Abstract
BACKGROUND
Increased susceptibility to cognitive impairment or psychosis in adulthood is associated with adolescent drug abuse. Studies in adults have identified impairments in attention and memory, and changes in EEG, as common consequences of ketamine abuse. In contrast, the effects of ketamine on the juvenile brain have not been extensively tested. This is a significant omission, since abuse of ketamine is often observed within this age group.
OBJECTIVES
Juvenile mice (4–6 weeks of age) were administered ketamine (20 mg/kg) for 14 days. EEG was assessed in response to auditory stimulation both at one week following ketamine exposure at 7 weeks of age (juvenile) and again at 12 weeks of age (adult). EEG was analyzed for baseline activity, event-related power and event-related potentials (ERPs).
RESULTS
While no effects of ketamine exposure were observed during the juvenile period, significant reductions in amplitude of the P20 ERP component and event-related gamma power were seen following ketamine when re-tested as adults. In contrast, reductions in event–related theta were seen in ketamine-exposed mice at both time points.
CONCLUSIONS
Age related deficits in electrophysiological components such as P20 or event-related gamma may be due to an interruption of normal neural maturation. Reduction of NMDAR signaling during adolescence leads to delayed-onset disruption of gamma oscillations and the P20 component of the ERP. Further, delayed onset of impairment following adolescent ketamine abuse suggests that methods could be developed to detect and treat the early effects of drug exposure prior to the onset of disability.
Keywords: NMDA, gamma, oscillations, schizophrenia, theta, drug-abuse
1. INTRODUCTION
Ketamine is associated with a high prevalence of abuse, especially in adolescents. Current estimates in the United States suggest that 1% of 8th graders and approximately 2% of 12th graders have used ketamine within the previous year. Outside of the US, increased prevalence of adolescent ketamine use has been reported in South America, Europe and Asia. Indeed, ketamine has recently become the most commonly abused substance amongst adolescents in Hong Kong (UNODC, global drug report). As such, it is likely that adolescent ketamine abuse will remain a serious global health concern for the immediate future.
Ketamine abuse in adults has been shown to produce lasting cognitive and psychiatric changes. Frequent users show poorer performance on spatial working memory and pattern recognition memory tasks as well as increased dissociative and affective symptoms (Morgan et al., 2010). These deficits overlap with decreases in prefrontal grey matter and increases in white matter abnormalities (Liao et al., 2011, 2010) as well as increased dopamine D1 receptor up-regulation (Narendran et al., 2005). In adult rodents, chronic ketamine treatment produces lasting disruptions in working memory (Rushforth et al., 2011), attentional set-shifting (Nikiforuk and Popik, 2012), long-term memory (Amann et al., 2009; Featherstone et al., 2012), reversal learning (Featherstone et al., 2012) and social interaction (Becker and Grecksch, 2004; Becker et al., 2003), consistent with disruption of higher-order executive function and memory. Indeed, long-term ketamine use is associated with decreased cortical activity and increased glutamate concentration (Chatterjee et al., 2012; Yu et al., 2012).
Despite the widespread use of ketamine by adolescents, little is known about the long-term consequences of sustained adolescent ketamine abuse on neural development and adult cognition. Relative to adult animals, neonatal rodents and primates show heightened sensitivity to the apoptotic effects of acute and chronic ketamine (Scallet et al., 2004; Slikker et al., 2007; Young et al., 2005; Zou et al., 2009), and display deficits in learning and memory following early ketamine exposure that extend into adulthood (Fredriksson et al., 2007; Huang et al., 2012; Paule et al., 2011). The limited number of studies conducted in adolescent animals to date have suggested an attenuated effect of ketamine relative to adult animals (Wiley et al., 2008). To our knowledge, no longitudinal studies have assessed the effects of prolonged adolescent ketamine exposure on adult brain function. tudies with other drugs of abuse have identified the adolescent period as a time of increased vulnerability to later drug-induced cognitive impairment or psychotic-like symptoms in adulthood (Pope et al., 2003), suggesting that the adolescent brain is especially malleable to the negative effects of exposure to drugs of abuse.
The current study examined the effects of chronic ketamine exposure in adolescent mice on electroencephalographic (EEG) indices of brain function, including Event-Related Potentials (ERPs), baseline power and event-related power, both at one week following cessation of exposure and during adulthood. EEG measures are particularly useful for addressing the neurodevelopmental effects of ketamine since there are clear developmental changes on EEG measures (Uhlhaas and Singer, 2011), EEG measures are relatively immune to potentially confounding changes in motor or non-auditory sensory development, and have been very well characterized following ketamine treatment (Amann et al., 2009; Connolly et al., 2004; Ehrlichman et al., 2009, 2008; Featherstone et al., 2012; Majewski-Tiedeken et al., 2008; Maxwell et al., 2006; Siegel et al., 2003). Additionally, several EEG phenomena are altered in disorders thought to be due to abnormal NMDA receptor mediated glutamate signaling, such as schizophrenia (Adler et al., 1998; Gonzalez-Burgos et al., 2011; Johannesen et al., 2008; Roach and Mathalon, 2008; Siegel et al., 1984; Uhlhaas and Singer, 2010). EEG oscillations are highly regulated by GABA and glutamate systems, both of which undergo substantial development during the adolescent period (Behrens and Sejnowski, 2009; Huppe-Gourgues and O'Donnell, 2012; Tseng and O'Donnell, 2005; Wang and Gao, 2009). Deficits in EEG power in schizophrenia are prominent in brain areas that are critical for cognitive performance, and thus, may reflect alterations in neural processes underlying cognitive function (Lewis and Gonzalez-Burgos, 2006). Additionally, increases in power within both the theta and gamma frequency range occur during performance of attention, working and long term memory tasks, further supporting a link between the generators of these rhythms and neural mechanisms for associated cognitive processes (Duzel et al., 2010; Lega et al., 2012; Tesche and Karhu, 2000).
2. METHODS
2.1 Subjects
C3H/HeHsd male mice (Jackson Laboratories, Bar Harbor, ME.) were used. Mice were purchased at 21 days of age and were given one week to acclimate to the vivarium prior to the start of experimentation. Mice received 14 daily injections of ketamine starting at 4 weeks (28 days) of age. Implantation of electrodes took place 2 days following the last injection (44 days), with EEG recording performed following recovery from surgery (51 days). Mice were housed in a room maintained at 22 (±2) °C and were kept on a 12:12 light/dark cycle (lights on at 08:00). Standard laboratory mouse chow and water were available ad libitum over the duration of the experiment. All procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and the local Institutional Laboratory Animal Care and Use Committee (IACUC) guidelines.
2.2. Injections
Mice received either 0.9% Saline (n=10) or 20 mg/kg Ketamine (n=12), injected I.P. daily over a 14 day period. All injections were administered in a 0.1 ml/kg volume.
2.3. Electrode implantation
Electrode implantation took place under 1% isoflurane anesthesia. A series of three small holes were drilled into the skull at −1.8, −0.8 and +0.2 mm AP to bregma; and 2.65 mm lateral. A positive electrode was placed into right hippocampal region at 1.8 mm posterior, 2.65 mm lateral, and 2.75 mm dorsal to bregma. A reference electrode was lowered onto the surface of the ipsilateral cortex at 0.2 mm anterior, 2.75 mm lateral, and 0.75 mm dorsal. A ground electrode was lowered onto the cortical surface between the positive and reference electrodes at 0.8 mm posterior, 2.75 mm lateral, and 0.75 mm dorsal. Ethyl cyanoacrylate (Loctite, Henkel KGaA, Duesseldorf, Germany) and dental cement (Ortho Jet, Lang Dental, Wheeling IL, USA) were used to secure the electrodes to the skull. One week was given to allow for recovery before EEG testing.
2.4. EEG recording and analysis
The EEG testing apparatus consisted of 8 standard mouse cages, modified to allow placement of a speaker on the top of the cage for delivery of auditory stimuli, located within a Faraday cage. Auditory stimuli were generated by Micro1401 hardware and Spike2, version 6.0 software (Cambridge Electronic Design, Cambridge, UK). EEG was recorded during auditory click presentations (8.5 sec inter-stimulus interval) of a white noise tone (10 msec duration, 85 dB). Mice received a total of 300 clicks. To minimize the effects of stress, animals were given 15 minutes to acclimate to the ERP apparatus prior to each ERP recording session. ERPs were analyzed using Spike2 software (CED, Cambridge, UK).
2.4.1. Baseline Power
Raw EEG was recorded for a 60 sec period prior to the start of auditory stimuli. The fast Fourier transformation function native to Spike2 was used to decompose power into 0.81 Hz bins (Hanning window). Absolute Gamma was quantified as the average of EEG power between 30 and 80 Hz, while Relative Gamma was quantified as the sum of EEG power between 30 to 80 Hz divided by the total sum of EEG power between 1 and 120 Hz. Absolute Theta was quantified as average of EEG power 4 to 12 Hz, while Relative Theta was defined as the sum of EEG power between 4 and 12 Hz divided by the total sum of EEG power between 1 and 120 Hz.
2.4.2. Event-Related Spectral Power (ERSP)
Data were processed using EEGLAB (Schwartz Center for Computational Neuroscience) to create a time-frequency measure for power. Three hundred single-trial epochs, ranging from minus1 to plus 2 sec relative to click onset, were extracted from continuous EEG recorded during clicks and subjected to further analysis. Power was calculated using Morlet wavelets in 116 logarithmically spaced frequency bins between 4 and 120 Hz, with wavelet cycle numbers ranging from 2 to 10 (Delorme and Makeig, 2004). Power was expressed in decibels (dB) as logµv10. The frequency band between 4 and 12 Hz was defined as theta, and 30 to 80 Hz as gamma. Theta was quantified as the average power between 0 and 200 msec, while gamma power was quantified as the average from 0 to 60 msec.
2.4.3. Event-related Potentials
The amplitude of each ERP waveform component was quantified as the change in amplitude relative to the previous point of inflection. These included the P20 (maximum value between 15 and 35 msec) and the N40 (minimum value occurring between 25 and 60 msec). Latency was calculated separately for each component (P20, N40) and was defined as the time point at which the maximum (for positive components) or minimum (for negative components) deflection occurred within the relevant time interval.
3. RESULTS
3.1. Baseline Power
3.1.1. Theta
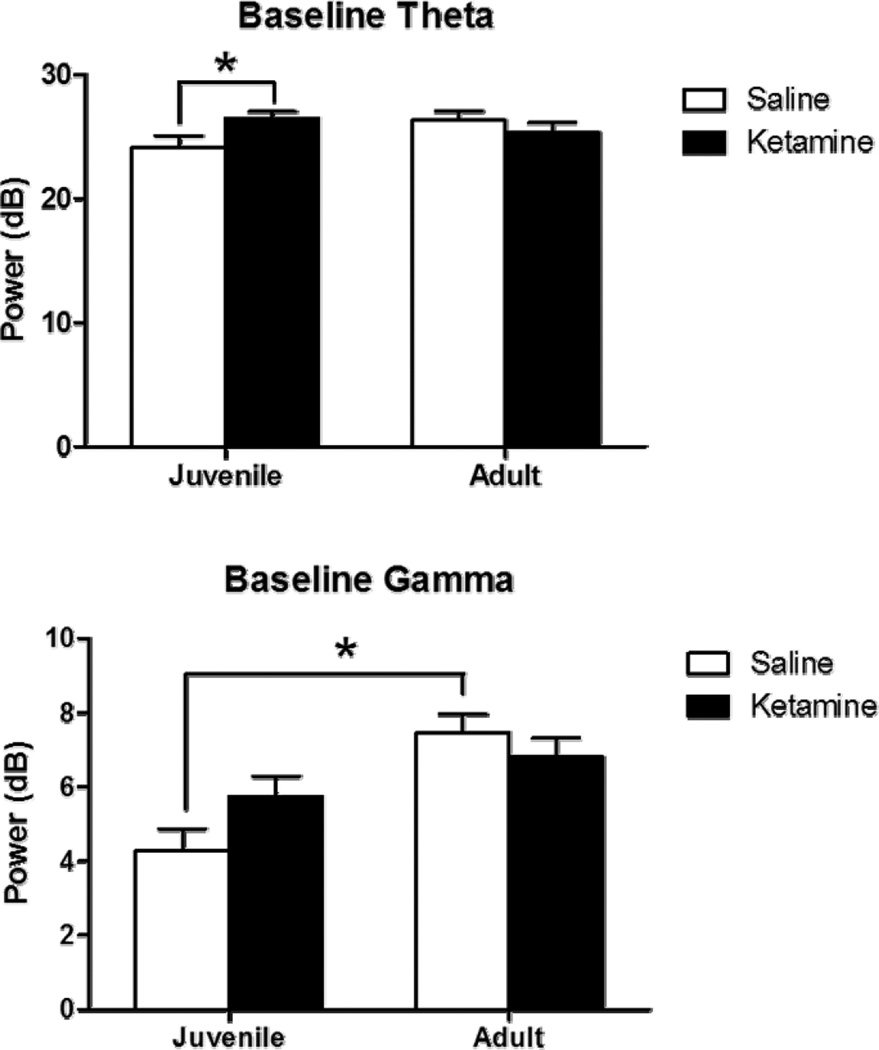
Absolute baseline theta did not significantly vary as a function of either age or treatment, but a significant interaction occurred between these two variables [F(1,16)=4.9, p<0.05]. Post hoc tests showed a significant increase in absolute baseline theta in ketamine-treated mice when assessed as juveniles (p<0.05), but this was not seen in the same mice when assessed as adults. A significant decrease in relative theta power occurred as a function of age [F(1,16)=41.1, p<0.05]. Chronic ketamine did not affect relative theta baseline power, nor was a significant interaction seen between drug treatment and age.
3.1.2. Gamma
Absolute gamma increased as a function of age [F(1,16)=21.5, p<0.01] and a significant interaction was observed between age and treatment [F(1,16)=5.2, p<0.05]. Post hoc analyses showed a significant increase in absolute gamma power in ketamine-, as compared to saline-treated mice when assessed as juveniles (p<0.01), but these groups did not differ when assessed as adults. Additionally, a significant increase in absolute gamma power was found in saline-treated mice as a function of age (p<0.001). This age-related increase was not present in animals treated with ketamine as juveniles. Relative gamma power increased as a function of age [F(1,16)=25.2, p<0.05], and this was significantly modified by chronic ketamine treatment [F(1,16)=6.7, p<0.05]. Post hoc analyses showed a significant increase in gamma power as a function of age in saline-treated, but not ketamine-treated, mice (p<0.001).
3.2. Event Related Spectral Power (ERSP)
3.2.1. Theta
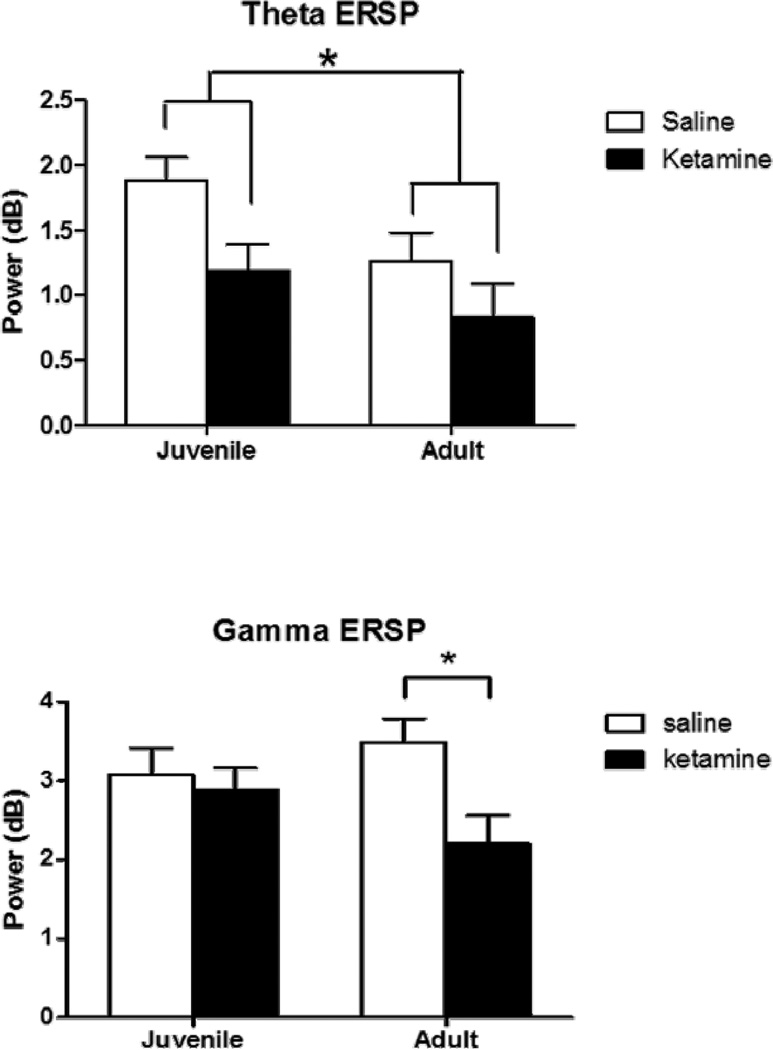
Adult mice showed significantly lower theta ERSP [F(1,16)=6.7, p<0.05], suggesting an age related decrease in this measure. Chronic ketamine significantly decreased theta power [F(1,16)=4.6, p<0.05]. The interaction between age and ketamine treatment was not significant, suggesting that ketamine disrupted theta ERSP at both developmental time points.
3.2.2. Gamma
A significant interaction was observed between age and drug treatment [F(1,16)=4.6, p<0.05] in chronic-treated animals, driven primarily by a decrease in gamma power in adult ketamine-treated mice relative to adult saline-treated mice (p<0.028). In contrast, no effect of chronic ketamine treatment was observed in juvenile mice.
3.3. Event-related Potentials
3.3.1. P20 amplitude
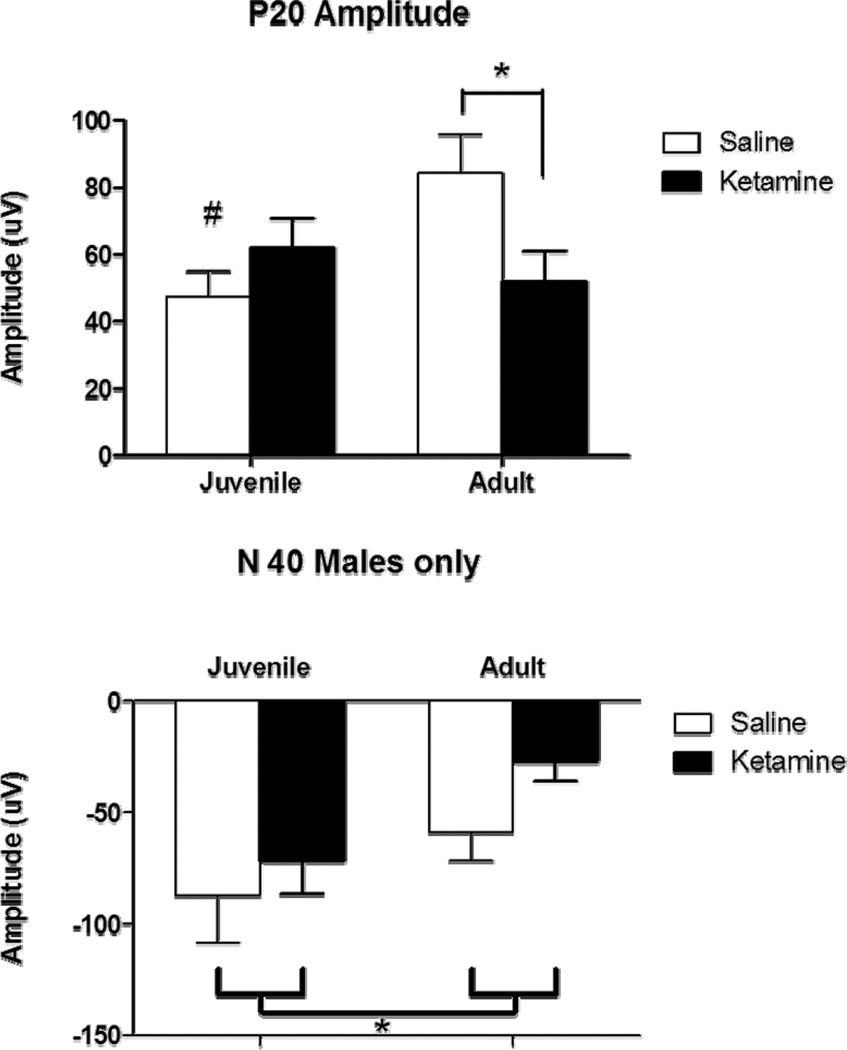
The effect of chronic ketamine treatment on P20 amplitude was dependent upon age [F(1,16)=5.7, p<0.05], with ketamine-treated mice showing significantly lower P20 amplitude relative to saline-treated mice when assessed in adulthood (p<0.05) but not earlier. This suggests that chronic ketamine treatment during adolescence induced an alteration in P20 amplitude that only emerged as a function of maturation. Thus, in saline-treated mice, a significant increase was seen in P20 amplitude as a function of increasing age (p<0.05), while a similar change failed to emerge following chronic ketamine treatment.
3.3.2. N40 amplitude
A significant decrease in N40 amplitude occurred as a function of increasing age [F(1,16)=10.5, p<0.05). No other effects were significant.
4. DISCUSSION
Adolescent drug abuse has received increasing attention with several recent reports suggesting that the use of illicit drugs during this time period can have long-term detrimental consequences for normal adult cognition (Pope et al., 2003). Despite consistent rates of abuse by adolescents little is known about the long-term effects of ketamine on neural and cognitive development. In the present study, mice that received chronic ketamine exposure during adolescence showed disruptions across a wide range of EEG measures when assessed during adulthood. These included a loss of age-related increase in P20 amplitude as well as a decrease in event-related gamma power (ERSP). Importantly, while differences were observed on these measures during adulthood, impairing effects of ketamine were not found when EEG was assessed during the juvenile period, suggesting the deficits observed in adults may have occurred due to a disruption of normal neural maturation. Adolescence is marked by developmental change in brain structure and neurotransmitter function that may be susceptible to the effects of chronic ketamine exposure. Gamma oscillations are strongly regulated by both GABA and glutamate systems (Carlen et al., 2011; Ehrlichman et al., 2009; Gandal et al., 2012; Gonzalez-Burgos et al., 2011; Lazarewicz et al., 2010; Lewis et al., 2005, 2004; Uhlhaas and Singer, 2010) and these systems undergo marked transformation during the adolescent period (Behrens and Sejnowski, 2009; Huppe-Gourgues and O'Donnell, 2012; Tseng and O'Donnell, 2005; Wang and Gao, 2009). Several recent reports have shown that event-related gamma power responses to both auditory and visual stimuli increase during the adolescent period (Rojas et al., 2006; Uhlhaas et al., 2009; Uhlhaas and Singer, 2011; Werkle-Bergner et al., 2009), raising the possibility that changes in event-related gamma could serve as an important biomarker of neurodevelopmental milestones and cognitive development. It is interesting to note that both baseline and event-related gamma showed strong age-related increases across the two developmental time points in the mice assessed here, suggesting considerable development of the gamma response during this time.
The pattern of results observed following juvenile ketamine treatment in the current study diverges from those in previous studies that have assessed chronic treatment during adulthood. Adult mice show lasting alterations in late component ERPs and event-related theta oscillations six months after cessation of chronic ketamine, with only modest reductions observed in event-related gamma oscillations (Amann et al., 2009; Featherstone et al., 2012; Maxwell et al., 2006). In the current study mice exposed to ketamine as juveniles showed reduced event-related theta immediately during the juvenile period, in contrast to the delayed effects seen on event-related gamma. This could suggest that the neural generators of theta develop relatively early, prior to the timing of ketamine exposure used here. Given the prominent role of theta in regulating gamma activity, it is possible that the changes observed on event-related gamma may have occurred due to these reductions in theta. It is also possible that normal levels of theta activity are necessary for proper development of adult gamma oscillatory responses to sensory stimuli.
The delayed emergence of the effects of ketamine on gamma power suggests a developmentally sensitive period during adolescence. Several reports have suggested that cannabis exposure during critical periods of adolescence is a significant risk factor for later emergence of cognitive impairment, especially when compared to adult drug use. While the question of whether these increases in sensitivity can be detected in rodent models of development remains to be seen, the present study clearly demonstrate that juvenile mice are sensitive to the effects of ketamine using EEG measures thought to be closely linked to pathological changes underlying impairment. Additionally, these data indicate that adolescent exposure to ketamine, and perhaps other drugs of abuse, may cause delayed onset deficits in adulthood. As such, future studies will address mechanisms to reverse such changes during the post abuse period. Such research would be useful in identifying methods to detect and treat the early effects of exposure to drugs of abuse prior to full emergence of these problems.
The time-course of the deficits observed here suggests that chronic ketamine treatment during adolescence might be an informative rodent model of the developmental changes underlying schizophrenia. Although schizophrenia is well known to emerge during the late adolescent or early adult period, few models of schizophrenia accurately address this aspect of the disease. Current studies are underway to more fully assess the overall pattern of behavioral and cognitive changes associated with early ketamine exposure.
Figure 1.
Baseline EEG following juvenile saline (white) or ketamine (black) exposure assessed at 7 weeks (juvenile) and 12 weeks (adult). Figures 1A and B show theta (4 to 12 Hz) while figures 1C and D depict gamma (30 to 80 Hz). Gamma was quantified as the average of EEG power between 30 and 80 Hz, while theta was quantified as the average power between 4 and 12 Hz. Baseline theta power was increased in chronic ketamine-treated mice when assessed at the juvenile time-point, but this was not seen when assessed in adulthood. Saline-treated mice showed an age-related increase in gamma power as a function of increasing age (p<0.05) and this was not observed in ketamine-treated mice. Asterisk shows significance at p>0.05.
Figure 2.
Event-related EEG (ERSP) following juvenile saline (white) or ketamine (black) exposure assessed at 7 weeks (juvenile) and 12 weeks (adult). Figure 2A shows event-related theta (4 to 12 Hz), while figure 2B shows event-related gamma (30 to 80 Hz). Ketamine treatment reduced event-related theta at both the juvenile and adult time points. Saline-treated mice showed an age related decrease in theta over the timespan of the ages assessed here. Likewise, saline-treated mice showed an age dependent increase in event-related gamma, which appeared to be blocked in ketamine-treated mice, suggesting that ketamine treatment in juvenile mice blocked normal maturation of event-related gamma. Asterisk shows significance at p>0.05.
Figure 3.
Event Related Potential following juvenile saline (white) or ketamine (black) exposure assessed at 7 weeks (juvenile) and 12 weeks (adult). Figure 3A shows the P20 component of the ERP, while figure 3B shows the N40 response. Saline-treated mice showed an age dependent increase in the P20 component, which appeared to be blocked in ketamine-treated mice, suggesting that ketamine treatment in juvenile mice blocked normal maturation of this response. Ketamine treatment reduced amplitude of the N40 component of the ERP at both the juvenile and adult time points. Saline-treated mice showed an age related decrease in N40 over the timespan of the ages assessed here. Asterisk shows significance at p>0.05.
Acknowledgments
Role of Funding Source: This study was supported by NIDA grant 5R01DA023210-02 to Steven J. Siegel.
Conflicts of Interest: Steven J. Siegel has received grants from Astellas, AstraZeneca, Abbott, NuPathe, Pfizer, and GlaxoSmithKline that are unrelated to the content of this manuscript. Robert Featherstone has received grant support from Astellas that is unrelated to the content of this manuscript. Steven Siegel has served as a consultant to Abbott, NuPathe, and Lundbeck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
None of the authors have a financial relationship with the organization that sponsored the research outlined in the present manuscript.
Contributors: i) RE Featherstone designed and conducted the study, performed data analysis and helped write and prepare the manuscript.
ii) L Nagy helped write and prepare the manuscript
iii) CG Hahn helped write and prepare the manuscript,
iv) S Siegel designed the study, helped write and prepare the manuscript
REFERENCES
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Amann LC, Halene TB, Ehrlichman RS, Luminais SN, Ma N, Abel T, Siegel SJ. Chronic ketamine impairs fear conditioning and produces long-lasting reductions in auditory evoked potentials. Neurobiol. Dis. 2009;35:311–317. doi: 10.1016/j.nbd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:1267–1277. doi: 10.1016/j.pnpbp.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry. 2011;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Verma R, Ganguly S, Palit G. Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology. 2012;63:1161–1171. doi: 10.1016/j.neuropharm.2012.05.041. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem. Res. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Duzel E, Penny WD, Burgess N. Brain oscillations and memory. Curr. Opin. Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, Turetsky BI, Siegel SJ. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J. Cogn. Neurosci. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RS, Siegel SJ. Subchronic ketamine treatment leads to permanent changes in EEG cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol. Dis. 2012;47:338–346. doi: 10.1016/j.nbd.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, Thieu T, Anderson R, Pierce RC, Jonak G, Gur RE, Carlson G, Siegel SJ. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr. Psychiatry Rep. 2011;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Liu Y, Jin W, Ji X, Dong Z. Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKC gamma-ERK signaling pathway in the developing brain. Brain Res. 2012;1476:164–171. doi: 10.1016/j.brainres.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Huppe-Gourgues F, O'Donnell P. D(1)-NMDA receptor interactions in the rat nucleus accumbens change during adolescence. Synapse. 2012;66:584–591. doi: 10.1002/syn.21544. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Bodkins M, O'Donnell BF, Shekhar A, Hetrick WP. Perceptual anomalies in schizophrenia co-occur with selective impairments in the gamma frequency component of midlatency auditory ERPs. J. Abnorm. Psychol. 2008;117:106–118. doi: 10.1037/0021-843X.117.1.106. [DOI] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J. Cogn. Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H, Liu T, Chen X, Hao W, Fletcher PC. Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol. Psychiatry. 2011;69:42–48. doi: 10.1016/j.biopsych.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain. 2010;133:2115–2122. doi: 10.1093/brain/awq131. [DOI] [PubMed] [Google Scholar]
- Majewski-Tiedeken CR, Rabin CR, Siegel SJ. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 2008;92:217–227. doi: 10.1016/j.drugalcdep.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J. Pharmacol. Exp. Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, Kegeles LS, Talbot PS, Huang Y, Hwang DR, Khenissi L, Cooper TB, Laruelle M, Abi-Dargham A. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am. J. Psychiatry. 2005;162:2352–2359. doi: 10.1176/appi.ajp.162.12.2352. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. Effects of quetiapine and sertindole on subchronic ketamine-induced deficits in attentional set-shifting in rats. Psychopharmacology (Berl.) 2012;220:65–74. doi: 10.1007/s00213-011-2487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol. Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bulletin. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale PD, Kleman MR, Benkers TL, Carlson JP, Reite ML. Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin. Neurophysiol. 2006;117:110–117. doi: 10.1016/j.clinph.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology. 2011;36:2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol. Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch. Gen. Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol. Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc. Natl. Acad. Sci. U S A. 2000;97:919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb. Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc. Natl. Acad. Sci. U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr. Bull. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkle-Bergner M, Shing YL, Muller V, Li SC, Lindenberger U. EEG gamma-band synchronization in visual coding from childhood to old age: evidence from evoked power and inter-trial phase locking. Clin. Neurophysiol. 2009;120:1291–1302. doi: 10.1016/j.clinph.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. Age-dependent differences in sensitivity and sensitization to cannabinoids and 'club drugs' in male adolescent and adult rats. Addict. Biol. 2008;13:277–286. doi: 10.1111/j.1369-1600.2007.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br. J. Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li Q, Wang D, Shi L, Lu G, Sun L, Wang L, Zhu W, Mak YT, Wong N, Wang Y, Pan F, Yew DT. Mapping the central effects of chronic ketamine administration in an adolescent primate model by functional magnetic resonance imaging (fMRI) Neurotoxicology. 2012;33:70–77. doi: 10.1016/j.neuro.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol. Sci. 2009;108:149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]