Abstract
Background
HIV-infected women face several risk factors for postpartum depression (PPD). We aimed to describe the prevalence and cumulative incidence of PPD in the low-income setting of Malawi, and to determine the association between maternal and infant HIV and PPD.
Methods
This longitudinal cohort study included 156 HIV-uninfected and 373 HIV-infected Malawian women enrolled 10-14 weeks after delivery who returned at 6, 9, 12, 15 and 18 months for follow-up visits. PPD was assessed at all visits. The prevalence of PPD at all visits was estimated using the Edinburgh Postnatal Depression Scale (EPDS). Association between PPD at 10-14 weeks and maternal and infant HIV status was assessed using log binomial regression. Cumulative incidence of PPD was assessed using Kaplan-Meier curves.
Results
Prevalence of PPD was highest (11%) at 10-14 weeks postpartum, and decreased to 2.9% at 18 months. There was no association between maternal HIV status and PPD (prevalence ratio 1.18, 95% CI 0.68, 2.08). Among HIV-infected women, prevalence of PPD was higher among women whose infants had acquired HIV (prevalence ratio 2.0 (95% CI 1.1, 3.6)). The cumulative probability of experiencing PPD over the first 12 months post-partum was estimated to be 33.5% for HIV-infected mothers with HIV-infected infants vs. 22.5% for HIV-infected mothers with uninfected infants and 23.2% for HIV-uninfected mothers.
Conclusions
PPD prevalence did not differ between HIV infected and uninfected mothers, but was increased among women with an HIV-infected infant. Our findings suggest it may be important to monitor PPD among women with HIV-infected infants.
Keywords: HIV, Postpartum Depression, Infant, Postnatal Depression
Introduction
Maternal mental health has been recognized as an important public health priority by the World Health Organization 1. One of the most vulnerable times for a mother's mental health is in the first 12 months postpartum, with minor and major depression frequently occurring after delivery, especially in the first three months postpartum 2. Symptoms of postpartum depression (PPD) include tearfulness, feelings of guilt, loss of appetite, feelings of inadequacy, fatigue and irritability 3. Identification of PPD is important as untreated postpartum depression can have negative effects on mother-infant interactions as well as consequences for the newborn child, who may experience emotional and cognitive problems later in life 4-6.
In high income settings the prevalence of PPD is estimated to be 13% 2, 7. One review of PPD included 23 studies from 10 African countries and found that the majority of studies reported estimated prevalence of PPD that were higher than those in high-income countries 9. Another review of 35 studies of pre- and postnatal psychological wellbeing in Africa estimated the prevalence of depression to be 11.3% (95% CI 9.5, 13.1) during pregnancy and 18.3% (95% CI 17.6, 19.1) during the postpartum period 8. . Similarly, a 19.8% (95% CI 19.5, 20.0) postnatal prevalence of common mental disorders was found in a review of 13 studies in low and lower-middle-income countries 10. A higher prevalence of postpartum depression of 31.3% (95% CI 21.3%, 43.5%) was also found in a review of studies of women living in the rural areas of developing countries 11.
Maternal illness, infant illness and financial stress have all been identified as factors increasing the risk of perinatal depression in low and lower-middle income countries 10. HIV-infected women in the resource-limited setting of sub-Saharan Africa are thus faced with a variety of risk factors for PPD. In addition to concern over their own health, HIV-infected women may experience anxiety over the health of their infected infant or the ongoing risk of transmission of HIV to the infant if the child is uninfected 12-15. They may also experience stress due to HIV-associated stigma or anticipation of stigma 16. Additionally, HIV-infected households often face increased levels of socioeconomic stressors due to the cost of healthcare and the impact that a chronic illness can have on the ability of family members to work 17, 18. Research on the prevalence of perinatal depression in HIV-infected women has been limited, with the majority focused on the prenatal period 12, 13, 19-30. A cross-sectional study, which found an association between common mental disorder in the postpartum period in Malawi, suggests there is value in closer examination of PPD in particular and the association with HIV 19.
We assessed the prevalence of PPD in HIV-uninfected women and in HIV-infected women (both those with HIV-infected and HIV-uninfected infants), and aimed to determine the association between HIV infection and PPD in Blantyre, Malawi.
Methods
Study population and study procedures
The data analyzed in this study arose from a community-based cohort study examining the effects of HIV on pediatric neurodevelopment. The study was conducted at two healthcare centers in the Blantyre region of Malawi between May 2008 and March 2012. Both clinics have prevention of mother-to-child transmission (PMTCT) programs. One center is an urban primary care facility that does not initiate or administer ART and refers those in need of ART to a hospital. The other center is located in a semi-rural area and serves as a primary and secondary care facility with on-site ART services.
As part of recruitment procedures for the parent study, early infant diagnosis (EID) services were established at these two healthcare facilities. At 6 weeks of age (or earliest visit thereafter), infants were tested for HIV and all HIV-exposed infants were referred for cotrimoxazole prophylaxis. Women were asked to return with their infant for HIV test results 4 weeks later. Three groups of women were enrolled for the parent study: HIV-uninfected women, HIV-infected women with uninfected infants, and HIV-infected women with infected infants. Data from the enrolment visit at 10 weeks and study visits at 14 weeks and 6, 9, 12, 15, and 18 months post-delivery were examined in the current analyses. At the 10-14 week visit, clinical, demographic and socioeconomic data were collected.
Screening for maternal postpartum depression was performed using the Edinburgh Postnatal Depression Scale (EPDS) 31, the most widely used screening questionnaire for PPD. The EPDS has been shown to have good test-retest reliabilities as well as good sensitivity for detecting major depression 32, and has been used in several studies in sub-Saharan Africa 33-35. The EPDS is a 10-item self-report questionnaire, which takes approximately 5 minutes to complete and asks the mother to rate how she has felt over the past 7 days. Each question has 4 possible answers and is scored 0-3 points, for a total score of 0-30. A cut-point of >12, as was used in this study, is recommended to detect probable depression, with a >9 cut-point to detect possible depression 31. The questionnaire was administered by the study nurses in the native language of Chichewa.
Statistical Analysis
Baseline EPDS was defined as the score at 10 weeks; the 14 week visit data were used when 10 week data were not available. Follow-up EPDS scores were collected at 6, 9, 12, 15 and 18 month visits including a window of +/- one month. EPDS data from mothers of HIV-exposed children who seroconverted during follow-up (n=21) were excluded from analysis at the time of the infant's first positive HIV test.
All statistical analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC). We calculated the prevalence of PPD at each visit overall and by mother and infant HIV status. After the 10-14 week visit, incident PPD was calculated as the proportion of women with a new PPD diagnosis at 6, 9, and 12 months following delivery, among women with previous data but no previous EPDS score greater than 12.
The cumulative probability of PPD-free survival was examined by Kaplan-Meier curves stratified by the mother and infant's HIV exposure status and included only women who had EPDS data from the 10-14 week visit. Women were censored at the time of a first missed visit. Changes in EPDS scores over time were examined by stratifying women based on the quartile of 10-14 week EPDS score.
To assess the association at 10-14 weeks postpartum between maternal HIV status and PPD and between infant HIV status among HIV-infected women and PPD, we calculated prevalence ratios (PRs) and 95% confidence intervals (CIs) using multivariable log binomial regression to account for the high (11%) frequency of the outcome 36 . Multivariable models contained the exposure (either maternal HIV or infant HIV infection), the outcome (PPD at 10-14 weeks after delivery) and covariables found to be associated with the exposure or with PPD based on bivariate analysis (p<0.20) or known from the literature to be associated with the exposure or with PPD. The following covariables were explored for associations with PPD: maternal age, maternal marital status, maternal education, whether the mother reported experiencing any problems during the pregnancy (yes or no), whether the father was living with the child, electricity in the home, mobile phone in the home, ownership of any land by the household, whether there was more than one separate room for sleeping, whether the mother perceived she had enough money to meet her needs, whether the mother reported she was hungry because she did not have enough food, infant gender, and whether there were other children in the household. We assessed the association between each pair of confounders to avoid colinearity. In the case of collinear variables (OR of ≥3), only one variable was retained based on relationships with other variables being explored. To construct the final model, we used a manual, backward elimination, change-in-estimate strategy. Potential confounders were removed from the preliminary full model in order of p-value magnitude. If the exposure-outcome association changed by less than 10% overall and in all strata of an interacting variable, a given covariable was not retained 37.
Ethics Approval
The University of Malawi College of Medicine Research and Ethics Committee and the University of North Carolina at Chapel Hill Institutional Review Board approved the study. Informed consent was obtained prior to conducting any study-related procedures.
Results
A total of 556 children were enrolled in the parent study between May 2008 and July 2011. There were 492 mothers with EPDS data available at the 10-14 week visit, 389 at 6 months, 353 at 9 months, 326 at 12 months, 287 at 15 months and 276 at 18 months. Overall 156 HIV-uninfected and 373 HIV-infected women contributed data on EPDS. At the 10-14 week visit, 338 (68.9%) women were HIV-infected and 74 (15%) infants were HIV-infected. The mean age of the mothers at the time of enrolment was 27.1 years (standard deviation 5.3). Demographic and socioeconomic characteristics were similar between HIV-infected and uninfected mothers, with the exception of lower levels of education and household land ownership and older age among HIV-infected women (Table 1). When comparing HIV-infected women with and without infected infants, women with uninfected infants were more likely to be taking antiretrovirals but were comparable in other measured characteristics.
Table 1. Demographic and socioeconomic characteristics by HIV status of the mother and infant at baseline.
| HIV-infected mothers | p value | ||||
|---|---|---|---|---|---|
|
|
|
||||
| With HIV-infected child N=74 | With HIV-uninfected, child N=264 | Mother and child HIV-uninfected N=154 | HIV-infected vs. uninfected mothers | Among HIV-infected mothers: HIV-infected vs. uninfected infants | |
| Household characteristics | |||||
| Insufficient food (past week) | 30 (40.5%) | 102 (41.1%) | 56 (38.1%) | 0.55 | 0.93 |
| Missing | 0 | 16 | 7 | ||
| Insufficient money (past week) | 51 (68.9%) | 188 (76.7%) | 114 (78.1%) | 0.46 | 0.17 |
| Missing | 0 | 19 | 8 | ||
| House has electricity | 28 (37.8%) | 102 (40.6%) | 56 (37.6%) | 0.62 | 0.67 |
| Missing | 0 | 13 | 5 | ||
| At least one mobile phone | 44 (66.7%) | 165 (67.1%) | 110 (75.3%) | 0.07 | 0.95 |
| Missing | 8 | 18 | 8 | ||
| Any land owned by household | 37 (63.8%) | 141 (65.6%) | 93 (79.5%) | 0.005 | 0.80 |
| Missing | 16 | 49 | 37 | ||
| More than one sleeping room | 49 (67.1%) | 182 (74.6%) | 109 (74.2%) | 0.77 | 0.21 |
| Missing | 1 | 20 | 7 | ||
| Father lives with child | 57 (78.1%) | 195 (78.3%) | 120 (82.2%) | 0.33 | 0.97 |
| Missing | 1 | 15 | 8 | ||
| No other children in household | 13 (19.7%) | 40 (16.2%) | 34 (23.3%) | 0.11 | 0.50 |
| Missing | 8 | 17 | 8 | ||
| Maternal characteristics | |||||
| Married or living with partner | 63 (85.1%) | 210 (83.7%) | 133 (89.3%) | 0.13 | 0.76 |
| Missing | 0 | 13 | 5 | ||
| Completed secondary education | 28 (37.8%) | 122 (48.6%) | 95 (63.8%) | 0.0004 | 0.10 |
| Missing | 0 | 13 | 5 | ||
| Currently employed | 8 (10.8%) | 41 (16.4%) | 13 (8.8%) | 0.06 | 0.24 |
| Missing | 0 | 14 | 6 | ||
| Currently on ART | 2 (2.7%) | 74 (30.7%) | n/a | - | <0.0001 |
| Missing | 0 | 23 | |||
| Hospitalized since delivering | 4 (5.5%) | 10 (4%) | 4 (2.8%) | 0.41 | 0.59 |
| Missing | 1 | 14 | 9 | ||
| Any problems during pregnancy | 11 (15.3%) | 44 (18.0%) | 17 (11.9%) | 0.13 | 0.59 |
| Missing | 2 | 20 | 11 | ||
| Mean age (standard deviation) | 27.1 (5.7) | 28.4 (5.0) | 24.9 (4.8) | <0.0001 | 0.07 |
| Missing | 0 | 11 | 5 | ||
| Infant characteristic | |||||
| Infant gender (male) | 34 (45.9%) | 134 (50.8%) | 82 (53.2%) | 0.47 | 0.46 |
| Missing | 0 | 0 | 0 | ||
| Hospitalized since birth | 9 (13%) | 11 (4.2%) | 8 (5.4%) | 0.79 | 0.02 |
| Missing | 5 | 3 | 7 | ||
Prevalent and incident PPD
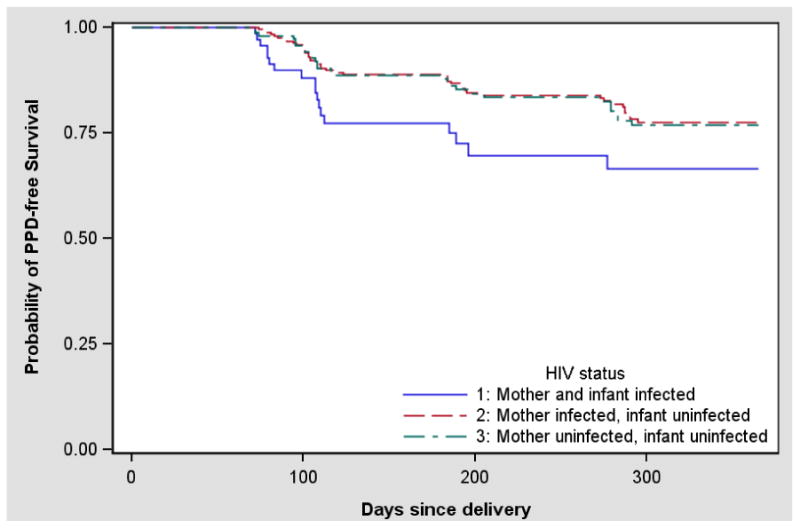
During the first 12 months following delivery, the overall prevalence of PPD ranged from 7.4% to 11%, with the highest prevalence at the visit at 10-14 weeks postpartum (Table 2). At that visit, the prevalence of PPD was higher among HIV-infected women with infected infants (18.9%) compared to HIV-infected women with uninfected infants (9.5%, p=0.03) and HIV-uninfected women (9.7%, p=0.05) (Figure 1). There was no statistically significant difference between the prevalence of PPD when considering only the mother's HIV status (p=0.55). The cumulative probability of PPD-free survival is illustrated in Figure 2, and demonstrates the higher frequency of depression among women with HIV-infected infants (Wilcoxon p=0.03). Overall, the cumulative probability of experiencing PPD over the first 12 months post-partum was estimated to be 33.5% for HIV-infected mothers with HIV-infected infants vs. 22.5% for HIV-infected mothers with uninfected infants and 23.2% for HIV-uninfected mothers.
Table 2. Prevalence of postpartum depression (PPD) by maternal and infant HIV status.
| HIV-infected mothers | |||||
|---|---|---|---|---|---|
|
|
|||||
| Time since delivery | With HIV-infected child | With HIV-uninfected child | All | HIV-uninfected mothers | Total |
| 10-14 weeks | 14/74 | 25/264 | 39/338 | 15/154 | 54/492 |
| (18.9%) | (9.5%) | (11.5%) | (9.7%) | (11.0%) | |
| 6 months | 6/51 | 17/221 | 23/272 | 7/117 | 30/389 |
| (11.8%) | (7.7%) | (8.5%) | (6.0%) | (7.7%) | |
| 9 months | 7/47 | 20/198 | 27/245 | 10/108 | 37/353 |
| (14.9%) | (10.1%) | (11.0%) | (9.3%) | (10.5%) | |
| 12 months | 2/39 | 15/185 | 17/224 | 7/102 | 24/326 |
| (5.1%) | (8.1%) | (7.6%) | (6.9%) | (7.4%) | |
| 15 months | 3/40 | 10/151 | 13/191 | 5/96 | 18/287 |
| (7.5%) | (6.6%) | (6.8%) | (5.2%) | (6.3%) | |
| 18 months | 1/29 | 3/152 | 4/181 | 4/95 | 8/276 |
| (3.4%) | (2.0%) | (2.2%) | (4.2%) | (2.9%) | |
Figure 1. Prevalence of postpartum depression (PPD) at the 10-14 week visit by HIV status of mother and infant*.
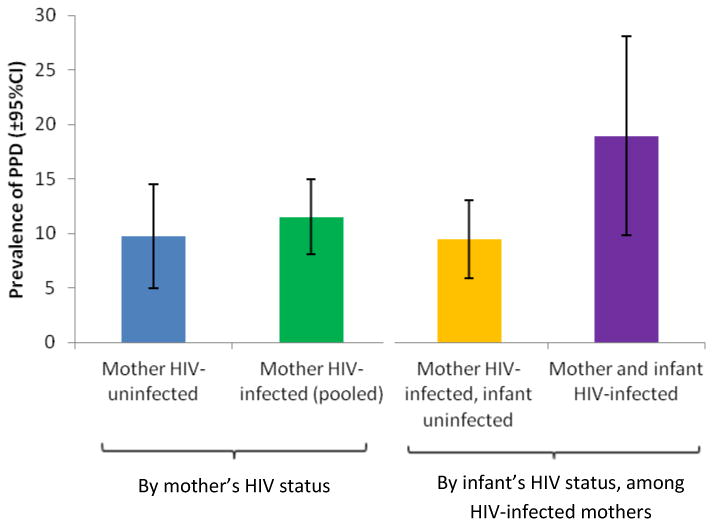
*The prevalence ratio for the association between mother's HIV status and PPD was 1.18 (95% CI 0.68, 2.08); among HIV-infected women only, the association between infant's HIV status and PPD was 2.0 (95% CI 1.1, 3.6)
Figure 2. Probability of Postpartum depression-free survival by maternal and infant HIV status.
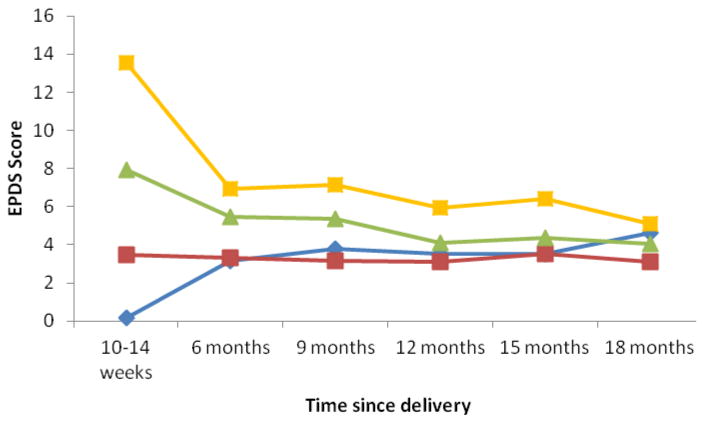
While women in the 2 highest baseline quartiles for PPD, and especially women in the highest quartile, showed substantial improvement by 6 months, regression to the mean was gradual after that (Fig 3). Incident PPD was observed in 5.6% (18/323) of previously non-depressed mothers at 6 months, in 6.4% (19/295) at 9 months, and in 2.7% (7/258) at 12 months. Meanwhile, among mothers with PPD at the 10-14 week visit, 72.2% (26/36) resolved by 6 months; among mothers with PPD at the 6 month visit, 51.9% (14/27) had resolved by 9 months; and among mothers with PPD at the 9 month visit, 73.3% (22/30) had resolved by 12 months. Among 41 women with PPD at the 10-14 week visit and at least one follow-up in the first year, 46.3% (19/41) experienced PPD at at least one additional visit.
Figure 3. EPDS score over 18 months of follow-up by quartile of baseline EPDS.
Association between maternal HIV, infant HIV and PPD
At the 10-14 week visit the prevalence ratio (PR) for the association between maternal HIV and PPD was 1.18 (95% CI 0.68, 2.08). None of the covariates explored (mother's employment status, insufficient money, mothers' education, electricity in the household, mother's marital status, mother's age, any problems during the current pregnancy and the child's gender) met the pre-specified criteria for confounding. The crude prevalence ratio for the association of infant HIV infection status and PPD among HIV-infected mothers was 2.0 (95% CI 1.1, 3.6). Due to the small number of women taking antiretrovirals in the HIV-infected infant group, maternal antiretroviral status could not be included in the multivariate model, but PPD was not associated with maternal antiretroviral status among HIV-infected women with uninfected infants (PR=1.05, 95% CI 0.45, 2.48).
Discussion
Overall, we found a prevalence of PPD of 11% at 10-14 weeks postpartum among HIV-infected and HIV-uninfected women found, which is at the lower end of the range of PPD reported for low-income countries 8, 10. PPD prevalence was not different between HIV infected and uninfected mothers. Three smaller studies examining the association between maternal HIV infection and mental health in the postpartum period in Africa have reported conflicting results 19-21. A cross-sectional study in Malawi of 314 women assessed at a median of 10 months postpartum found an increased risk of common mental disorders in HIV infected women (beta 1.29, p=0.046). In contrast, a cohort study assessed 272 Zambian women and found that HIV was not associated with mental morbidity in the first 16 weeks postpartum. A cross-sectional study of 179 women in Zimbabwe assessed at 6 weeks postpartum estimated an almost two-fold increase in PPD, but the difference was not statistically significant (aOR 1.95, 95% CI 0.76, 4.99). Only the study in Zimbabwe specifically investigated PPD, and infant HIV status was not known in any of these studies.
An important strength of our study was the cohort design with accurate monitoring of infant HIV status between age 6 weeks and 18 months. Among HIV-infected women, having an HIV-infected infant was associated with a two-fold increase in PPD (PR 2.0, 95% CI 1.1, 3.6) compared to HIV infected women whose infant was free of HIV infection. This finding is in line with other studies that suggested that the risk for PPD may be more associated with illness of the mother and infant rather than solely the mother's HIV status 19. In this study, 10-14 weeks was the time at which the mother was informed of the infant's HIV test results. Even though the EPDS asks about feelings over the past week, it is possible that the women's feelings about the infant's HIV status influenced the responses they gave on the EPDS. It is therefore possible that the higher prevalence of PPD represents a reaction to the news of the infant's status. There is also evidence that infant illness is associated with higher prevalence of PPD 38, 39, and we found a higher proportion of HIV-infected infants had been hospitalized since birth. Illness of the child and reaction to the child's diagnosis could thus be contributing to higher prevalence of PPD among women with HIV-infected children. Alternatively, the higher prevalence of PPD observed among women with HIV-infected infants could also have been due to depression that started before or during pregnancy, a known risk factor for PPD 40. Depression before or during the pregnancy could influence the mother's uptake of antiretroviral treatment or adherence to PMTCT protocols during pregnancy and after the birth of the child. Likewise, there may be sociodemographic and clinical risk factors that increase a woman's susceptibility to depression in the perinatal period and a child's risk of HIV acquisition. These issues warrant further examination.
The fact that approximately 6% of women presented with new PPD at 6 and 9 months suggests that it may be important to continue to monitor women for PPD over the first year following delivery. While most common in the first 3 months postpartum, some women may not exhibit signs of PPD until later in the first year 2, particularly in the context of chronic stressors and inadequate support 41. Repeated screenings to detect PPD may be particularly valuable given that depression has been associated with progression of HIV in the general population 42, 43. However, special attention must be paid to the sensitivity and specificity of the screening tool as well as to the costs and consequences of managing false positives and false negatives 44-47.
In this study there was no intervention offered for women exhibiting signs of PPD. Although women may have sought out help for their depression, Malawi has limited mental health resources 48 and it is unlikely that any formal treatment was provided to the majority of women suffering from PPD. The fact that 46% of women with PPD at the 10-14 week visit exhibited PPD at a later visit suggests there may be a need for PPD resources for women exhibiting PPD, especially among women whose infant is diagnosed with HIV infection.
While the relatively large sample size, longitudinal design, good retention and accurate knowledge of infant HIV status are important strengths, our study has important limitations. We did not have access to information about the mother's mental health history or her feelings about the pregnancy, factors associated with PPD in some studies 34, 35, 38, 49, 50. We did not administer the EPDS before the mother was informed of the infant's HIV status. The EPDS is only a screening tool, not a clinical diagnosis of depression; however, the instrument has been widely validated and applied 9. It is also possible that women experiencing PPD may have been less likely to enroll in the study which may have resulted in underestimates of PPD prevalence. Finally, infant death, which was experienced by 40 women in this study, led to discontinuation in the parent study. Consequently, we did not have follow-up information after the child's death, a time during which these women may have been at high risk of developing PPD. Our estimate of incident PPD may therefore have been underestimated.
In conclusion, in this cohort of Malawian women we found that HIV-infection of the child, but not of the mother, was associated with a two-fold increase in prevalence of PPD at 10-14 weeks postpartum. Future studies assessing the continuum of depression during and following pregnancy as well as specifically examining PPD before and after a mother learns of her child's HIV test results will help to further elucidate the relationship between infant HIV infection and maternal PPD. A qualitative study among women with HIV-infected children could also provide valuable insight. Meanwhile it may be important to examine the feasibility and clinical value of routinely screen women whose infant is diagnosed with HIV and to consider including care and support for PPD as part of their HIV care and treatment package.
Acknowledgments
Anna Dow and Annelies Van Rie contributed to the study design, analysis and manuscript development. Queen Dube contributed to study design and manuscript development. Brian Pence contributed to analysis and interpretation of the data and manuscript development. All authors approved the final draft of the manuscript. The authors would like to thank Chawanangwa Chirambo and the CHIDEV clinical officers and nurses in Blantyre, the staff at the Malawi-Liverpool-Wellcome Trust, and Elizabeth Cromwell and Jill Lebov at UNC. We would also like to thank all of the participating women and children, who made this study possible. Research supported by the Fogarty International Center and NICHD under Award Number R01HD053216. MLW is supported by a core grant from the Wellcome Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sources of Funding: Research supported by the Fogarty International Center and NICHD under Award Number R01HD053216. The Malawi-Liverpool-Wellcome Trust is supported by a core grant from the Wellcome Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.World Health Organization. Improving Maternal Mental Health. Geneva: 2008. Available at http://www.who.int/mental_health/prevention/suicide/Perinatal_depression_mmh_final.pdf. [Google Scholar]
- 2.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 3.Robinson GE, Stewart DE. Postpartum disorders. In: Stotland NL, Stewart DE, editors. Psychological aspects of women's health care. 2nd. Washington, DC: American Psychiatric Press, Inc.; 2001. [Google Scholar]
- 4.Beck CT. The effects of postpartum depression on maternal-infant interaction: a meta-analysis. Nurs Res. 1995;44:298–304. [PubMed] [Google Scholar]
- 5.Murray L, Stein A. The effects of postnatal depression on the infant. Baillieres Clin Obstet Gynaecol. 1989;3:921–933. doi: 10.1016/s0950-3552(89)80072-0. [DOI] [PubMed] [Google Scholar]
- 6.Stein A, Gath DH, Bucher J, Bond A, Day A, Cooper PJ. The relationship between postnatal depression and mother-child interaction. Br J Psychiatry. 1991;158:46–52. doi: 10.1192/bjp.158.1.46. [DOI] [PubMed] [Google Scholar]
- 7.O'Hara MW, Swain AM. Rates and risk of postpartum depression- a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- 8.Sawyer A, Ayers S, Smith H. Pre- and postnatal psychological wellbeing in Africa: a systematic review. J Affect Disord. 2010;123:17–29. doi: 10.1016/j.jad.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Parsons CE, Young KS, Rochat TJ, Kringelbach ML, Stein A. Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. Br Med Bull. 2011;101:57–79. doi: 10.1093/bmb/ldr047. [DOI] [PubMed] [Google Scholar]
- 10.Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012;90:139G–149G. doi: 10.2471/BLT.11.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villegas L, McKay K, Dennis CL, Ross LE. Postpartum depression among rural women from developed and developing countries: a systematic review. J Rural Health. 2010;27:278–288. doi: 10.1111/j.1748-0361.2010.00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Kwalombota M. The effect of pregnancy in HIV-infected women. AIDS Care. 2002;14:431–433. doi: 10.1080/09540120220123829. [DOI] [PubMed] [Google Scholar]
- 13.Bennetts A, Shaffer N, Manopaiboon C, et al. Determinants of depression and HIV-related worry among HIV-positive women who have recently given birth, Bangkok, Thailand. Soc Sci Med. 1999;49:737–749. doi: 10.1016/s0277-9536(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 14.Koricho AT, Moland KM, Blystad A. Poisonous milk and sinful mothers: the changing meaning of breastfeeding in the wake of the HIV epidemic in Addis Ababa, Ethiopia. Int Breastfeed J. 2010;5:12. doi: 10.1186/1746-4358-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarus R, Struthers H, Violari A. Hopes, fears, knowledge and misunderstandings: responses of HIV-positive mothers to early knowledge of the status of their baby. AIDS Care. 2009;21:329–334. doi: 10.1080/09540120802183503. [DOI] [PubMed] [Google Scholar]
- 16.Cuca YP, Onono M, Bukusi E, Turan JM. Factors associated with pregnant women's anticipations and experiences of HIV-related stigma in rural Kenya. AIDS Care. 2012;24:1173–1180. doi: 10.1080/09540121.2012.699669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauliere A, Toure S, Alexandre PK, et al. The financial burden of morbidity in HIV-infected adults on antiretroviral therapy in Cote d'Ivoire. PLoS One. 2010;5:e11213. doi: 10.1371/journal.pone.0011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71:147–155. [PubMed] [Google Scholar]
- 19.Stewart RC, Bunn J, Vokhiwa M, et al. Common mental disorder and associated factors amongst women with young infants in rural Malawi. Soc Psychiatry Psychiatr Epidemiol. 2010;45:551–559. doi: 10.1007/s00127-009-0094-5. [DOI] [PubMed] [Google Scholar]
- 20.Chibanda D, Mangezi W, Tshimanga M, et al. Postnatal depression by HIV status among women in Zimbabwe. J Womens Health (Larchmt) 2010;19:2071–2077. doi: 10.1089/jwh.2010.2012. [DOI] [PubMed] [Google Scholar]
- 21.Collin SM, Chisenga MM, Kasonka L, et al. Factors associated with postpartum physical and mental morbidity among women with known HIV status in Lusaka, Zambia. AIDS Care. 2006;18:812–820. doi: 10.1080/09540120500465061. [DOI] [PubMed] [Google Scholar]
- 22.Stranix-Chibanda L, Chibanda D, Chingono A, et al. Screening for psychological morbidity in HIV-infected and HIV-uninfected pregnant women using community counselors in Zimbabwe. J Int Assoc Physicians AIDS Care (Chic) 2005;4:83–88. doi: 10.1177/1545109706286555. [DOI] [PubMed] [Google Scholar]
- 23.Ross R, Sawatphanit W, Zeller R. Depressive symptoms among HIV-positive pregnant women in Thailand. J Nurs Scholarsh. 2009;41:344–350. doi: 10.1111/j.1547-5069.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 24.Bernatsky S, Souza R, de Jong K. Mental health in HIV-positive pregnant women: results from Angola. AIDS Care. 2007;19:674–676. doi: 10.1080/09540120601012705. [DOI] [PubMed] [Google Scholar]
- 25.Blaney NT, Fernandez MI, Ethier KA, Wilson TE, Walter E, Koenig LJ. Psychosocial and behavioral correlates of depression among HIV-infected pregnant women. AIDS Patient Care STDS. 2004;18:405–415. doi: 10.1089/1087291041518201. [DOI] [PubMed] [Google Scholar]
- 26.Rubin LH, Cook JA, Grey DD, et al. Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. J Womens Health (Larchmt) 2011;20:1287–1295. doi: 10.1089/jwh.2010.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapetanovic S, Christensen S, Karim R, et al. Correlates of perinatal depression in HIV-infected women. AIDS Patient Care STDS. 2009;23:101–108. doi: 10.1089/apc.2008.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rochat TJ, Richter LM, Doll HA, Buthelezi NP, Tomkins A, Stein A. Depression among pregnant rural South African women undergoing HIV testing. JAMA. 2006;295:1376–1378. doi: 10.1001/jama.295.12.1376. [DOI] [PubMed] [Google Scholar]
- 29.Cyimana A, Andrews B, Ahmed Y, Vwalika B. HIV/AIDS and Postnatal Depression at the University Teaching Hospital, Lusaka, Zambia. Med J Zambia. 2010;37:78–83. [PMC free article] [PubMed] [Google Scholar]
- 30.Manikkam L, Burns JK. Antenatal depression and its risk factors: an urban prevalence study in KwaZulu-Natal. S Afr Med J. 2012;102:940–944. doi: 10.7196/samj.6009. [DOI] [PubMed] [Google Scholar]
- 31.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 32.Boyd RC, Le HN, Somberg R. Review of screening instruments for postpartum depression. Arch Womens Ment Health. 2005;8:141–153. doi: 10.1007/s00737-005-0096-6. [DOI] [PubMed] [Google Scholar]
- 33.Abiodun OA. Postnatal depression in primary care populations in Nigeria. Gen Hosp Psychiatry. 2006;28:133–136. doi: 10.1016/j.genhosppsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Adewuya AO. Early postpartum mood as a risk factor for postnatal depression in Nigerian women. Am J Psychiatry. 2006;163:1435–1437. doi: 10.1176/ajp.2006.163.8.1435. [DOI] [PubMed] [Google Scholar]
- 35.Owoeye AO, Aina OF, Morakinyo O. Risk factors of postpartum depression and EPDS scores in a group of Nigerian women. Trop Doct. 2006;36:100–103. doi: 10.1258/004947506776593341. [DOI] [PubMed] [Google Scholar]
- 36.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 38.Alami KM, Kadri N, Berrada S. Prevalence and psychosocial correlates of depressed mood during pregnancy and after childbirth in a Moroccan sample. Arch Womens Ment Health. 2006;9:343–346. doi: 10.1007/s00737-006-0154-8. [DOI] [PubMed] [Google Scholar]
- 39.Nakku JE, Nakasi G, Mirembe F. Postpartum major depression at six weeks in primary health care: prevalence and associated factors. Afr Health Sci. 2006;6:207–214. doi: 10.5555/afhs.2006.6.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–157. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Seguin L, Potvin L, St-Denis M, Loiselle J. Depressive symptoms in the late postpartum among low socioeconomic status women. Birth. 1999;26:157–163. doi: 10.1046/j.1523-536x.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 42.Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44:470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 44.Hewitt CE, Gilbody SM. Is it clinically and cost effective to screen for postnatal depression: a systematic review of controlled clinical trials and economic evidence. BJOG. 2009;116:1019–1027. doi: 10.1111/j.1471-0528.2009.02148.x. [DOI] [PubMed] [Google Scholar]
- 45.Kagee A, Tsai AC, Lund C, Tomlinson M. Screening for common mental disorders in low resource settings: reasons for caution and a way forward. Int Health. 2013;5:11–14. doi: 10.1093/inthealth/ihs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009;339:b5203. doi: 10.1136/bmj.b5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rochat TJ, Tomlinson M, Newell ML, Stein A. Detection of antenatal depression in rural HIV-affected populations with short and ultrashort versions of the Edinburgh Postnatal Depression Scale (EPDS) Arch Womens Ment Health. 2013 doi: 10.1007/s00737-013-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob KS, Sharan P, Mirza I, et al. Mental health systems in countries: where are we now? Lancet. 2007;370:1061–1077. doi: 10.1016/S0140-6736(07)61241-0. [DOI] [PubMed] [Google Scholar]
- 49.Gausia K, Fisher C, Ali M, Oosthuizen J. Magnitude and contributory factors of postnatal depression: a community-based cohort study from a rural subdistrict of Bangladesh. Psychol Med. 2009;39:999–1007. doi: 10.1017/S0033291708004455. [DOI] [PubMed] [Google Scholar]
- 50.Hanlon C, Medhin G, Alem A, et al. Impact of antenatal common mental disorders upon perinatal outcomes in Ethiopia: the P-MaMiE population-based cohort study. Trop Med Int Health. 2009;14:156–166. doi: 10.1111/j.1365-3156.2008.02198.x. [DOI] [PubMed] [Google Scholar]


