Abstract
Many intracellular bacterial pathogens reside within a membrane-bound compartment. The biogenesis of these vacuolar compartments is complex, involving subversion of host cell secretory pathways by bacterial proteins. In recent years it has become clear that disruption of vacuole biogenesis may result in membrane rupture and escape of bacteria into the host cell cytosol. Correct modulation of the host cell cytoskeleton, signalling molecules such as small GTPases and the lipids of the vacuole membrane have all been shown to be critical in the maintenance of vacuole integrity. Increasing evidence suggests that vacuole rupture may result from aberrant mechanical forces exerted on the vacuole, possibly due to a defect in vacuole expansion.
Introduction
In order to survive, bacterial pathogens that replicate within the cells of their hosts must adopt lifestyles that both exploit and maintain intracellular niches. This is no simple task because eukaryotic organisms have evolved efficient strategies to destroy invading microbes. Intracellular bacteria enter the host cell via phagocytosis and the nascent phagosome is directed into the endocytic pathway. Phagosomes bearing nonpathogens will follow a path that fuses with the lysosomal compartment resulting in bacterial degradation. Pathogens have developed different strategies to avoid this fate. A few pathogenic bacteria including Shigella, Listeria, and Francisella, escape from the phagosome into the cytosol of the host cell. Although these pathogens avoid the endocytic pathway and the challenge of residing in a nutrient poor vacuolar compartment, they face other challenges in the cytosol notably the cytoplasmic innate immune system. Most of the characterized intracellular pathogens choose to remain within a membrane-bound compartment and modify this niche to facilitate their survival and replication. In recent years it has become apparent that in addition to establishing a replication vacuole, intravacuolar pathogens also actively promote the maintenance and integrity of these vacuoles. Here we review recent advances in the understanding of how pathogen-containing vacuoles are maintained.
Establishment of a replication vacuole
In order to survive and replicate within a vacuole, bacterial pathogens must direct the nature of this compartment to suit their needs. The specifics of this process vary from species to species but there are common strategies utilized by all vacuolar bacterial pathogens. Intracellular bacterial pathogens utilize specialized secretion systems to deliver bacterial effector proteins into the host cell. Many of these effector proteins modify the vacuolar niche directly or indirectly to allow the establishment of a replication vacuole. By targeting host cell proteins and lipids, effector proteins alter the identity of the nascent phagosome and promote selective interactions with endosomal membrane compartments. These selective interactions alter the trafficking of the pathogen-containing vacuole such that it avoids the toxic effects of fusing with lysosomes yet maintains constant interactions with host cell vesicles to allow vacuole expansion and nutrient acquisition [1].
Maintenance of a replication vacuole
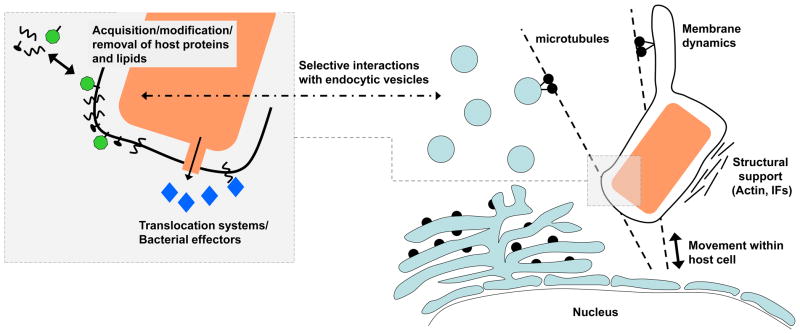
The formation of a replication vacuole requires a complex interplay of host pathways and bacterial factors. Disruption of this process leads to the failure to establish a replication niche. Incorrect targeting of the vacuole may result in routing of the membrane bound compartment into the default endocytic pathway, resulting in destruction of the bacterial pathogen or the formation of a nonpermissive vacuole. However, it has become apparent that disruption of vacuole assembly can result in a compartment that has many features of permissive replication vacuole, but has compromised integrity, resulting in pathogen release into the cytoplasm (Fig. 1).
Fig 1. Maintenance of vacuole stability.
Vacuolar pathogens translocate effector proteins into the host cell to modify the vacuolar niche and subvert host cell pathways. Effectors direct the acquisition, modification and removal of host proteins and lipids of the vacuole membrane. Changes in lipid and protein composition alter the identity of the vacuole leading to selective interactions with endocytic vesicles. Interactions with the host cytoskeleton determine the subcellular localization of the vacuole, provide structural support and may facilitate vesicle recruitment. The dynamics of the vacuole membrane are affected by changes in the membrane lipids and interactions with microtubules and microtubule motors. Vacuole integrity is closely linked to vacuole biogenesis and subversion of these events frequently leads to vacuole rupture. In addition, the vacuole membrane may be compromised by the specialized secretion systems used to translocate effector proteins into the host cell.
Role of the cytoskeleton in vacuole stability
Vacuole trafficking within eukaryotic cells is dependent upon the cell cytoskeleton and cytoskeletal motors. Therefore it is not surprising that pathogen-containing vacuoles (PCV) are tightly associated with cytoskeletal components, and that modulation of the cytoskeleton is a key strategy that facilitates vacuole formation. Host cell vesicles and organelles are transported on cytoskeletal networks, which can be manipulated to allow vesicle recruitment to the PCV. The cytoskeleton also determines the localization of the PCV within the host cell. Indeed many intracellular pathogens traffic to a perinuclear location after phagocytosis, which may facilitate interactions between the PCV and secretory vesicles necessary to maintain a functional replication vacuole. Correct modulation of the cytoskeleton has been shown to be required for expansion of both the Salmonella and Chlamydia vacuoles [2,3]. Finally, it has been shown that some PCVs are surrounded by a scaffold of F-actin and other cytoskeletal components that provide structural support for the vacuole [3,4].
The interaction of the Salmonella-containing vacuole (SCV) with the host cytoskeleton is highly complex and tightly controlled. Shortly after uptake, the SCV traffics to the microtubule organizing center (MTOC) in a manner dependent on both microtubules and actin [2,5,6]. A feature of the SCV is the formation of membrane filaments that extend from the SCV to the cell periphery. Transport to the MTOC, retention in this location and production of filaments require the coordinated efforts of several translocated effectors. SopB activates myosin II to promote retrograde movement of the SCV [6], whereas SifA, SseF, SseG, PipB2 and SopD regulate the binding of microtubule motors to the SCV. SseF and SseG promote retention of the SCV at the MTOC by recruiting dynein [7]. PipB2 and SopD2 recruit kinesin-1 to the SCV [8,9]; the presence of high levels of kinesin-1 on the SCV causes vacuole instability [10] but another bacterial effector, SifA, recruits kinesin-1-interacting protein SKIP to promote the transport of membrane filaments and vesicles toward the cell periphery [10,11]. This delicate balance of interactions with cytoskeletal motors is critical for maintenance of SCV integrity. Inhibition of myosin II, kinesin-1 or dynein leads to loss of SCV integrity as does the absence of sifA [5,6,12]. Schroeder et al. recently demonstrated that the accumulation of kinesin-1 on the SCV resulting from the absence of sifA can be prevented by the further deletion of sopD2 and pipB2 [9]. The authors observed a novel type of tubule extending from the SCV that is absent in the presence of SopD2 suggesting that SopD2 suppresses the formation of a novel type of tubule extending from the SCV that can compensate for the lack of SifA-induced filaments.
Both the Salmonella and Chlamydia vacuoles are surrounded by a network of F-actin [3,4]. This network is required for vacuole stability of both these pathogens as treatment with F-actin polymerisation inhibitors leads to the loss of integrity [3,4]. The role of the F-actin network has been studied most closely in the case of Chlamydia infection. It has been shown that F-actin and intermediate filaments combine to form a structural scaffold surrounding the Chlamydia inclusion that maintains the integrity of the inclusion [3]. The secreted chlamydial protease CPAF was shown to process intermediate filament proteins to form a filamentous structure that is able to support expansion of the inclusion [3]. More recently, it has been demonstrated that CPAF activity is required for inclusion integrity [13]. This may reflect its role in intermediate filament protein processing although it also targets many other host and bacterial proteins that may play a role in vacuole maintenance.
Role of small GTPases in vacuole stability
During phagocytosis of particles and formation of a membrane bound compartment, host cell proteins are acquired that regulate the trafficking of this compartment. With sequential membrane fusion and fission events, the repertoire of membrane-associated host proteins changes and directs the fate of the organelle. Foremost among these host cell proteins are the small GTPases. Small GTPases regulate host cell functions, including membrane trafficking, by cycling between inactive GDP-bound and active GTP-bound states. In their active state, small GTPases interact with downstream effectors that promote membrane transport. Not surprisingly, many vacuolar pathogens regulate the recruitment and activation state of host cell GTPases to alter the trafficking of their vacuole [1,14]. Salmonella enterica Typhimurium initially recruits Rab5, a marker of early endosomes to its vacuole [15,16]. Rab5 is subsequently replaced with Rab7 following fusion with late endosomes [17]. This process has been shown to be important in vacuole stability as expression of constitutively-active Rab5 or dominant-negative Rab7 cause loss of vacuole integrity and release of S. Typhimurium into the cytosol [18]. Legionella pneumophila recruits GTPases Rab1 and ARF1 which are typically present on early secretory vesicles and are required for formation of a replication vacuole [19,20]. Legionella utilizes at least 5 translocated effectors to modulate Rab1 function on the vacuolar surface [21]. Recently LidA, an effector that enhances Rab1 recruitment, has been implicated in vacuole stability. A Legionella strain lacking 2 translocated effectors, LidA and WipB, was shown to have decreased replication vacuole stability and a corresponding decrease in intracellular survival [22].
Role of lipids in vacuole stability
In addition to targeting the proteins found on the vacuole membrane, intravacuolar pathogens also target the lipids of the vacuolar membrane. Changes in membrane lipids alter the membrane identity of the organelle affecting trafficking. Recruiting or altering lipids is also important for metabolism, membrane dynamics and the tethering of effector proteins to the vacuole [23–25]. Several pathogens change the phosphoinositol signature found on their vacuoles. For instance, Mycobacterium tuberculosis depletes PI(3)P to prevent phagosome maturation, whereas S. enterica Typhimurium generates PI(3)P to promote vacuole fusion with late endosomes [23]. L. pneumophila utilizes phosphoinositides PI(4)P and PI(3)P to anchor bacterial effector proteins to the vacuole [26]. Although they play a key role in vacuole biogenesis, the involvement of phosphoinositides in vacuole stability has not been investigated.
In addition to phosphoinositides, pathogens also recruit or exclude other lipids from their vacuolar membranes. Chlamydia trachomatis recruits host cell sphingomyelin and cholesterol to its inclusion both of which are incorporated by the bacterium and are required for replication [27,28]. C. trachomatis has been shown to recruit sphingomyelin from both vesicular and non-vesicular sources for different purposes. Sphingomyelin from non-vesicular sources is required for bacterial replication whereas vesicular sphingomyelin is involved in inclusion expansion and is required for stability of the inclusion [29,30]. It has been suggested that a failure to acquire sphingomyelin and other host lipids prevents inclusion expansion to accommodate the replicating chlamydiae and results in inclusion fragmentation [29].
A final example of lipids affecting vacuole stability is the modulation of cholesterol levels by the S. enterica Typhimurium translocated effector SseJ. SseJ is a phospholipase that is activated by RhoA and exhibits glycerophospholipid:cholesterol acyltransferase (GCAT) activity leading to the production of cholesterol esters and the depletion of cholesterol from the SCV [31–33]. The activity of SseJ has been shown to destabilize the SCV membrane in the absence of correct interactions with cytoskeletal motors (described above). Additional deletion of sseJ inhibits the vacuole rupture phenotype of a sifA mutant [34] and the absence of sseJ reduces the vacuole destabilizating affect of myosin II inhibition [6]. Cholesterol affects the fluidity of membranes and it has been suggested that SseJ-dependent cholesterol depletion may contribute to tubulation of the SCV by increasing membrane fluidity while also increasing susceptibility to cytoskeleton motor-dependent forces that cause membrane rupture [32]. Interestingly, PlaA, a Legionella protein with homology to SseJ, has been shown to regulate stability of the LCV. PlaA is exported by the type 2 secretion system and has been shown to cleave phospholipids and lysophospholipids and to esterify cholesterol [35,36]. Similarly to SseJ, PlaA destabilizes the vacuole membrane in the absence of another Legionella effector, SdhA [37].
Vacuole disruption as a survival strategy
A few intracellular pathogens including Listeria and Shigella, escape a membrane-bound compartment shortly after uptake to avoid the restrictive environment of a phagosome targeted to the endocytic pathway. However it is becoming clear that pathogens thought to exclusively replicate within membrane-bound compartments may also exhibit a cytoplasmic stage of replication. It has been known for several years that a small percentage of Salmonella escape from the vacuole and reside in the cytosol of epithelial cells [38]. Recent work has shown that the bulk of Salmonella replication observed during growth in epithelial cells occurs in the cytosol; the significance of this during infection is unknown although it may play a role in bacterial dissemination [39,40]. Escape from the vacuole has also been observed for Mycobacterial species and L. pneumophila suggesting that an extra-vacuolar stage occurs in other bacterial infections [41–43]. The frequency and outcome of vacuole disruption during disease for both of these organisms is unknown. It is likely that the choice of intracellular niche reflects a complex balance between the need to hide from cytoplasmic immune responses and the restrictive nature of life in a vacuole [44].
It is also clear that pathogen-containing vacuoles exhibit some permeability during infection to allow the translocation of bacterial effector proteins into the host cytoplasm. This permeability is achieved through the function of specialized secretion systems forming pores in the vacuole membrane that effectors pass through. M. tuberculosis was recently shown to permeabilize its phagosome membrane during the early stages of infection via the ESX-1 secretion system [45]. This permeability results in the release of M. tuberculosis DNA into the host cytoplasm and the triggering of the cytosolic innate immune system. A similar immune response is seen during infection with other vacuolar pathogens that possess specialized secretion systems, suggesting that selective permeabilization of the vacuole membrane is a general strategy [46,47].
Advances in detecting vacuole disruption
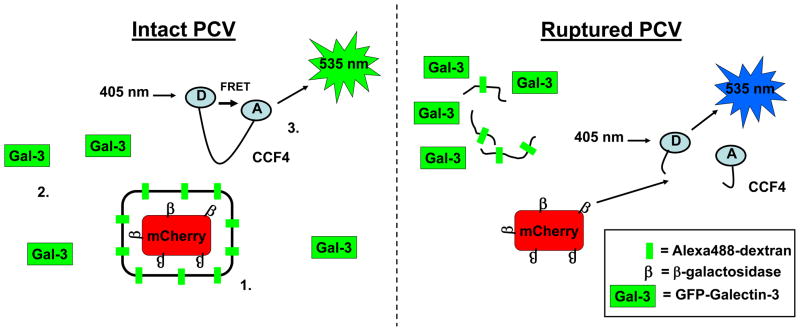
Most studies examining the integrity of pathogen-containing vacuoles utilize transmission electron microscopy to directly visualize membranes or immunofluorescent microscopy to detect a marker of the vacuole membrane such as LAMP1 or Chlamydial Inc proteins [e.g. 3,12]. An alternate approach is to selectively permeabilize infected cells such that antibodies can pass the plasma membrane and detect cytoplasmic bacteria but are excluded from membrane-bound compartments [12,37]. There are significant limitations with these techniques including the possibility of artefacts resulting from sample processing and the inability to visualize vacuole integrity in real time using live cells. To address these limitations, Enninga and colleagues have developed two novel approaches that allow live visualization of membrane integrity and as such are suitable for high throughput and kinetic assays (Fig. 2). The first assay takes advantage of the observation that cytosolic galectins are specifically recruited to the remnants of ruptured vacuoles [48,49]. Cells expressing GFP-Galectin 3 have been successfully used to follow the escape of Shigella into the cytosol in real time [50,51]. The second technique also utilizes fluorescent microscopy to detect vacuole rupture and takes advantage of the ability of Gram negative bacteria to display β-lactamases on their surface. Infected cells can be preloaded with the fluorescent β-lactamase substrate CCF4. If the bacteria have access to the cytosol, CCF4 is cleaved and there is a change in fluorescence due to a loss of FRET. This technique has been used to study vacuolar escape of Shigella and M. tuberculosis [42,50] and is suitable for detection by flow cytometry making it appropriate for high throughput studies [52]. A third live-cell imaging approach was developed by Steele-Mortimer and colleagues [39]. The authors utilized fluorescent-tagged dextran and mCherry expressing Salmonella. The dextran labels the endocytic compartment of infected cells and accumulates within the SCV. SCV integrity was tracked using live fluorescent microscopy to detect colocalization of Alexa488-Dextran and mCherry Salmonella (Fig. 2).
Fig 2. Detection of vacuole rupture.
Three recent techniques allow the detection of vacuole rupture in real time on a single cell basis. (1) Alexa488-Dextran marks the vacuole membrane and colocalization with bacteria expressing mCherry can be detected by fluorescence microscopy. (2) GFP-tagged Galectin-3 is specifically recruited to the remnants of ruptured vacuoles. (3) Following vacuole disruption, surface-expressed β-lactamases can cleave cytosolic CCF4. Cleavage of CCF4 relieves the intramolecular FRET and causes a change in fluorescence emission.
Conclusion and Perspectives
With our increasing understanding of PCVs, it is clear that a frequent outcome of aberrant vacuole biogenesis and expansion is the loss of vacuole integrity and the release of pathogenic bacteria into the cytoplasm. The formation of a replication vacuole is a tightly regulated process involving both bacterial effector proteins and host pathways. Many of these effector proteins and host pathways have been shown to be required for intracellular replication of bacterial pathogens but their role in vacuole maintenance has not be investigated. As we move forward dissecting the biology of PCVs it is important to revisit these old studies and investigate the effect of disrupting these systems on vacuolar integrity.
The mechanisms of vacuole disruption are largely unclear. They may involve biochemical disruption by host or bacterial enzymes or mechanical disruption caused by aberrant vacuole expansion to accommodate bacterial replication or inappropriate forces exerted by cytoskeletal motors. Much of the evidence to date supports a mechanical model of vacuole rupture and the biophysics involved in vacuole biology is an important consideration.
Finally, given that vacuole disruption often results in triggering of the cytosolic immune system and destruction of the pathogen, it is likely that pathogen-containing vacuoles are themselves targeted by the host. Indeed interferon-inducible GTPases target pathogen-containing vacuoles to inhibit pathogen replication via several mechanisms including fusion with lysosomes or autophagosomes [53]. An outcome of recruitment of interferon-inducible GTPases by the Toxoplasma gondii parasitophorous vacuole is membrane disruption [54]. It remains to be seen whether this is also occurs during infection with bacterial pathogens.
Highlights.
Many pathogens direct the formation of a specialized vacuolar niche
Vacuole assembly and trafficking involve modulation of host proteins and lipids
Disruption of vacuole assembly often leads to loss of vacuole integrity
Aberrant mechanical forces may cause vacuole rupture
Acknowledgments
We thank Dr. Sina Mohammadi for reviewing the manuscript. EAC was supported by HHMI, and RRI is an Investigator of HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alix E, Mukherjee S, Roy CR. Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol. 2011;195:943–952. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison RE, Brumell JH, Khandani A, Bucci C, Scott CC, Jiang X, Finlay BB, Grinstein S. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol Biol Cell. 2004;15:3146–3154. doi: 10.1091/mbc.E04-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar Y, Valdivia RH. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 2008;4:159–169. doi: 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Méresse S, Unsworth KE, Habermann A, Griffiths G, Fang F, Martínez-Lorenzo MJ, Waterman SR, Gorvel JP, Holden DW. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 5.Guignot J, Caron E, Beuzón C, Bucci C, Kagan J, Roy C, Holden DW. Microtubule motors control membrane dynamics of Salmonella-containing vacuoles. J Cell Sci. 2004;117:1033–1045. doi: 10.1242/jcs.00949. [DOI] [PubMed] [Google Scholar]
- 6.Wasylnka JA, Bakowski MA, Szeto J, Ohlson MB, Trimble WS, Miller SI, Brumell JH. Role for myosin II in regulating positioning of Salmonella-containing vacuoles and intracellular replication. Infect Immun. 2008;76:2722–2735. doi: 10.1128/IAI.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrahams GL, Müller P, Hensel M. Functional dissection of SseF, a type III effector protein involved in positioning the salmonella-containing vacuole. Traffic Cph Den. 2006;7:950–965. doi: 10.1111/j.1600-0854.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 8.Henry T, Couillault C, Rockenfeller P, Boucrot E, Dumont A, Schroeder N, Hermant A, Knodler LA, Lecine P, Steele-Mortimer O, et al. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc Natl Acad Sci U S A. 2006;103:13497–13502. doi: 10.1073/pnas.0605443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder N, Henry T, de Chastellier C, Zhao W, Guilhon A-A, Gorvel J-P, Méresse S. The virulence protein SopD2 regulates membrane dynamics of Salmonella-containing vacuoles. PLoS Pathog. 2010;6:e1001002. doi: 10.1371/journal.ppat.1001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucrot E, Henry T, Borg J-P, Gorvel J-P, Méresse S. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308:1174–1178. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]
- 11.Dumont A, Boucrot E, Drevensek S, Daire V, Gorvel J-P, Poüs C, Holden DW, Méresse S. SKIP, the host target of the Salmonella virulence factor SifA, promotes kinesin-1-dependent vacuolar membrane exchanges. Traffic Cph Den. 2010;11:899–911. doi: 10.1111/j.1600-0854.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 12.Beuzón CR, Méresse S, Unsworth KE, Ruíz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen I, Bednar MM, Amin V, Davis BK, Ting JPY, McCafferty DG, Valdivia RH. The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence. Cell Host Microbe. 2011;10:21–32. doi: 10.1016/j.chom.2011.06.008. The authors designed a cell-permeable peptide inhibitor of the chlamydial protease CPAF. They used this peptide to demonstrate that CPAF activity is required for inclusion integrity. The peptide inhibitor of CPAF may represent a potential antichlamydial therapeutic by targeting inclusion stability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ham H, Sreelatha A, Orth K. Manipulation of host membranes by bacterial effectors. Nat Rev Microbiol. 2011;9:635–646. doi: 10.1038/nrmicro2602. [DOI] [PubMed] [Google Scholar]
- 15.Mallo GV, Espina M, Smith AC, Terebiznik MR, Alemán A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J Biol Chem. 2001;276:23607–23615. doi: 10.1074/jbc.M101034200. [DOI] [PubMed] [Google Scholar]
- 17.Méresse S, Steele-Mortimer O, Finlay BB, Gorvel JP. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brumell JH, Tang P, Zaharik ML, Finlay BB. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar typhimurium in the cytosol of epithelial cells. Infect Immun. 2002;70:3264–3270. doi: 10.1128/IAI.70.6.3264-3270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 20.Derré I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neunuebel MR, Machner MP. The taming of a Rab GTPase by Legionella pneumophila. Small GTPases. 2012;3:28–33. doi: 10.4161/sgtp.18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor TJ, Boyd D, Dorer MS, Isberg RR. Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen. Science. 2012;338:1440–1444. doi: 10.1126/science.1229556. Using a novel genetic screening strategy that combines bacterial insertion mutants with RNA interference targeting host pathways, the authors identify Legionella effectors and host pathways that contribute to vacuole integrity in a functionally redundant manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber SS, Ragaz C, Hilbi H. Pathogen trafficking pathways and host phosphoinositide metabolism. Mol Microbiol. 2009;71:1341–1352. doi: 10.1111/j.1365-2958.2009.06608.x. [DOI] [PubMed] [Google Scholar]
- 24.Van der Meer-Janssen YPM, van Galen J, Batenburg JJ, Helms JB. Lipids in host-pathogen interactions: pathogens exploit the complexity of the host cell lipidome. Prog Lipid Res. 2010;49:1–26. doi: 10.1016/j.plipres.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg BE, Grinstein S. Pathogen destruction versus intracellular survival: the role of lipids as phagosomal fate determinants. J Clin Invest. 2008;118:2002–2011. doi: 10.1172/JCI35433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilbi H, Weber S, Finsel I. Anchors for effectors: subversion of phosphoinositide lipids by legionella. Front Microbiol. 2011;2:91. doi: 10.3389/fmicb.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Ooij C, Kalman L, van Ijzendoorn, Nishijima M, Hanada K, Mostov K, Engel JN. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol. 2000;2:627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 28.Hackstadt T, Scidmore MA, Rockey DD. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci U S A. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson DK, Gu L, Rowe RK, Beatty WL. Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog. 2009;5:e1000664. doi: 10.1371/journal.ppat.1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elwell CA, Jiang S, Kim JH, Lee A, Wittmann T, Hanada K, Melancon P, Engel JN. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011;7:e1002198. doi: 10.1371/journal.ppat.1002198. Chlamydiae require host sphingomyelin for replication and for inclusion growth and stability. The authors separate these requirements and show that Chlamydia intercepts both vesicular and non-vesicular pathways to acquire sphingomyelin. Inclusion growth and stability are dependent on vesicular trafficking of sphingomyelin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nawabi P, Catron DM, Haldar K. Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol Microbiol. 2008;68:173–185. doi: 10.1111/j.1365-2958.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 32.Lossi NS, Rolhion N, Magee AI, Boyle C, Holden DW. The Salmonella SPI-2 effector SseJ exhibits eukaryotic activator-dependent phospholipase A and glycerophospholipid : cholesterol acyltransferase activity. Microbiol Read Engl. 2008;154:2680–2688. doi: 10.1099/mic.0.2008/019075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christen M, Coye LH, Hontz JS, LaRock DL, Pfuetzner RA, Megha, Miller SI. Activation of a bacterial virulence protein by the GTPase RhoA. Sci Signal. 2009;2:ra71. doi: 10.1126/scisignal.2000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz-Albert J, Yu X-J, Beuzón CR, Blakey AN, Galyov EE, Holden DW. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 35.Flieger A, Neumeister B, Cianciotto NP. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect Immun. 2002;70:6094–6106. doi: 10.1128/IAI.70.11.6094-6106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang C, Rastew E, Hermes B, Siegbrecht E, Ahrends R, Banerji S, Flieger A. Zinc metalloproteinase ProA directly activates Legionella pneumophila PlaC glycerophospholipid:cholesterol acyltransferase. J Biol Chem. 2012;287:23464–23478. doi: 10.1074/jbc.M112.346387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc Natl Acad Sci U S A. 2012;109:3481–3486. doi: 10.1073/pnas.1121286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birmingham CL, Brumell JH. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy. 2006;2:156–158. doi: 10.4161/auto.2825. [DOI] [PubMed] [Google Scholar]
- 39.Malik-Kale P, Winfree S, Steele-Mortimer O. The bimodal lifestyle of intracellular Salmonella in epithelial cells: replication in the cytosol obscures defects in vacuolar replication. PloS One. 2012;7:e38732. doi: 10.1371/journal.pone.0038732. The authors developed protocols to study intracellular replication on a single-cell basis in real time. They used these protocols to show that the bulk of intracellular replication can be attributed to the small percentage of bacteria that escape the vacuole and replicate within the cytosol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci. 2010;107:17733–17738. doi: 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 42.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8:e1002507. doi: 10.1371/journal.ppat.1002507. Using a CCF4-based reporter system to detect cytoplasmic Mycobacterium tuberculosis, the authors show that M. tuberculosis escapes from the vacuole after 3 to 4 days of infection. This work demonstrates that CCF4 can be used to detect vacuole rupture even after several days of vacuole biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molmeret M, Bitar DM, Han L, Kwaik YA. Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during the last stages of intracellular infection of macrophages and Acanthamoeba polyphaga. Infect Immun. 2004;72:4040–4051. doi: 10.1128/IAI.72.7.4040-4051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welin A, Lerm M. Inside or outside the phagosome? The controversy of the intracellular localization of Mycobacterium tuberculosis. Tuberc Edinb Scotl. 2012;92:113–120. doi: 10.1016/j.tube.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monroe KM, McWhirter SM, Vance RE. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosales-Reyes R, Aubert DF, Tolman JS, Amer AO, Valvano MA. Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages. PloS One. 2012;7:e41726. doi: 10.1371/journal.pone.0041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost M-C, Lafont F, et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. 2010;12:530–544. doi: 10.1111/j.1462-5822.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- 49.Thurston TLM, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482:414–418. doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray K, Bobard A, Danckaert A, Paz-Haftel I, Clair C, Ehsani S, Tang C, Sansonetti P, Tran GVN, Enninga J. Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell Microbiol. 2010;12:545–556. doi: 10.1111/j.1462-5822.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- 51.Ehsani S, Santos JC, Rodrigues CD, Henriques R, Audry L, Zimmer C, Sansonetti P, Tran Van Nhieu G, Enninga J. Hierarchies of host factor dynamics at the entry site of Shigella flexneri during host cell invasion. Infect Immun. 2012;80:2548–2557. doi: 10.1128/IAI.06391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nothelfer K, Dias Rodrigues C, Bobard A, Phalipon A, Enninga J. Monitoring Shigella flexneri vacuolar escape by flow cytometry. Virulence. 2011;2:54–57. doi: 10.4161/viru.2.1.14666. [DOI] [PubMed] [Google Scholar]
- 53.Kim B-H, Shenoy AR, Kumar P, Bradfield CJ, MacMicking JD. IFN-Inducible GTPases in Host Cell Defense. Cell Host Microbe. 2012;12:432–444. doi: 10.1016/j.chom.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii Parasitophorous Vacuole by IFNγ-Inducible Immunity-Related GTPases (IRG Proteins) Triggers Necrotic Cell Death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]