJ, Janz D. Neuropathological findings in primary generalized epilepsy: A study of eight cases. Epilepsia. 1984;25:8–21. doi: 10.1111/j.1528-1157.1984.tb04149.x. [DOI] [PubMed] [Google Scholar]
- 11.Vok EE, Prayson RA. Hamartomas in the setting of chronic epilepsy: A clinicopathologic study of 13 cases. Hum Pathol. 1997;28:227–232. doi: 10.1016/s0046-8177(97)90111-8. [DOI] [PubMed] [Google Scholar]
- 12.Wieser HG. ILAE Commission report: Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 13.Donner EJ, Smith CR, Snead OC., III Sudden unexplained death in children with epilepsy. Neurology. 2001;57:430–434. doi: 10.1212/wnl.57.3.430. [DOI] [PubMed] [Google Scholar]
- 14.Leestma JE, Kalelkar MB, Teas SS, Jay GW, Hughes JR. Sudden unexpected death associated with seizures: Analysis of 66 cases. Epilepsia. 1984;25:84–88. doi: 10.1111/j.1528-1157.1984.tb04159.x. [DOI] [PubMed] [Google Scholar]
- 15.Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365:1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- 16.Kinney HC, Armstrong DL, Chadwick AE, et al. Sudden death in toddlers associated with developmental abnormalities of the hippocampus: A report of five cases. Pediatr Dev Pathol. 2007;10:208–223. doi: 10.2350/06-08-0144.1. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama J, Arinami T. Molecular genetics of febrile seizures. Epilepsy Res. 2006;70(Suppl. 1):S190–S198. doi: 10.1016/j.eplepsyres.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Poduri A, Wang Y, Gordon D, et al. Novel susceptibility locus at chromosome 6q16.3–22.31 in a family with GEFS+ Neurology. 2009;73:1264–1272. doi: 10.1212/WNL.0b013e3181bd10d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vestergaard M, Pedersen MG, Ostergaard JR, Pedersen CB, Olsen J, Christensen J. Death in children with febrile seizures: A population-based cohort study. Lancet. 2008;372:457–463. doi: 10.1016/S0140-6736(08)61198-8. [DOI] [PubMed] [Google Scholar]
- 20.Sillanpaa M, Camfield PR, Camfield CS, et al. Inconsistency between prospectively and retrospectively reported febrile seizures. Dev Med Child Neurol. 2008;50:25–28. doi: 10.1111/j.1469-8749.2007.02006.x. [DOI] [PubMed] [Google Scholar]