Abstract
Senescence-associated β-galactosidase (SA-β-gal) activity is widely used as a marker of cellular senescence and as an indicator of organismal aging. Here we report that SA-β-gal activity is present in the visceral endoderm layer of early post-implantation mouse embryos in predictable patterns that vary as the embryo progresses in development. However, determination of the mitotic index and analysis of the expression of Cdkn1a (p21), a marker of senescent cells, do not indicate cellular senescence. Instead, analysis of embryos in culture revealed the presence of SA-βgal activity in apical vacuoles of visceral endoderm cells likely a reflection of acidic β-galactosidase function in these organelles. This feature serves as a practical marker of the dynamics of the visceral endoderm that can be applied to developmental as well as functional studies of early mammalian embryos.
Keywords: Mouse, embryo, SA-β-gal, yolk sac, apical vacuoles
INTRODUCTION
Normal somatic cells do not indefinitely proliferate in culture. At the end of their limited replicative lifespan, they enter a permanent growth arrest of cell division by a process termed cellular senescence (Campisi, 2007; Hayflick and Moorhead, 1961). Some characteristics of senescent cells include an increase in cell size (Bowman et al., 1975; Meek et al., 1977; Schneider et al., 1977; Simons, 1967), growth arrest typical at G1-phase (Di Leonardo et al., 1994; Herbig et al., 2004; Ogryzko et al., 1996; Serrano et al., 1997) and apoptosis resistance (Campisi and d’Adda di Fagagna, 2007). Senescent cells also show altered gene expression, including increased expression of the Cdk inhibitor proteins p21 and p16 (Braig and Schmitt, 2006; Campisi, 2001), which function in parallel signaling pathways to maintain senescence growth arrest (Ben-Porath and Weinberg, 2005; Espinosa et al., 2003; Jackson and Pereira-Smith, 2006). The first and also the most extensively used biomarker of senescent cells is senescence-associated β-galactosidase (SA-β-gal) staining (Campisi and d’Adda di Fagagna, 2007; Dimri et al., 1995; Lee et al., 2006). SA-β-gal activity is typically measured by in situ staining under acidic conditions using a chromogenic substrate.
During an experiment designed to ectopically induce senescence in transgenic mouse embryos, we noticed that wild-type control embryos assayed for SA-β-gal activity developed staining in the visceral endoderm, an extra-embryonic component of the developing conceptus. This observation prompted us to expand these studies and explore the pattern of acidic β-gal activity in early post-implantation embryos.
A systematic analysis of embryos dissected at stages spanning embryonic days 5.5 and 9.5 (E5.5–E9.5) revealed that SA-β-gal activity marks the visceral endoderm in predictable patterns that vary as the embryo progresses in development. This activity was first observed in the whole visceral endoderm layer of embryos at E5.5, approximately one day before the appearance of the primitive streak. After that, it was gradually restricted to extra-embryonic regions of the conceptus and by primitive streak stages it marked the extra-embryonic and posterior visceral endoderm. Later, at gastrulation stages and during early organogenesis, SA-β-gal activity was detectable solely in the visceral endoderm component of the visceral yolk sac.
Determination of the mitotic index of visceral endoderm cells using phospho-Histone H3 immunostaining and analysis of the expression of Cdkn1a (p21) did not reveal evidence of senescence in visceral endoderm cells. Instead they showed that visceral endoderm cells are actively proliferating. Moreover, we detected expression of Cdkn1a in the primitive streak, a region of high cellular proliferation. Analysis of embryos co-cultured with rhodamine dextran to mark endocytotic vesicles in combination with fluorescent SA-β-gal staining, revealed the presence of SA-β-gal activity in apical vacuoles an organelle that has lysosomal activity.
From these studies, we conclude that the SA-β-gal activity observed in visceral endoderm cells is not related to senescence but likely represents acidic β-galactosidase activity present in apical vacuoles and associated with the nutritional function of visceral endoderm at early post-implantation stages.
RESULTS
SA-β-gal staining marks the visceral endoderm
To characterize the extent of SA-β-gal activity in early post-implantation mouse embryos, we conducted β-galactosidase assays at pH 6.0 in embryos dissected between E5.5 and E9.5.
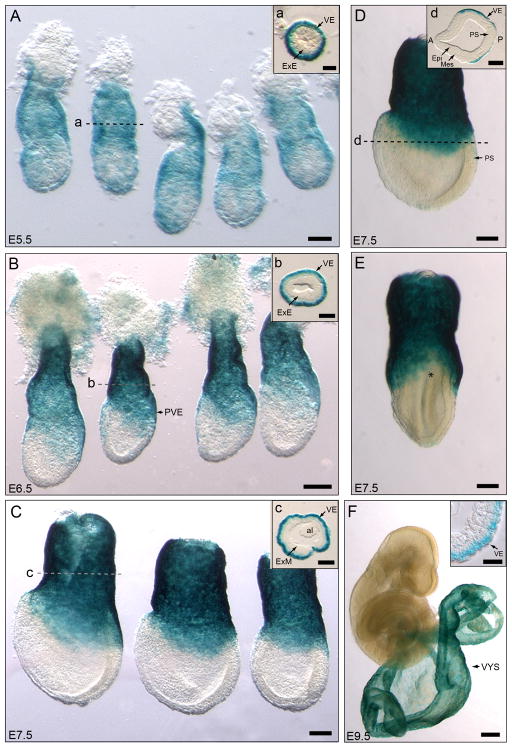
DVE stage embryos dissected at E5.5 (n=10) showed widespread visceral endoderm staining that included both the embryonic and the extra-embryonic visceral endoderm (Fig. 1A). Acidic β-gal staining was gradually restricted to the extra-embryonic visceral endoderm as the embryo progressed in development. At E6.5, coincident with the appearance of the primitive streak, acidic β-galactosidase staining marked only the extra-embryonic and posterior visceral endoderm with no labeled cells detected overlaying the rest of the epiblast region (n= 25)(Fig. 1B). The staining in the posterior visceral endoderm region covered about one third to one half of the length of the epiblast and tapered anteriorly around the circumference of the embryo along the epiblast/extra-embryonic ectoderm boundary. The epiblast and extra-embryonic ectoderm remained clear of staining (Fig. 1B).
Figure 1. SA-β-gal activity marks the visceral endoderm and yolk sac of early post-implantation mouse embryos.
A–E. Pattern of SA-β-gal staining in embryos dissected between E5.5 and E7.5. A. SA-β-gal staining marks the whole visceral endoderm in E5.5 embryos. B. At E6.5, the staining is restricted to the extra-embryonic and posterior visceral endoderm. C. In E7.5 embryos SA-β-gal activity is confined to the extra-embryonic and lateral visceral endoderm. D, E. Side (D) and posterior (E) views of the same E7.5 embryo illustrate the lack of staining in the anterior and posterior sides (marked by asterisk in E) of the embryo. Histological sections in A–C show that only the visceral endoderm layer is labeled. Section in D shows that the lateral wings of visceral endoderm are labeled but not the anterior or posterior visceral endoderm. The dotted lines represent the approximate level of the sections shown. F. SA-β-gal positive cells are restricted to the visceral endoderm layer of the visceral yolk sac in E9.5 embryos. The insert shows a section through the visceral yolk sac showing the apically labeled visceral endoderm epithelium on the outer layer and the non-labeled derivatives of the extra-embryonic mesoderm in the inner side. Abbreviations: VE, visceral endoderm; ExE, extra-embryonic ectoderm; PVE, posterior visceral endoderm, ExM, extra-embryonic mesoderm; al, allantois; A, anterior; P, posterior; Epi, epiblast; Mes, mesoderm, PS, primitive streak; VYS, visceral yolk sac. Scale bars: 50 μm in A; 25 μm in a and in insert f; 100 μm in B, b, C, c, D, d and E; 250 μm in F.
At E7.5, acidic β-galactosidase-positive visceral endoderm cells were confined to the extra-embryonic region (n= 32) (Fig. 1C–E). The area of staining extended over the sides of the embryo but was excluded from the anterior and posterior regions of the embryo (Fig. 1D, E). At later stages, acidic β-galactosidase staining was restricted to the visceral endoderm layer of the yolk sac in embryos dissected at E8.5 (n= 5, not shown) and E9.5 (n= 8) (Fig. 1F).
In summary, SA-β-gal activity initially marks the whole visceral endoderm of early post-implantation embryos but as development progresses it becomes restricted to the extra-embryonic and posterior visceral endoderm and later is limited to the visceral endoderm layer of the visceral yolk sac.
Visceral endoderm cells are not senescent cells
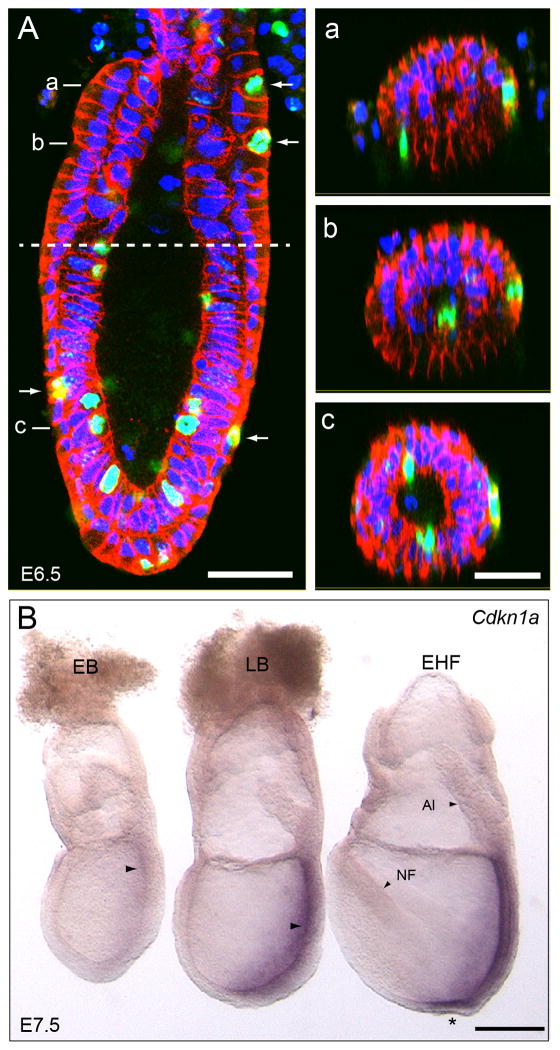
The presence of SA-β-gal staining in visceral endoderm cells prompted us to investigate if these cells showed other hallmarks of senescent cells. Senescent cells cease to proliferate and arrest in G1 (Di Leonardo et al., 1994; Herbig et al., 2004; Ogryzko et al., 1996; Serrano et al., 1997), therefore, we assessed the proliferative status of visceral endoderm cells by determining their mitotic index using anti phospho Histone H3 (pHH3) antibodies (Wei et al., 1999). We immunostained embryos dissected at E6.5 and counted the cells in mitosis in extra-embryonic and embryonic regions of the conceptus (Fig. 2A). Mitotic cells were distributed randomly in the visceral endoderm. Our results revealed a mitotic index of 2.06% in the extra-embryonic and 3.05% in the embryonic visceral endoderm regions. These results indicate that visceral endoderm cells are actively cycling instead of the expected G1 arrest characteristic of senescent cells.
Figure 2. Detection of cells in mitosis and analysis of Cdkn1a (p21) expression.
A. Sagital confocal section of an E6.5 embryo showing visceral endoderm cells in mitosis (arrows) labeled with an anti-phospho-Histone H3 antibody (green). Cell-cell boundaries are marked by E-cadherin (red) and nuclei with DAPI (blue). The boundary between the embryonic and extra-embryonic regions of the conceptus is marked by a dashed line. Panels a-c show transverse confocal sections of the embryo shown in A. The approximate location of the sections is indicated in panel A. B. Analysis of the expression of Cdkn1a in embryos dissected at E7.5. Cdk1a is expressed in the primitive streak (arrows) and epiblast component of the node (marked by an asterisk) but not in the extra-embryonic or posterior visceral endoderm. Abbreviations: EB, Early allantoic bud stage; LB, Late allantoic bud stage; EHF, Early Headfold stage; NF, neural fold; Al, Allantois. Scale Bars: 50 μm in A and c and 200 μm in B. Panels a-c are shown at the same scale.
We also analyzed the expression of Cdkn1a (p21), a common marker of senescent cells. P21 acts as a negative regulator of cell cycle progression by binding and inhibiting complexes formed between cyclin E and Cdk2. To determine if the expression of p21 coincided with the pattern of SA-β-gal activity observed in the visceral endoderm, we conducted wholemount in situ expression analyses of Cdkn1a, in E6.5 and E7.5 embryos.
At E6.5, we were not able to detect expression of Cdkn1a in any tissue of the conceptus (n= 5, not shown). At E7.5 (n=7), staining was weakly apparent in the primitive streak region of embryos at early bud stages (Fig. 2B). At a slightly later stage (late bud stage), the staining was clearly discernible along the entire length of the primitive streak region. This was also the case of embryos at early and late head fold stages. At these later stages, Cdkn1a expression was also evident in the epiblast layer of the node (Fig. 2B). In neither case, however, we observed Cdkn1a expression in the visceral endoderm layer of the conceptus.
The presence of cycling cells and the absence of Cdkn1a expression in visceral endoderm suggest that the SA-β-gal activity observed in early post-implantation embryos is not indicative of cellular senescence.
SA-β-gal activity marks apical vacuoles of visceral endoderm cells
The presence of SA-β-gal activity in visceral endoderm cells but absence of other senescence markers prompted us to investigate the origin of SA-β-gal staining in our embryos. Previous reports have suggested that the SA-β-gal assay reflects high lysosomal activity in senescence cells (Kurz et al., 2000). In addition, using human fibroblasts deficient for GLB1, the gene coding for lysosomal β-galactosidase, Lee and co-workers demonstrated that SA-β-gal staining reflects the activity of lysosomal β-galactosidase (Lee et al., 2006). Since the mouse visceral endoderm contains large apical vacuoles that exhibit lysosomal characteristics (Kawamura et al., 2012), we sought to determine if the SA-β-gal staining observed in visceral endoderm cells corresponded to acidic β-galactosidase activity present in apical vacuoles.
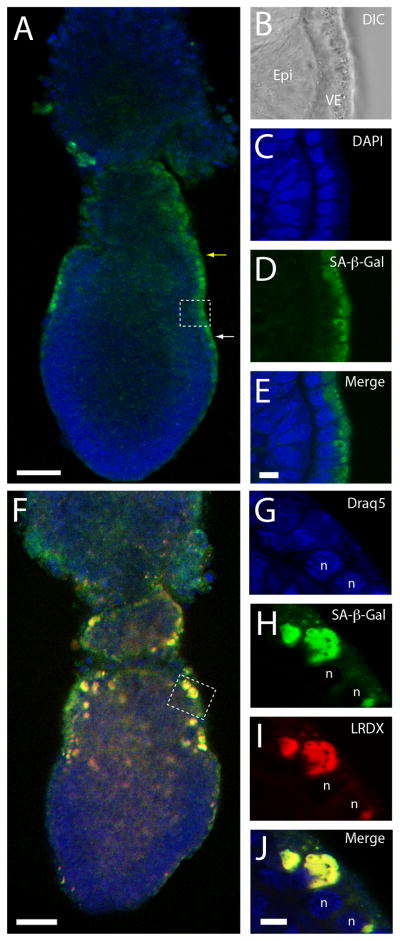
To determine if the observed SA-β-gal activity corresponded to acidic β-galactosidase present in apical vacuoles, we marked apical vacuoles in cultured E6.5 embryos and assayed the labeled embryos for SA-β-gal activity. To detect SA-β-gal activity, we adapted a protocol that utilizes the fluorescent β-galactosidase substrate (C12FDG), to detect SA-β-gal activity in live cells (Debacq-Chainiaux et al., 2009) for use in living mouse embryos. Using this protocol we were able to confirm the results obtained using the traditional SA-β-gal staining protocol. As expected, staining was restricted to extra-embryonic and posterior visceral endoderm in E6.5 embryos (Fig. 3A) and to the apical surface of visceral endoderm cells (Fig. 3B–E).
Figure 3. SA-β-gal activity marks apical vacuoles of visceral endoderm cells.
A. Detection of SA-β-gal activity in E6.5 embryos using C12FDG, a fluorescent β-galactosidase substrate. SA-β-gal staining (green) labels the extra-embryonic (yellow arrow) and posterior (white arrow) visceral endoderm. B–E. DIC (B) and confocal section (C–E) images of the area marked with a square in A. SA-β-gal activity is present in the apical surface of the visceral endoderm layer. F–J. Detection of SA-β-gal activity in apical vacuoles of visceral endoderm cells. F. E6.5 embryo assayed for SA-β-gal activity and incubated in lysinated Rhodamine-dextran (LRDX) to label apical vacuoles. G–J. Confocal sections of the region marked with a square in F showing nuclei marked with Draq5 (G), SA-β-gal activity using C12FDG (H), apical vacuoles marked with LRDX (I) and a merge of the three labels (J). Abbreviations: Epi, epiblast; VE, visceral endoderm; n, nucleus. Scale bars: 50 μm in A and F and 10 μm in E and J. Sections B–E and G–H are shown at the same scale.
To confirm the presence of SA-β-gal staining in apical vacuoles, we conducted a functional assay to mark apical vacuoles in E6.5 embryos and then assayed the embryos for fluorescent SA-β-gal activity. To label apical vacuoles, we incubated embryos in lysinated rhodamine dextran (LRDX) in culture media for 15 minutes (Kawamura et al., 2012). After the period of incubation the embryos were chased for a period of 15 minutes to allow transport of LRDX from endosomes to apical vacuoles. After this incubation period, the embryos were assayed for fluorescence SA-β-gal activity and imaged using confocal microscopy. Our results showed co-localization of green fluorescence produced by the fluorescent SA-β-gal assay and red fluorescence provided by LRDX in the apical vacuoles (Fig. 3F–J and Supp. Fig. 1).
These results indicate that the observed SA-β-gal activity in the visceral endoderm correspond to acidic β-gal activity present in the apical vacuoles.
DISCUSSION
Since first described in 1995 (Dimri et al., 1995), the SA-β-gal assay has been widely used as a biomarker of senescent cells and recently has been shown to be a developmental mechanism of mid-gestation mouse embryos (Munoz-Espin et al., 2013; Storer et al., 2013). Our studies, however, reveal that SA-β-gal staining marks the visceral endoderm of early post-implantation embryos, a non-senescent cell population. Determination of the mitotic index showed that visceral endoderm cells are actively cycling, confirming previous studies (Stuckey et al., 2011). In addition, analysis of the expression of the senescence marker p21 did not reveal expression in the visceral endoderm but rather on the highly proliferative primitive streak. Previous studies have shown that SA-β-gal staining can be encountered in non-senescent situations. For example, a small number of β-galactosidase positive cells at pH 6.0 were found in growing 3T3 fibroblast cultures (Yegorov et al., 1998) and acidic β-galactosidase staining was found in the glomerular basement membrane of kidney cells (Melk et al., 2003).
It has been suggested that senescence-associated β-galactosidase activity is derived from lysosomal β-galactosidase that reflects increased lysosomal biogenesis in senescent cells (Campisi and d’Adda di Fagagna, 2007; Kurz et al., 2000; Lee et al., 2006). We showed that the SA-β-gal activity was present in the apical vacuoles of visceral endoderm cells, an organelle with lysosomal activity (Kawamura et al., 2012). Therefore we believe that the observed SA-β-gal activity corresponds to acidic β-gal activity present in these organelles.
The visceral endoderm has a nutritional role at post-implantation stages before the establishment of the maternal-embryo circulation through the placenta (Zohn and Sarkar, 2010). A recent study has shown that the visceral endoderm endocytoses nutrients by a process of microautophagy in which endosomes are engulfed by large apical vacuoles (Kawamura et al., 2012). Hence, the high acidic β-galactosidase activity of visceral endoderm cells likely reflects the trophic activity of the visceral endoderm mediated by the apical vacuoles and lysosomes. Interestingly, acidic β-gal activity may be a characteristic of tissues involved in nutrient absorption since it has been found in intestinal cells (Going et al., 2002; Liu et al., 2007).
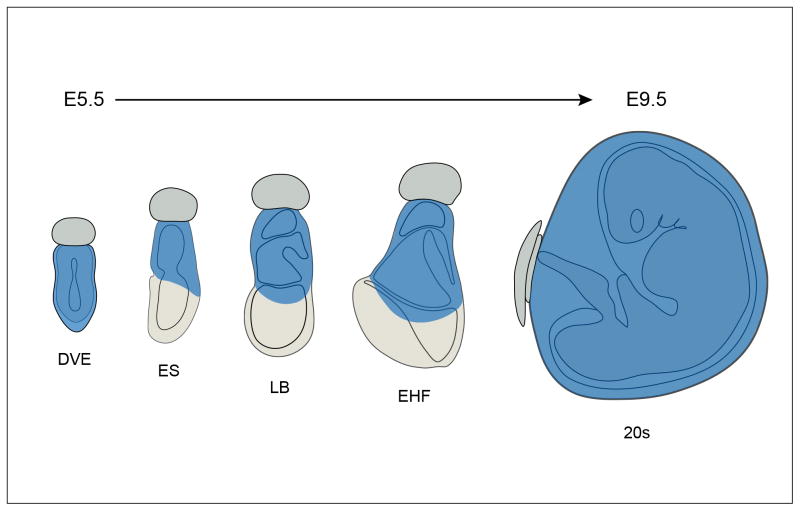
Our results show that SA-β-gal activity marks the visceral endoderm of early post-implantation embryos, in predictable patterns, initially in the whole visceral endoderm at E5.5, then in the extra-embryonic and posterior visceral endoderm as the embryo progresses towards gastrulation and later in the visceral yolk sac as the embryo proceeds through the initial stages of organogenesis (see Fig. 4). This stereotyped pattern of staining resembles the pattern of expression of several markers of the visceral endoderm such as transthyretin, (also present in the liver), and alphafetoprotein, the embryonic equivalent of albumin, a prototype liver marker. (Kwon and Hadjantonakis, 2009; Viotti et al., 2011). These characteristics coupled with previous notions that the visceral endoderm mimics the function of the fetal large intestine and liver (Meehan et al., 1984) reinforce the view that SA-β-gal staining reflects the nutritional role of the visceral endoderm and suggest a shift of the source of embryo nutrition from the whole visceral endoderm layer to the extra-embryonic visceral endoderm and then to the visceral yolk sac as the embryo progresses in development.
Figure 4. Schematic representation of SA-β-gal staining between E5.5 and E9.5.
SA-β-gal staining (shown in blue) marks the entire visceral endoderm at DVE stage (~E5.5) but it is gradually restricted to the extra-embryonic and posterior visceral endoderm by primitive streak stages (~E6.5). At gastrulation stages (~E7.5) SA-β-gal staining becomes restricted to the extra-embryonic portion of the conceptus, and eventually, it is solely confined to the visceral yolk sac (E9.5). Abbreviations: DVE, Distal Visceral endoderm stage; ES, early streak stage; LB, Late allantoic bud stage; EHF, Early head folds stage; 20s, 20 somite stage.
Kwon and co-workers reported that descendants of the embryonic visceral endoderm are incorporated into the definitive endoderm layer of the embryo (Kwon et al., 2008). This process involves a functional transition of visceral endoderm cells into definitive endoderm (Kwon et al., 2008). Our results suggest that lost of acidic β-galactosidase activity in embryonic visceral endoderm precedes this transition since acidic β-galactosidase staining is not evident in the embryonic visceral endoderm at the time that definitive endoderm cells emanating from the primitive streak intercalate between the visceral endoderm layer of the embryo.
A number of molecular changes found in senescent cells in vitro resemble those occurring in somatic cells during the process of aging in vivo (Campisi and d’Adda di Fagagna, 2007; Krishnamurthy et al., 2004; Nielsen et al., 1999) and SA-β-gal activity has been associated with organismal aging (Melk et al., 2003; Mishima et al., 1999; Pendergrass et al., 1999; Sigal et al., 1999). It seems paradoxical then that SA-β-gal activity is present at early stages of embryogenesis. This is also the case of p21, a marker of senescence whose transcripts are present in the primitive streak, a region with high levels of cell proliferation during gastrulation (Snow, 1977; Stuckey et al., 2011). Our study shows that these two markers are not only indicators of senescence or aging but can mark nutritional or highly proliferative tissues. Our study also indicates that analysis of these two markers can indicate different cellular processes at different stages of development as shown by studies conducted at mid-gestation stages where SA-β-gal activity and p21 indicate cellular senescence (Munoz-Espin et al., 2013; Storer et al., 2013).
METHODS
Mouse strains and embryo collection
Embryos were obtained from crosses between CD-1 mice (Charles River Laboratories). Dissections were conducted in DMEM (Dulbecco’s Modified Eagle Medium) (Invitrogen, Cat. No. 31600-034) containing 10% heat inactivated fetal bovine serum (Atlanta Biologicals, Cat. No. S11150), penicillin (100 U/ml), streptomycin (100 μg/ml) (Invitrogen, Cat. No. 15140-122) and 20 mM HEPES (Fisher, Cat. No. BP310). Embryos were staged according to morphological landmarks (Downs and Davies, 1993; Rivera-Perez et al., 2010). Embryos were also described in terms of dissection time. In this case, the middle of the dark cycle that preceded the morning in which the copulation plug was observed was considered the beginning of gestation.
SA-β-gal staining
Dissected embryos were rinsed twice in PBS, fixed for 5–10 minutes at room temperature in 2% formaldehyde and 0.2% glutaraldehyde in PBS and washed three times in PBS for 3 minutes. After the fixation and washing steps, they were incubated at 37°C in a bacterial incubator in a freshly prepared solution containing 1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl P3-D-galactoside), 40 mM citric acid, 150 mM sodium phosphate, pH 6.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 50 mM NaCl and 2 mM MgCl2. Staining was allowed to proceed for 5–10 h or a maximum 16 h.
Histology
Embryos assayed for acidic β-galactosidase activity were rinsed in PBS, re-fixed for 1 h in 4% paraformaldehyde, equilibrated in gradients of 10, 20 and 30% sucrose in PBS and mounted in OCT compound. Cryosections were obtained every 20 μm and mounted in 70% glycerol/PBS.
Whole-Mount in situ Hybridization
Embryos were fixed overnight in 4% paraformaldehyde prepared in PBS. After fixation, they were dehydrated in methanol series and stored in 100% methanol at −20°C. Hybridization was done at 70°C as previously described (Rivera-Perez and Magnuson, 2005). The Cdkn1a probe consisted of a 1,030 bp fragment that comprised part of the coding sequence and part of the 3′ UTR of splice variant 1 of Cdkn1a. The probe also recognizes part of the coding sequence and full 3′UTR or splice variants 2 (256 bp) and 3 (234 bp) of Cdkn1a.
Immunofluorescence
Embryos obtained from CD1 crosses were fixed for 1 h in 4% paraformaldehyde prepared in PBS. After fixation, embryos were washed 3 times in PBS for 10 min and incubated in blocking solution (5% goat serum, 0.5% Triton X-100 and 1% bovine serum albumin in PBS) for 1 h. Subsequently, the embryos were incubated with primary antibody diluted 1:500 for 2 hours. After primary antibody staining, the embryos were washed three times in PBT (0.5% Triton X-100, 1% BSA in PBS) and incubated for 1 h at room temperature in secondary antibody diluted 1:500 in PBT. Embryos were then rinsed 3 times in PBT for 10 min, once in PBS for 5 min, counterstained with DAPI, washed twice in PBS for 5 min and mounted in 50% Glycerol/PBS. Primary antibodies: Anti phospho-Histone H3 (EMD Millipore catalog number: 06-570) and rat anti E-cadherin IgG (Zymed catalog number 13-1900). Secondary antibodies: Alexa-fluor 488 Goat anti Rabbit IgG (Molecular probes Cat. No. A11008), Alexa-fluor 594 Goat anti Rat IgG (Molecular Probes, Cat. No. A-11007). Confocal images were obtained using a Leica TCS SP5 confocal laser scanning microscope using a 20x/0.7 HC PL APO Lbd. BI objective. Z-stacks were obtained at either 2 or 4 μm intervals. Stacks were then merged and reconstructed using ImageJ 1.46n software.
Determination of mitotic index
To calculate the mitotic index the number of cells in M phase, marked with anti-phospho Histone H3 antibody, was scored using ImageJ onthogonal views function. Labeled cells located in the visceral endoderm layer over the epiblast (embryonic visceral endoderm) or over the extra-embryonic ectoderm (extra-embryonic visceral endoderm) were scored. The mitotic index is defined as the ratio between the number of cells in mitosis and the total number of visceral endoderm cells (indicated by nuclear staining using DAPI) in each region. Five embryos were scored for a total of 4282 cells, 1987 of them in the extra-embryonic visceral endoderm region.
Fluorescent SA-β-gal and endocytic labeling
Embryos obtained from CD-1 crosses were cultured in a 1:1 mixture of DMEM and rat serum (Rivera-Perez et al., 2010) containing 2 mg/ml lysinated rhodamine dextran (Dextran, Tetramethyl Rhodamine, 70 MW Lysine fixable. Molecular Probes, catalog number D-1818) and 20 mM C12FDG (5-Dodecanoylaminofluorescein Di-β-D-Galactopyranoside. Molecular Probes, catalog number D-2893) for 15 min. After the initial culture, the embryos were rinsed twice in culture medium (1:1 DMEM/rat serum) and incubated again in the same media for 15 min. After the secondary culture period, the embryos were rinsed twice in PBS and fixed for 10 minutes in 4% paraformaldehyde at room temperature. After fixation, the embryos were rinsed twice in PBS for 10 minutes each, stained with Draq5 diluted 1:5000 (1 μM), washed twice in PBS for 5 min and mounted in a solution containing 50% Glycerol in PBS.
Supplementary Material
Sequential series of optical sections acquired as xy images through an E6.5 embryo. Each frame is an overlay of laser scanning confocal images showing SA-β-gal fluorescence staining, apical vacuoles labeled with lysinated Rhodamine-dextran and nuclei stained with Draq5.
Acknowledgments
This work was supported by NIH grants GM87130 and GM94874 to JAR-P.
We thank Hong Zhang for critically reading the manuscript, Gary Schwarting and Roger Davis labs for help with cryosections and Jeff Nickerson for help with confocal microscopy. This work was supported by NIH grants GM87130 and GM94874 to JAR-P.
References
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Bowman PD, Meek RL, Daniel CW. Aging of human fibroblasts in vitro. Correlations between DNA synthetic ability and cell size. Exp Cell Res. 1975;93:184–190. doi: 10.1016/0014-4827(75)90438-3. [DOI] [PubMed] [Google Scholar]
- Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 2006;66:2881–2884. doi: 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging and cancer cell biology, 2007. Aging Cell. 2007;6:261–263. doi: 10.1111/j.1474-9726.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Espinosa JM, Verdun RE, Emerson BM. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell. 2003;12:1015–1027. doi: 10.1016/s1097-2765(03)00359-9. [DOI] [PubMed] [Google Scholar]
- Going JJ, Stuart RC, Downie M, Fletcher-Monaghan AJ, Keith WN. ‘Senescence-associated’ beta-galactosidase activity in the upper gastrointestinal tract. J Pathol. 2002;196:394–400. doi: 10.1002/path.1059. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006;66:8356–8360. doi: 10.1158/0008-5472.CAN-06-1752. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Sun-Wada GH, Aoyama M, Harada A, Takasuga S, Sasaki T, Wada Y. Delivery of endosomes to lysosomes via microautophagy in the visceral endoderm of mouse embryos. Nat Commun. 2012;3:1071. doi: 10.1038/ncomms2069. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113 (Pt 20):3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Hadjantonakis AK. Transthyretin mouse transgenes direct RFP expression or Cre-mediated recombination throughout the visceral endoderm. Genesis. 2009;47:447–455. doi: 10.1002/dvg.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Meehan RR, Barlow DP, Hill RE, Hogan BL, Hastie ND. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984;3:1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek RL, Bowman PD, Daniel CW. Establishment of mouse embryo cells in vitro. Relationship of DNA synthesis, senescence and malignant transformation. Exp Cell Res. 1977;107:277–284. doi: 10.1016/0014-4827(77)90350-0. [DOI] [PubMed] [Google Scholar]
- Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Mishima K, Handa JT, Aotaki-Keen A, Lutty GA, Morse LS, Hjelmeland LM. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci. 1999;40:1590–1593. [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed Cell Senescence during Mammalian Embryonic Development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999;79:1137–1143. [PubMed] [Google Scholar]
- Ogryzko VV, Hirai TH, Russanova VR, Barbie DA, Howard BH. Human fibroblast commitment to a senescence-like state in response to histone deacetylase inhibitors is cell cycle dependent. Mol Cell Biol. 1996;16:5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass WR, Lane MA, Bodkin NL, Hansen BC, Ingram DK, Roth GS, Yi L, Bin H, Wolf NS. Cellular proliferation potential during aging and caloric restriction in rhesus monkeys (Macaca mulatta) J Cell Physiol. 1999;180:123–130. doi: 10.1002/(SICI)1097-4652(199907)180:1<123::AID-JCP14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Jones V, Tam PP. Culture of whole mouse embryos at early postimplantation to organogenesis stages: developmental staging and methods. Methods Enzymol. 2010;476:185–203. doi: 10.1016/S0076-6879(10)76011-0. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schneider EL, Mitsui Y, Au KS, Shorr SS. Tissue-specific differences in cultured human diploid fibroblasts. Exp Cell Res. 1977;108:1–6. doi: 10.1016/s0014-4827(77)80002-5. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR, Jr, Reid LM, Gupta S. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol. 1999;276:G1260–1272. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- Simons JW. The use of frequency distributions of cell diameters to characterize cell populations in tissue culture. Exp Cell Res. 1967;45:336–350. doi: 10.1016/0014-4827(67)90184-x. [DOI] [PubMed] [Google Scholar]
- Snow MHL. Gastrulation in the mouse: Growth and regionalization of the epiblast. J Embryol Exp Morphol. 1977;42:293–303. [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Stuckey DW, Clements M, Di-Gregorio A, Senner CE, Le Tissier P, Srinivas S, Rodriguez TA. Coordination of cell proliferation and anterior-posterior axis establishment in the mouse embryo. Development. 2011;138:1521–1530. doi: 10.1242/dev.063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti M, Nowotschin S, Hadjantonakis AK. Afp::mCherry, a red fluorescent transgenic reporter of the mouse visceral endoderm. Genesis. 2011;49:124–133. doi: 10.1002/dvg.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Yegorov YE, Akimov SS, Hass R, Zelenin AV, Prudovsky IA. Endogenous beta-galactosidase activity in continuously nonproliferating cells. Exp Cell Res. 1998;243:207–211. doi: 10.1006/excr.1998.4169. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 2010;88:593–600. doi: 10.1002/bdra.20705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequential series of optical sections acquired as xy images through an E6.5 embryo. Each frame is an overlay of laser scanning confocal images showing SA-β-gal fluorescence staining, apical vacuoles labeled with lysinated Rhodamine-dextran and nuclei stained with Draq5.