Abstract
Ultra high-performance liquid chromatography (UHPLC) with evaporative light scattering (ELS) detection was used for the quantification of steroidal saponins and diosgenin from the rhizomes or tubers of various Dioscorea species and dietary supplements that were purported to contain Dioscorea. The analysis was performed on an Acquity UPLC™ system with an UPLC™ BEH Shield RP18 column using a gradient elution with water and acetonitrile. Due to their low UV absorption, the steroidal saponins were observed by evaporative light scattering detection. The twelve compounds could be separated within 15 minutes using the developed UHPLC method with detection limits of 5–12 μg/mL with 2μL injection volume. The analytical method was validated for linearity, repeatability, accuracy, limits of detection (LOD) and limits of quantification (LOQ). The Relative Standard Deviations (RSD) for intra- and inter-day experiments were less than 3.1 %, and the recovery efficiency was 97–101 %. The total content of standard compounds was found to be in the range from 0.01–14.5% and 0.9–28.6 mg daily intake for dry plant materials and solid commercial preparations, respectively. UHPLC-mass spectrometry with a quadrupole mass analyzer and ESI source was used only for confirmation of the identity of the various saponins. The developed method is simple, rapid and especially suitable for quality control analysis of commercial products.
Keywords: Dioscorea villosa L, Dioscorea spp, UHPLC-ELSD, steroidal saponins, diosgenin
INTRODUCTION
Yam is the common name for rhizomes of plants from the genus Dioscorea which are widely distributed all over the world. Dioscorea is a genus in the monocotyledonous family Dioscoreaceae (Sautour M et al., 2006). Several species of this genus serve as staple crops in many parts of the world (Mabberley DJ et al., 1997) (Martin FW et al., 1974). Pharmacological studies have shown that some steroidal saponins have biological activities contributing to the efficacy of this herb (Liu JQ et al., 2010). The most well-known species of this genus is Dioscorea villosa, also called wild yam. The rhizomes and roots of this plant are used for its phyto-estrogenic properties such as treatment of menstrual complaints, and rheumatoid arthritis (Braun L et al., 2007) (Chang DG, 2006) (Soffa VM, 1996). Many reports have indicated that diosgenin (the aglycon of the yam steroidal saponin) could be used as a precursor for the partial synthesis of steroidal based drugs, e.g. progesterone and testosterone (Chen Y et al., 1994) (Morgan B, 1997).
Over 50 steroid saponins have been isolated and characterized from various Dioscorea species. Steroidal saponins (furostanol and spirostanol glycosides) are reportedly to be the major physiologically active constituents in yam (Sautour M et al., 2007). The major saponins included two furanostane types - methyl parvifloside and protodeltonin - as well as two spirostane types–deltonin and glucosidodeltonin (zingiberensis I). Minor saponins that were reported by Sautour (Sautour M et al., 2007), were methylprotodioscin, disoscin and prosapogenin A (Hayes PY et al., 2007). Two flavan-3-ol glycosides have also been isolated from D. villosa (Sautour M et al., 2006).
Analytical methods for the determination of steroidal compounds using high-speed countercurrent chromatography-ELSD and HPLC-ELSD have been reported for Dioscorea spp (Yoon K-D et al., 2012) (Yang D-J et al., 2003). Recently, Lin (Lin J-T et al., 2008) used an HPLC-ELSD method for the determination of saponins from different parts of D. pseudojaponica. Fingerprint analysis of D. nipponica using HPLC-ELSD was developed (Liu C-Z et al., 2007). A LC–MS method has been used to characterize steroidal saponins from D. zingiberensis (Zhua J et al., 2010) and D. panthaica (Li R et al., 2006). An HPTLC method for the determination of diosgenin from in-vitro cultures and rhizomes of D. deltoidea has been described (Amir M et al., 2012). Theerasin (Theerasin S et al., 2009) used reverse phase liquid chromatography for the separation of phenolic compounds in D. hispida. Even though these analytical methods have been published for Dioscorea species, to-date no report employing UHPLC-ELSD for fingerprinting and quantification of these twelve steroidal saponins and diosgenin from Dioscorea species has appeared in the literature. Although these previously developed methods can be used for the analysis of a few marker compounds, they often involve long run times (about 30 min for 3 compounds analyzed and over 60 minutes for 6 compounds analyzed). Their detection limits were also found to be high. In the current study, a simple, rapid economical, and precise UHPLC method has been developed for chemical profiling and quantification of steroidal saponins.
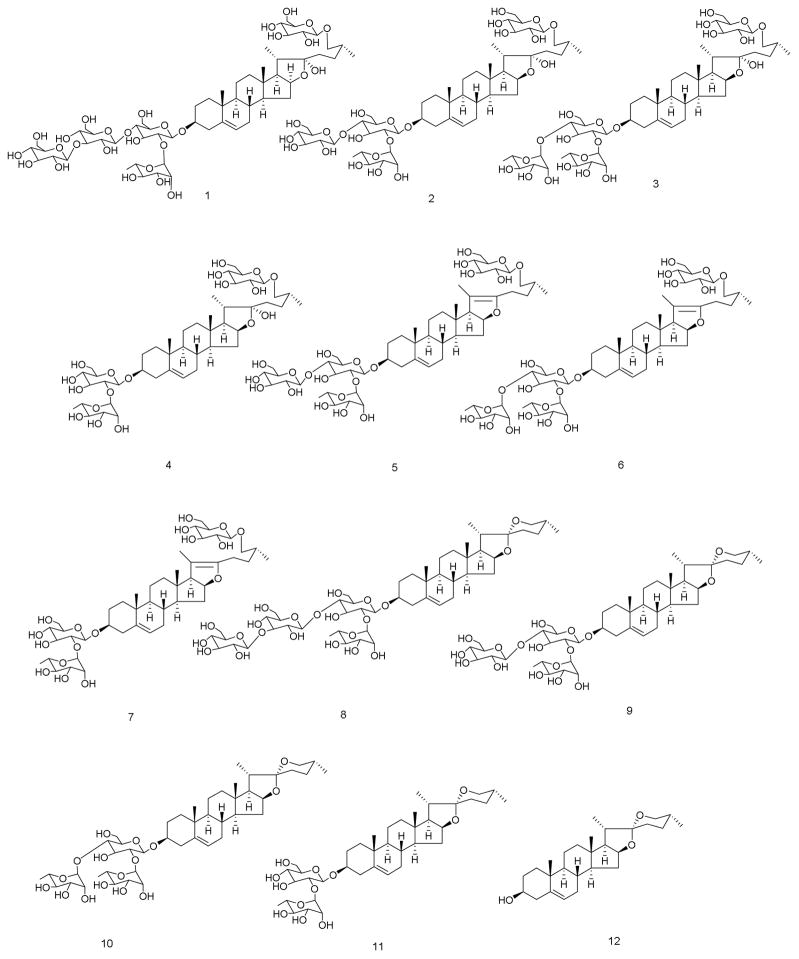
The newly developed UHPLC method for quantitative determination of eleven steroidal saponins [parvifloside (1), protodeltonin (2), protodioscin (3), protobioside (4), huangjiangsu A (5), pseudoprotodioscin (6), 26-O-β-D-glucopyranosyl-3β,26-diol-25(R)-furost-5,20(22)-dien-3-O-α-L-rhamnopyranosyl(1→2)-O-β-D-glucopyranoside (7), zingiberensis saponin I (8), deltonin (9), dioscin (10), progenin III (11), and a sapogenin, diosgenin (12)] (Figure 1) from the rhizomes and roots of Dioscorea species and has been found to require shorter retention times while maintaining good resolution and sensitivity. Detection of the saponins was achieved by the use of an ELS detector. A highly sensitive UHPLC-MS method was also used to identify and confirm the structure of compounds in Dioscorea samples and dietary supplements that claimed to contain D. villosa.
Figure 1.
Structures of compounds: parvifloside (1), protodeltonin (2), protodioscin (3), protobioside (4), huangjiangsu A (5), pseudoprotodioscin (6), 26-O-β-D-glucopyranosyl-3β,26-diol-25(R)-furost-5,20(22)-dien-3-O-α-L-rhamnopyranosyl(1→2)-O-β-D-glucopyranoside (7), zingiberensis saponin I (8), deltonin (9), dioscin (10), progenin III (11), diosgenin (12)
EXPERIMENTAL
Instrumentation and Chromatographic Conditions
UHPLC-ELSD
All analyses were performed on a Waters Acquity UPLC™ system (Waters Corp., Milford, MA, USA) that included a binary solvent manager, sampler manager, column compartment, PDA (Waters Acquity model code UPD), ELS detector (Waters Acquity model code UPE), and MS detector (Waters Acquity model SQD), all controlled by a Waters Empower 2 data station. An Acquity UPLC™ BEH Shield RP18 column (100mm×2.1mm I.D., 1.7μm) was used. The column and sample temperature were maintained at 40 °C and 15 °C, respectively. The column was equipped with a LC-18 guard column (Vanguard 2.1 × 5 mm, Waters Corp., Milford, MA, USA). The mobile phase consisted of water (A) and acetonitrile (B), both containing 0.05% formic acid at a flow rate of 0.27 mL/min, which were used with the following gradient: 0 min, 75 % A: 25 % B in next 6 min to 60 % A:40 % B, then for 4 min 23 % A:77 % B, then for 3 min 5 % A:95 % B and to 100 % B in next 2 min. Separation was followed by a 3 min washing procedure with 100 % B and a re-equilibration period of 3.5 min. A strong needle wash solution (95/5; acetonitrile/water) and a weak needle wash solution (10/90; acetonitrile/water) were used. The total run time for analysis was 15 minutes. The injection volume was 2 μL. The ELS detector was set at 50 °C with a gain of 400 and the nitrogen pressure was 45 psi.
The effluent from the LC column was directed into the ESI probe. Mass spectrometer conditions were optimized. The source and the desolvation temperatures were maintained at 150 and 350 °C, respectively. The probe (capillary), cone and extractor voltages were fixed at 3.0kV, 35V and 3 V, respectively. Nitrogen was desolvation (650 L/hr) and drying gas (25 L/hr). Compounds were confirmed in both positive and negative modes. Mass spectra were obtained at a dwell time of 0.1 s in single ion monitoring (SIM) and 500 Da/sec of scan rate.
Standards and chemicals
Twelve standards were used as reference compounds. The steroidal saponins, parvifloside (1), protodeltonin (2), protobioside (4), huangjiangsu A (5), pseudoprotodioscin (6), 26-O-β-D-glucopyranosyl-3β,26-diol-25(R)-furost-5,20(22)-dien-3-O-α-L-rhamnopyranosyl(1→ 2)-O-β-D-glucopyranoside (7), zingiberensis saponin I (8), deltonin (9), dioscin (10) and progenin III (11) were isolated at the National Center for Natural Products Research (NCNPR), University of Mississippi, University, Mississippi, USA. The identity and purity of these compounds as confirmed by chromatographic (TLC, HPLC) methods and the analysis of the spectral data (IR, 1D- and 2D-NMR, ESI-HRMS) as well as comparison with published spectral data (Munafo JP Jr et al., 2010) (Yoshikawa M et al., 2007) (Shao Y et al., 1997) (Dong M et al., 2001) (Wang CY et al., 2009) (Shen P et al., 2002) (Silva BP et al., 1998) (Hu K et al., 1996) (Zheng Q-A et al., 2004) (Ali Z et al., 2013a, 2013b). The purity of these compounds was found to be greater than 90%. The purity of the standards was confirmed by injecting concentrated standards (1 mg/mL) with detection by PDA, ELSD and MS. Protodioscin (3) was purchased from Chromadex (Santa Ana, CA, USA). Diosgenin (12) was purchased from MP Biomedicals Inc, (Solon, OH, USA). Acetonitrile and formic acid were of HPLC grade purchased from Fisher Scientific (Fair Lawn, NJ, USA). Water for the mobile phase was purified using a Milli-Q system (Millipore).
Plant materials
Tubers of Dioscorea cayennensis Lam. (# 9462, 9166, 11612), Dioscorea rotundata Poir. (# 9463, 9167), Dioscorea opposita Thunb. (# 9464, 9165), Dioscorea bulbifera L. (# 11609), Dioscorea deltoidea Wall. ex Griseb. (#11614), tubers of Dioscorea quaternata Walter (#11616), Dioscorea spp. (#11618, 11619), along with rhizomes of Dioscorea caucasica Lipsky (# 9169, 11610) and Dioscorea villosa L. (# 9800) were obtained from the cultivated, living collection of the NCNPR Maynard W Quimby Medicinal Plant Garden, University of Mississippi. All species of all plant samples were authenticated by Dr. Aruna Weerasooriya of the Maynard W Quimby Medicinal Plant Garden, 301 Insight Park Drive, The University of Mississippi. University, Mississippi, USA.
Rhizomes of D. villosa (# 5365, 8595), tubers of D. opposita (# 5987, 6082, 8694), rhizomes of D. alata (# 6814, 8886), tubers of D. trifida (# 7671) and D. cayennensis (# 7600), bulbils of D. bulbifera (#12962, 10229, 10234) were obtained from Missouri Botanical Garden, Missouri, USA. Tubers of D. nipponica (# 9577, 9578) and D. opposita (# 9579, 9580) were obtained from Harvard Medical School, USA. Tubers of D. batatas (# 9797), D. japonica (# 9798), and D. nipponica (# 9799) were obtained from South Korea.
Rhizomes of D. villosa (# 1591, 2011, 7557, 7558, 7559, 9161, 9336, 9412, 9413, 10219, 13064, 13065) were obtained from commercial source. Dietary supplements (#10205–10217) claiming to contain D. villosa or Dioscorea were purchased online. Specimens of all samples are deposited at the NCNPR’s botanical repository, The University of Mississippi, University, Mississippi, USA.
Preparation of Standard Solutions
Stock solutions of the standard compounds were prepared at a concentration of 2 mg/mL in methanol. Calibration curves were prepared at seven different concentration levels. The range of the concentrations were 25–550 μg/mL for compounds 1–7; 10–550 μg/mL for compounds 8–10 and 15– 550 μg/mL for compounds 11–12 using UHPLC-ELSD method.
Sample preparation
For capsules/pellets
Five pellets were weighed and then pulverized with a mortar and pestle. For capsules, five samples were weighed, opened and the contents mixed and triturated in a mortar and pestle.
For Solids
Dry plant samples (0.5 g) or an adequate amount of powdered pellets or capsules contents were weighed (average weight of dosage form) then sonicated in 2.5mL of methanol for 30 min followed by centrifugation for 15 min at 959 × g. The supernatant was transferred to a 10mL volumetric flask. The procedure was repeated four more times with 2.0 mL methanol and the respective supernatants were combined. The final volume was adjusted to 10mL with methanol and mixed thoroughly. Prior to injection, an adequate volume (ca. 2 mL) was passed through a 0.45μm PTFE membrane filter. The first 1.0mL was discarded and the remaining volume was collected in an LC sample vial. For plant samples where a few constituents were highly concentrated, the sample solutions were further diluted.
For Liquids
1.0 mL of sample solution was mixed with 9.0 mL of methanol, vortex for 30 seconds and sonicated for 30 minutes, vortexed for 30 sec and centrifuge for 10 minutes at 959 × g. The clear supernatant solution was used for analysis. Sample # 10209 required further dilution to 10 fold.
Validation procedure
The newly developed UHPLC method was validated in terms of precision, accuracy, and linearity according to ICH guidelines (ICH, 2005). Below limits of quantification (BLOQ) and limit of quantification (LOQ) were determined by injecting a series of dilute solutions with known concentrations for each standard. BLOQ and LOQ were defined as the signal-to-noise ratio equal to 2:1 or 3:1 and 10:1, respectively. The accuracy of the assay method was evaluated in triplicate using two concentration levels of 25 and 400 μg/ml. Intra- and inter-day variation of the assay was determined on 3 consecutive days with 3 repetitions each.
RESULTS AND DISCUSSION
Extraction
Sample preparation is of paramount importance to any reliable analysis. Different solvents (methanol, water and 70% ethanol) were tested for an exhaustive extraction of D. villosa (NCNPR accession # 13064). Each solvent was tested in triplicate to ensure reproducibility. The percentage of saponin compounds detected in each extract was then determined using the UHPLC-ELSD method. The best extraction solvent was methanol, which produced higher concentrations of the compounds than extractions with 70% ethanol or water. The methanol extract contained the highest percentage of all measured compounds (recovery was about >97 %). Lower percentages of saponin compounds were recovered in the 70% ethanol and water extracts (about 90 % and 60 %, respectively).
Chromatographic Conditions Optimization
Optimized chromatographic conditions were achieved after several trials with acetonitrile, formic acid, and water in different proportions for the mobile phase. A mobile phase containing water and acetonitrile, both containing formic acid with a constant flow rate at 0.27 mL/min on an Acquity UPLC™ BEH shield RP18 (100 mm × 2.1 mm, 1.7 μm i.d.) column using gradient elution at a fixed column temperature of 40 °C were found to be the optimal separation condition for the determination of compounds 1–12 in various samples. Baseline resolution was obtained and good peak shapes observed using these conditions described previously. The different columns tried included; Acquity UPLC™ Bridged Ethyl-siloxane/silica Hybrid (BEH) C18 (100 mm × 2.1 mm I.D., 1.7 μm), Acquity UPLC™ Bridged Ethyl-siloxane/silica Hybrid (BEH) C8 (straight chain alkyl column) (50 mm × 2.1 mm I.D., 1.7 μm), Acquity UPLC™ BEH C18 (50 mm × 2.1 mm I.D., 1.7 μm) and Acquity UPLC™ BEH Shield RP18 (embedded polar group column). Each provided a different combination of hydrophobicity, silanol activity, hydrolytic stability and chemical interaction with the analytes. Among these the BEH shield RP18 resolved peaks 1–12 adequately; other columns could not resolve compounds 2–4 and 9–10 satisfactorily under the same conditions. For a successful separation of the Dioscorea saponins, not only the separation conditions had to be carefully investigated, but also the method of detection. As these compounds have no chromophore, their UV absorption is very low and a sensitive detection even at 200 nm is not possible. The ELSD response provides an exponential relationship (log of response versus log of concentration was linear) and is proportionate to the mass of the observed compound, which proved to be a useful alternative for saponins analysis.
Stability of the Solutions
The stability of the solutions was tested with 12 standards and samples that were stored in a refrigerator (4 °C) for one week. No significant changes were observed in the concentrations of the components analyzed.
Method Validation
The validation study allowed the evaluation of the method for its suitability for routine analysis.
Linearity, Range, BLOQ and LOQ
Table 1 shows the calibration data for all twelve compounds, including the regression equations, correlation coefficients (r2>0.999), BLOQ and LOQ. Calibration plots for compounds 1–12 were obtained over the calibration range at seven concentration levels. Using regression analysis, calibration curves for steroidal saponins (15–550 μg/mL) was established.
Table 1.
Regression Equations, Correlation Coefficients (r2), BLOQ and Limit of Quantification (LOQ) determined using the LC-ELSD method
| Compounds | Regression Equation | r2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|
| 1 | Y = 1.66e+000X +2.40e+000 | 0.9999 | 12 | 25 |
| 2 | Y = 1.72e+000X + 2.12e+000 | 0.9998 | 12 | 25 |
| 3 | Y = 1.71e+000X + 2.03e+000 | 0.9999 | 12 | 25 |
| 4 | Y = 1.73e+000X + 2.14e+000 | 0.9999 | 12 | 25 |
| 5 | Y = 1.68e+000X + 1.99e+000 | 0.9996 | 12 | 25 |
| 6 | Y = 1.68e+000X + 2.00e+000 | 0.9999 | 12 | 25 |
| 7 | Y = 1.70e+000X + 2.03e+000 | 0.9999 | 12 | 25 |
| 8 | Y = 1.35e+000X + 2.89e+000 | 0.9996 | 5 | 10 |
| 9 | Y = 1.35e+000X + 2.91e+000 | 0.9996 | 5 | 10 |
| 10 | Y = 1.35e+000X + 2.93e+000 | 0.9993 | 5 | 10 |
| 11 | Y = 1.30e+000X + 3.04e+000 | 0.9997 | 7 | 15 |
| 12 | Y = 1.77e+000X + 2.06e+000 | 0.9997 | 7 | 15 |
Specificity
The specificity of the method was determined by injecting individual samples. No interference was observed for any of the components.
Precision
Intra- and inter-day variation of the analysis was determined for samples # 9413, 5365, 13064 and was lower than 5 %, with a maximum RSD of 3.0 %. The intra-day RSD for the replicates were between 0.07 and 3.05 % for compounds 1–12 using the developed UHPLC method. Similarly, the RSD for the day to day replicates were between 0.34–2.7 % (Table 2).
Table 2.
Intra- and inter-day precision of plant sample # 13064 assayed under optimized conditions for compounds 1–12 using the LC-ELSD method
| Compounds | Intra-Day (n=4) | Inter-Day(n=12) | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| 1 | 0.453 (1.30) | 0.457 (0.82) | 0.454 (0.78) | 0.455 (0.99) |
| 2 | 1.273 (0.41) | 1.279 (0.19) | 1.277 (0.27) | 1.276 (0.34) |
| 3 | 0.295 (1.37) | 0.292 (0.98) | 0.295 (0.80) | 0.294 (1.06) |
| 4 | 0.319 (0.74) | 0.317 (0.54) | 0.316 (0.47) | 0.317 (0.69) |
| 5 | 0.364 (0.85) | 0.366 (0.27) | 0.368 (0.26) | 0.366 (0.72) |
| 6 | 0.083 (2.32) | 0.081 (1.42) | 0.084 (2.80) | 0.083 (2.65) |
| 7 | 0.073 (2.05) | 0.074 (1.29) | 0.072 (2.08) | 0.073 (2.02) |
| 8 | 0.281 (0.96) | 0.277 (0.62) | 0.277 (0.54) | 0.278 (1.01) |
| 9 | 0.660 (0.79) | 0.661 (0.89) | 0.664 (0.07) | 0.662 (0.67) |
| 10 | 0.115 (1.23) | 0.115 (1.88) | 0.115 (0.44) | 0.115 (1.20) |
| 11 | 0.236 (0.69) | 0.238 (0.54) | 0.235 (0.35) | 0.236 (0.67) |
| 12 | 0.049 (3.05) | 0.048 (2.95) | 0.048 (1.98) | 0.049 (2.71) |
Values in mg/100 mg of plant sample; relative standard deviation are given in parentheses
Accuracy
The accuracy of the method was determined by spiking samples (#9413, 5365 and 13064) with a known amount of saponins. The plant sample was exhaustively extracted five times as discussed under the “experimental section”, dried and then spiked with known amounts of the standard compounds at two different concentrations, extracted again and then analyzed under optimized conditions. The accuracy of the assay method was evaluated in triplicate at two concentration levels, 25 and 400 μg/ml for all the standards in the sample. The percentage recovery of these samples ranged from 97.0 to 101.3 %.
Analysis of Plant Samples and Dietary Supplements
All plant samples (commercial and authenticated) were analyzed for the determination of steroidal saponins. The identification of these compounds in Dioscorea samples was based on the retention times and MS with those of standards. Spiking samples with standard compounds provided a further confirmation assay. To demonstrate the applicability of this method, several Dioscorea market products as well as other Dioscorea species were analyzed. Sixteen different authenticated and commercial samples of D. villosa were analyzed. Once a fingerprint method had been developed through the use of an authenticated plant sample (#5365), other samples of Dioscorea were tested to ensure the method’s usefulness. All samples of Dioscorea are listed in the experimental section. The method was validated by testing a number of populations within a single species of Dioscorea. The analyses of compounds 1–12 in the various samples are presented in Tables 3 and 4 and reflect the substantial diversity among the samples. Eleven authenticated and commercial samples (# 1591, 5365, 7557, 8595, 9412, 9413, 12152, 12166, 13064, 13065) of D. villosa contained compounds 1–12. Sample # 9161 showed the presence of 1–4, 8–12. Sample # 9800 contained compounds 1–6, 8–12. Compounds 1–3 and 8, 9, 11 displayed the most dominant peaks giving a range from 0.12–2.6%, 0.17–5.57%, below limit of quantification (BLOQ)-1.19%, 0.05–0.55%, 0.07–1.56%, and BLOQ-9.44%, respectively. Sample # 7559, 9336 contained compound 12 (diosgenin) only. All these samples were labeled as D. villosa. For the majority of the D. villosa samples, compounds 1–12 were detected. The total amount of saponins found in the D. villosa samples was in between BLOQ-14.5% (Table 3). Morgan (Morgan M., 2011) noted that some commercial extracts are standardized to diosgenin rather than dioscin, and that such extracts may be derived from other species (e.g. Chinese yam, D. oppositifolia L.) which may lack the full chemical profile of D. villosa.
Table 3.
Quantitative analysis (%) of triterpene saponins and sapogenin from different species of Dioscorea and commercial products. Parvifloside (1), protodeltonin (2), protodioscin (3), protobioside (4), huangjiangsu A (5), pseudoprotodioscin (6), 26-O-β-D-glucopyranosyl-3β,26-diol-25(R)-furost-5,20(22)-dien-3-O-α-L-rhamnopyranosyl(1→2)-O-β-D-glucopyranoside (7), zingiberensis saponin I (8), deltonin (9), dioscin (10), progenin III (11), diosgenin (12)
| Code # | Sample Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8595 | D. villosa | 0.56 | 1.96 | 0.37 | 0.51 | 0.11 | 0.01 | 0.02 | 0.35 | 0.96 | 0.10 | 9.44 | 0.13 | 14.52 |
| 5365 | 1.05 | 2.99 | 0.36 | 0.30 | 0.62 | 0.09 | 0.06 | 0.05 | 0.13 | 0.02 | 0.07 | 0.02 | 5.76 | |
| 7557 | 1.45 | 3.80 | 0.95 | 0.44 | 0.89 | 0.28 | 0.14 | 0.24 | 0.46 | 0.07 | 0.10 | 0.02 | 8.84 | |
| 9413 | 0.92 | 3.39 | 0.49 | 0.39 | 0.05 | 0.02 | 0.01 | 0.23 | 0.69 | 0.08 | 0.15 | 0.03 | 6.45 | |
| 12166 | 0.79 | 2.81 | 0.33 | 0.47 | 0.73 | 0.11 | 0.15 | 0.22 | 0.58 | 0.09 | 0.27 | 0.06 | 6.61 | |
| 13064 | 0.46 | 1.28 | 0.29 | 0.32 | 0.37 | 0.08 | 0.07 | 0.28 | 0.66 | 0.12 | 0.24 | 0.05 | 4.22 | |
| 13065 | 0.65 | 1.25 | 0.42 | 0.33 | 0.11 | 0.03 | 0.02 | 0.20 | 0.44 | 0.08 | 0.17 | 0.09 | 3.79 | |
| 9412 | 2.02 | 5.57 | 1.17 | 0.79 | BLOQ | 0.01 | 0.01 | 0.33 | 0.79 | 0.19 | 0.38 | 0.07 | 11.33 | |
| 1591 | 1.67 | 2.83 | BLOQ | 0.23 | 1.09 | 0.05 | 0.12 | 0.52 | 0.67 | BLOQ | 0.11 | 0.03 | 7.32 | |
| 10219 | 0.92 | 3.00 | 0.33 | 0.27 | 0.08 | BLOQ | BLOQ | 0.18 | 0.50 | 0.05 | 0.13 | 0.04 | 5.50 | |
| 12152 | 0.91 | 2.89 | 0.39 | 0.23 | 0.08 | BLOQ | BLOQ | 0.22 | 0.54 | 0.07 | 0.22 | 0.03 | 5.58 | |
| 9800 | 0.50 | 4.09 | 1.19 | 0.03 | 0.04 | 0.02 | ND | 0.14 | 0.65 | 0.18 | 0.02 | 0.03 | 6.89 | |
| 7558 | 1.68 | 4.47 | 0.60 | 0.99 | ND | ND | ND | 0.55 | 1.56 | 0.16 | 1.07 | 0.19 | 11.27 | |
| 9161 | 0.12 | 0.17 | 0.17 | 0.16 | ND | ND | ND | 0.05 | 0.07 | 0.04 | 0.10 | 0.03 | 0.91 | |
| 7559 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.23 | 3.23 | |
| 9336 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 2011 | Dioscorea spp (species not specified) | 0.32 | 3.00 | 0.49 | 0.23 | 0.62 | 0.12 | 0.07 | 0.15 | 0.81 | 0.16 | 0.08 | 0.04 | 6.09 |
| 7664 | ND | ND | BLOQ | ND | ND | ND | ND | ND | 0.09 | 0.04 | BLOQ | 0.01 | 0.14 | |
| 11618 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 11619 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 5987 | D. opposita | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.02 | 0.02 |
| 8694 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.02 | 0.02 | |
| 6082 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.01 | 0.01 | |
| 9579 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 9165 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 9580 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | |
| 7600 | D. cayennensis | ND | BLOQ | 0.41 | BLOQ | ND | ND | ND | ND | 0.14 | 0.24 | BLOQ | 0.01 | 0.80 |
| 9166 | ND | BLOQ | 0.10 | BLOQ | ND | ND | ND | ND | 0.06 | 0.06 | BLOQ | 0.01 | 0.23 | |
| 9462 | ND | 0.01 | 0.12 | BLOQ | ND | ND | ND | ND | 0.14 | 0.15 | BLOQ | BLOQ | 0.42 | |
| 11612 | ND | BLOQ | 0.03 | BLOQ | ND | ND | ND | ND | ND | 0.07 | BLOQ | 0.01 | 0.11 | |
| 7862 | D. bulbifera | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 10234 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 11609 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 9169 | D. caucasica | 2.64 | 1.00 | 0.09 | 0.11 | ND | ND | ND | 0.43 | 0.15 | BLOQ | ND | ND | 4.42 |
| 11610 | 0.47 | 1.90 | 0.44 | BLOQ | ND | ND | ND | 0.15 | 0.44 | 0.02 | ND | ND | 3.42 | |
| 8886 | D. alata | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.02 | 0.02 |
| 6814 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 9167 | D. rotundata | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 9463 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 9577 | D. nipponica | ND | ND | 3.67 | 0.98 | ND | 0.05 | ND | ND | ND | 1.62 | 0.02 | BLOQ | 6.34 |
| 9578 | ND | ND | 5.11 | 0.53 | ND | BLOQ | ND | ND | ND | 0.50 | BLOQ | BLOQ | 6.14 | |
| 9799 | ND | ND | 1.27 | BLOQ | ND | BLOQ | ND | ND | ND | 3.93 | 0.04 | BLOQ | 5.24 | |
| 9797 | D. batatas | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 9798 | D. japonica | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.07 | 0.07 |
| 7671 | D. trifida | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 11614 | D. deltoidea | BLOQ | ND | 1.78 | BLOQ | ND | ND | ND | ND | ND | 0.77 | 0.01 | BLOQ | 2.56 |
| 11616 | D. quaternata | 0.30 | 2.33 | 0.44 | 0.27 | ND | ND | ND | 0.04 | 0.34 | 0.05 | 0.08 | 0.03 | 3.88 |
| Commercial Products | ||||||||||||||
| 10211 | D. villosa | 0.61 | 2.20 | 0.20 | 0.36 | 0.45 | 0.06 | 0.08 | 0.28 | 0.67 | 0.06 | 0.20 | 0.02 | 5.19 |
| 10215 | 0.13 | 0.46 | 0.08 | 0.08 | 0.19 | 0.03 | 0.03 | 0.07 | 0.18 | 0.03 | 0.07 | 5.59 | 6.94 | |
| 10205 (mg/ml) | 7.89 | 2.71 | 0.45 | 0.5 | BLOQ | BLOQ | BLOQ | BLOQ | 0.22 | BLOQ | BLOQ | BLOQ | 11.77 | |
| 10210 | 0.13 | 0.34 | 0.03 | 0.07 | 0.06 | ND | ND | 0.03 | 0.08 | 0.02 | 0.03 | 0.21 | 1.0 | |
| 10206 | BLOQ | 0.34 | 8.20 | 0.22 | ND | 0.12 | ND | 0.02 | 1.08 | 0.06 | 0.10 | BLOQ | 10.14 | |
| 10209 (mg/mL) | 1.77 | 3.54 | 0.80 | 1.08 | ND | ND | ND | 0.62 | 1.71 | 0.16 | 0.63 | 0.03 | 10.34 | |
| 10213 | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | ND | ND | BLOQ | BLOQ | BLOQ | BLOQ | 0.88 | 0.88 | |
| 10207 | ND | ND | 3.51 | 0.41 | ND | 0.03 | ND | ND | ND | 3.74 | 0.05 | 0.06 | 7.8 | |
| 10208 | ND | ND | 5.47 | 1.18 | ND | ND | ND | ND | ND | 2.74 | 0.06 | 0.09 | 9.54 | |
| 10212 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 6.13 | 6.13 | |
| 10214 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ | |
| 10216 | D. opposita | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ |
| 10217 | Dioscorea spp | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | BLOQ | BLOQ |
ND = Not Detected
BLOQ = Below Limit of Quantification
Content in % (w/w for solid plant materials and solid commercial preparations, w/v for liquid preparations)
Table 4.
Content (mg/mL, w/v or mg/ave. wt of content, w/w) of steroidal saponins and diosgenin in various Dioscorea products and estimated maximum daily intakes to information provided on product labels
| Code # | Wt used for analysis | Serving size | Amount/serving size | Label claim | 12-Steroidal compounds content | Daily Intake (mg) |
|---|---|---|---|---|---|---|
| 10205 | 1 mL = 30 drops | 30 drops | 2000 mg | 1–2 mL thrice daily | 11.8 | 35.4* |
| 10206 | 425.6 mg | 2 capsules | 850 mg | 2 capsules daily | 10.14 | 20.3 |
| 10207 | 513.2 mg | 2 capsules | 995 mg | 2 capsules daily | 7.8 | 15.6 |
| 10208 | 442.3 mg | 1 capsule | 400 mg | 3 capsules daily | 9.54 | 28.6 |
| 10209 | 1 mL = 30 drops | - | - | 1–2 mL for 2–5 times/day | 10.34 | 20.7–51.7* |
| 10210 | 729.6 mg | 1 capsule | - | 1 capsule 2 times daily | 1.0 | 2 |
| 10211 | 469.3 mg | 1 capsule | 500 mg | 1 capsule 2 times daily | 5.19 | 10.4 |
| 10212 | 499.2 mg | 1 capsule | 200 mg | 1 capsule daily | 6.13 | 6.1 |
| 10213 | 986.5 mg | 1 capsule | 50 mg | 1 capsule daily | 0.88 | 0.9 |
| 10214 | 564.5 mg (15 pellets) | 5 pellets | - | 15 pellets/ daily | BLOQ | BLOQ |
| 10215 | 487.6 mg | 1 capsule | 500 mg (Dioscorea spp) | 1 capsule 2 times daily | 6.94 | 13.9 |
| 10216 | 300 mg | - | -(D. opposita) | - | BLOQ | - |
| 10217 | 499.8 mg | - | -(Dioscorea spp) | - | BLOQ | - |
mg/mL (w/v); BLOQ = Below Limit of Quantification
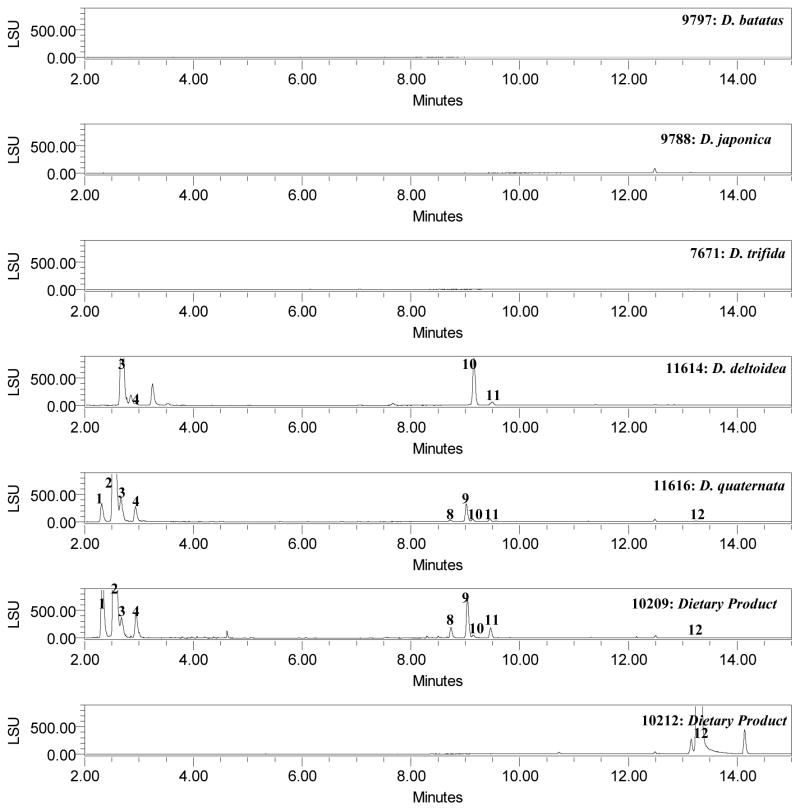
Dioscorea products are often used in the USA as dietary supplements. The currently developed method were used to analyze and compare these products. Thirteen market products (# 10205–10217) were analyzed, in comparison to sample # 9413, which was the original tuber powder used for the isolation of the standard compounds. As shown in Table 3, the products showed variations in the total content of compounds 1–12. Most of the commercial products (# 10205–10217) contained either tuber powder or the plant extract. The analysis revealed significant variations from product to product. Unlike the crude plant material, commercial supplements [#10207, 10216, 10210, 10212] are a mixture of wild yam root or wild yam root extract powder with other botanicals (black pepper fruit extract, guarana seed, green tea leaf, yerba mate leaf, ashwaganda, ginseng root, schisandra fruit, ginger root, cayenne fruit, rice flour or vegetable cellulose) along with dextrin, vitamins, minerals, excipients etc. Most of the products (#10206, 10208, 10211, 10213–10215, 10217) contained only Dioscorea as the botanical ingredient (wild yam root powder or extract). Two liquid preparations (#10205, 10209) contained whole rhizome of wild yam extract with alcohol and water. The estimated maximum daily intake (mg/day) = to the weight of saponin (mg) X dilution factor X the suggested maximum daily intake in capsules or drops/ weight (mg) of content in capsules or drops. For solid dosage forms, the suggested daily use varied from 2–6 capsules; due to the difference in the composition for these samples, the daily intake also may vary (BLOQ-28.6 mg) (Table 4). Total amounts of saponins that might be consumed daily were highest in sample #10208 and lowest in sample #10214 (Table 4) for solid dosage forms.
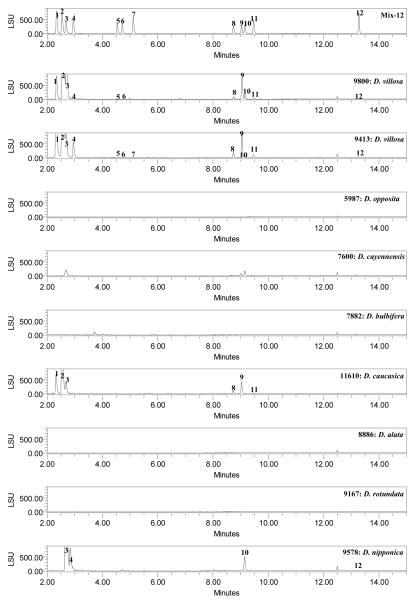
Finally, to verify this method’s ability to differentiate between Dioscorea species, 13 different species were analyzed. Fig. 2 shows the ELSD chromatograms of methanolic extracts of D. villosa, D. opposita, D. cayennensis, D. bulbifera, D. caucasica, D. alata, D. rotundata, D. nipponica, D. batatas, D. japonica, D. trifida, D. deltoidea, D. quaternata obtained with current method. All samples were distinguishable from D. villosa. Most of the D. opposita, D. japonica, D. alata samples, contained compound 12 only. All four samples of D. cayennensis (# 9462, 9166, 7600, 11612) contained compounds 2–3, 9–12. Samples of D. batatas (#9797), D. trifida (#7671), and D. rotundata (#9167, 9463) did not show for the presence of compounds 1–12. D. caucasica samples (#9169, 11610) showed for the presence of compounds 1–5 and 8–10. Samples of D. deltoidea (#11614) detected for the presence of compounds 1, 2–3, 10–12. Samples of D. quaternata (#11616) showed for the presence of compounds 1–4, and 8–12. D. quaternata, was reported as a unique species in the past (Shehbaz Al et al., 1989) (USDA NRCS 2002) (Wunderlin RP et al., 2003) but has recently been shown to be the same as D. villosa (Raz L, 2003). Four unknown samples of Dioscorea species were also analyzed. Of four samples, one sample (# 2011) showed for the presence of all 1–12 compounds which indicated that it may be D. villosa. One sample (# 7664) contained compounds 3, 9–12 and two samples (#11618, 11619) could detect for the presence of compound 12 only. The majority of the saponin components in D. villosa have higher concentrations than those of the other species of Dioscorea. Samples # 7559, 9336 which were ‘labeled’ as D. villosa did not show the complete fingerprint profile as compared to those of authentic samples. With the present method plant material or extracts can be tested to confirm or deny the presence of D. villosa which may help to detect possible spiking of plant material, or prevent the use of potentially mislabeled or misidentified “Dioscorea” material.
Figure 2.
UHPLC Chromatograms of standard mix, Dioscorea species and dietary supplements using ELSD
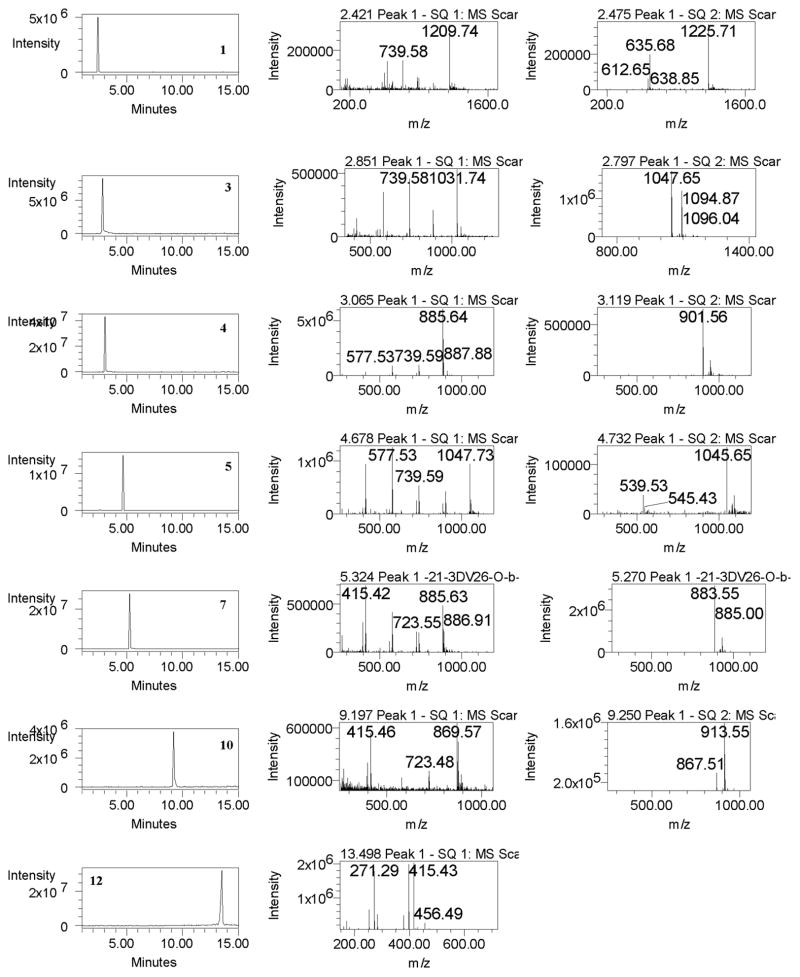
Additionally, an LC-MS method can provide a powerful qualitative technique for the selective determination of molecular masses of analytes. This method involved the use of the [M+H]+, [M+H-H2O]+ and [M+Na]+ ions. In a positive ion mode, the respective dehydrated species [M+H-H2O]+ at m/z 1209.5, 1047.5, 1031.5, 885.5 and protonated molecule [M+H]+ at m/z 1047.5, 1031.5, 885.5, 1047.5, 885.5, 869.5, 723.4 and 415.3 for compounds 1–12 were observed (Table 5). Further, the fragmentation patterns observed in the mass spectrum were useful for the characterization of compounds. The fragmentation ions were ascribed to the loss of one to two hexoses and water from the triterpene skeleton (Table 5). In (-)-ESI-MS, produced ions for the deprotonated molecule [M-H]− and because of the presence of formic acid in the mobile phase, the standards also gave abundant [M+HCOO]− ion. Figure 3 shows the selected ion monitoring (SIM) chromatograms in positive and negative ion modes for the standards. The retention times and selective mass spectra for compounds from various Dioscorea plant samples and products matched with the corresponding standard compounds.
Table 5.
Peak assignment for the analysis of compounds using UHPLC-MS Method
| Peak | RT | (+)-ESI-MS (m/z) [M+H]+ | MS Fragment ions (m/z) | (−)-ESI-MS (m/z) [M-H]− | Identities |
|---|---|---|---|---|---|
| 1 | 2.79 | 1209.5 [M+H-H2O]+ | 1063.5 [M+H-H2O-Rha]+ 901.5 [M+H-H2O-Rha-Glu]+ 739.4 [M+H-H2O-Rha-2Glu]+ 577.4 [M+H-H2O-Rha-3Glu]+ 415.3 [M+H-H2O-Rha-4Glu]+ |
1225.6 | parvifloside |
| 2 | 2.95 | 1047.5 [M+H-H2O]+ | 901.5 [M+H-H2O-Rha]+ 739.4 [M+H-H2O-Rha-glu]+ 577.4 [M+H-H2O-Rha-2glu]+ |
1063.5 | protodeltonin |
| 3 | 3.11 | 1031.5 [M+H-H2O]+ | 885.5 [M+H-H2O-rha]+ 739.4 [M+H-H2O-2Rha]+ 577.4 [M+H-H2O-2Rha-Glu]+ 415.3 [M+H-H2O-2Rha-2Glu]+ |
1047.5 | protodioscin |
| 4 | 3.36 | 885.5 [M+H-H2O]+ | 739.4 [M+H-H2O-Rha]+ 577.4 [M+H-H2O-Rha-Glu]+ 415.3 [M+H-H2O-Rha-2Glu]+ |
901.5 | protobioside |
| 5 | 4.95 | 1047.5 | 901.5 [M+H-Rha]+ 739.4 [M+H-Rha-Glu]+ 577.4 [M+H-2Glu-Rha]+ 415.3 [M+H-3Glu-Rha]+ 397.3 [M+H-3Glu-Rha-H2O]+ |
1045.5 | huangjiangsu A |
| 6 | 5.12 | 1031.5 | 885.5 [M+H-Glu]+ 739.4 [M+H-Glu-Rha]+ 577.4 [M+H-2Glu-Rha]+ 415.3 [M+H-2Glu-2Rha]+ 397.3 [M+H-2Glu-2Rha-H2O]+ |
1029.5 | pseudoprotodioscin |
| 7 | 5.52 | 885.5 | 739.4 [M+H-Rha]+ 577.4 [M+H-Glu-Rha]+ 415.3 [M+H-2Glu-Rha]+ 397.3 [M+H-2Glu-Rha-H2O]+ |
883.5 | 26-O-β-D-glucopyranosyl-3β,26-diol-25(R)-furost-5,20(22)-dien-3-O-α-L-rhamnopyranosyl(1→2)-O-β-D-glucopyranoside |
| 8 | 9.1 | 1069.5 [M+Na]+ | 885.4789 [M+H-Glu]+ 577.37 [M+H-2Glu-Rha]+ 415.32 [M+H-3Glu-Rha]+ 397.31 [M+H-3Glu-Rha-H2O]+ 271.20 [M+H-3Glu-Rha-C8H16O2-H2O]+ |
1045.5 | zingiberensis saponin I |
| 9 | 9.39 | 885.5 | 739.43 [M+H-Rha]+ 577.3696 [M+H-Glu-Rha]+ 415.3174 [M+H-2Glu-Rha]+ 397.3060 [M+H-2Glu-Rha-H2O]+ |
883.5 | deltonin |
| 10 | 9.51 | 869.5 | 723.43 [M+H-Rha]+ 577.37 [M+H-2Rha]+ 415.32 [M+H-Glu-2Rha]+ 271.20 [M+H-Glu-2Rha-C8H16O2]+ 253.19 [M+H-Glu-2Rha-C8H16O2-H2O]+ |
913.5 [M+HCOO]− | dioscin |
| 11 | 9.83 | 723.4 | 577.37 [M+H-Rha]+ 415.32 [M+H-Glu-Rha]+ 397.31 [M+H-Glu-Rha-H2O]+ 271.20 [M+H-Glu-Rha-C8H16O2]+ 253.19 [M+H-Glu-Rha-C8H16O2-H2O]+ |
721.4 767.4 [M+HCOO]− |
progenin III |
| 12 | 13.39 | 415.3 | 271.20 [M+H-C8H16O2]+ 253.19 [M+H-H2O-C8H16O2]+ |
- | diosgenin |
Figure 3.
SIM chromatograms (A) and mass spectra in positive (B) and negative (C) ion modes of standard compounds
CONCLUSIONS
The newly developed UHPLC-ELSD method for steroid saponins and diosgenin was found to result in shorter retention times while maintaining good resolution as compared to conventional HPLC. The developed method was validated for all parameters tested and successfully applied for the identification of 12 compounds in crude plant materials of Dioscorea species, and dietary supplements that claimed to contain wild yam. Additionally, it can also be used to differentiate Dioscorea species. The UHPLC analysis of various samples of Dioscorea species showed different profiles in terms of saponin content. Sixteen samples of D. villosa were found to contain varied concentrations of steroidal compounds and market products claiming to contain D. villosa revealed significant product-to-product variations. LC-mass spectrometry coupled with electrospray ionization (ESI) method was used for the identification of 12 compounds in plant samples and dietary supplements. This method involved the use of [M+H]+, [M+H-H2O]+ and [M+Na]+ ions in the positive and negative ion mode, peaks showed deprotonated molecules [M-H]− and a abundant [M+HCOO]− ions for standard compounds. Other significant peaks in positive ion mode resulted from the loss of one to two hexoses.
Figure 4.
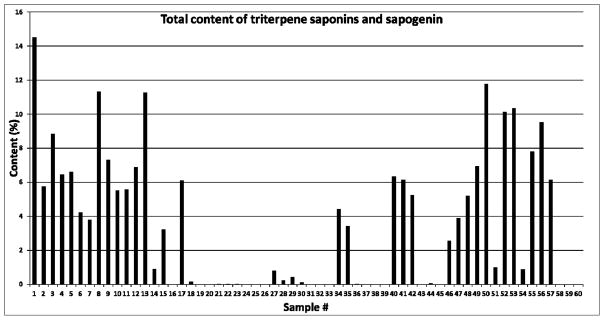
Total content (%) of triterpene saponins and sapogenin from different species of Dioscorea and commercial products using UHPLC-UV: Sample #1–16: D. villosa; #17–20: Dioscorea spp; #21–25: D. opposita; #27–30: D. cayennensis; #31–33: D. bulbifera; #34–35: D. caucasica; #36–37:D. alata; #38–39: D. rotundata; #40–42: D. nipponica; #43:D. batatas; #44: D. japonica; #45: D. trifida; #46: D. deltoidea; #47: D. quaternata; #48–61: Commercial products
Acknowledgments
This publication or project was made possible by Grant Number P50AT006268 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health. Additional funding was also provided by the United States Food and Drug Administration (FDA), Grant Number 5U01FD004246. The authors would like to thank Annette Ford and Julie R. Mikell for the extractions of plant samples and data checking. The authors would also like to thank Prof. Jinwoong Kim for providing the samples of D. batats (# 9797), D. japonica (# 9798) and D. nipponica (# 9799).
References
- Amir M, Ahmad A, Siddique NA, Mujeeb M, Ahmad S, Siddique WA. Development and validation of HPTLC method for the estimation of diosgenin in in vitro culture and rhizome of Dioscorea deltoidea. Acta Chromatogr. 2012;24:111–121. [Google Scholar]
- Ali Z, Smillie TJ, Khan IA. Cholestane steroid glycosides from the rhizomes of Dioscorea villosa (Wild Yam) Carbohydrate Research. 2013;370:86–91. doi: 10.1016/j.carres.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z, Smillie TJ, Khan IA. Two spirostan steroid glycoside fatty esters from Dioscorea cayenensis. Natural Product Communications. 2013;8:323–326. [PMC free article] [PubMed] [Google Scholar]
- Al-Shehbaz IA, Schubert BG. The Dioscoreaceae in the southeastern United States. J Arnold Arbor. 1989;70:57–95. [Google Scholar]
- Braun L, Cohen M. Herbs and Natural Supplements-An evidence-based guide. 2. Elsevier; Churchill Livingstone: 2007. p. 692. [Google Scholar]
- Chang DG. Nonhormonal therapies for hot flases in menopause. Am Fam Physician. 2006;73:457–464. [PubMed] [Google Scholar]
- Chen Y, Wu Y. Progress in research and manufacturing of steroidal sapogenins in China. J Herb Spic Med Plants. 1994;2:59–70. [Google Scholar]
- Dong M, Wu L, Chen Q, Wang B. Isolation and identification of steroidal saponins from Dioscorea panthaica Prain et burkill. Xaoxue Xuebao. 2001;36:42–45. [PubMed] [Google Scholar]
- Hayes PY, Lambert LK, Lehmann R, Penman K, Kitching W, De Voss JJ. Complete (1)H and (13)C assignments of the four major saponins from Dioscorea villosa (wild yam) Magn Reson Chem. 2007;45(11):1001–1005. doi: 10.1002/mrc.2071. [DOI] [PubMed] [Google Scholar]
- Hu K, Dong AJ, Yao XS, Kobayashi H, Iwasaki S. Antineoplastic agents. Part 1. Three spirostanol glycosides from rhizomes of Dioscorea collettii Hypoglauca. Planta Med. 1996;62:573–575. doi: 10.1055/s-2006-957978. [DOI] [PubMed] [Google Scholar]
- ICH. ICH Harmonized Tripartite Guidelines. Nov, 2005. Validation of Analytical Procedures: Text and Methodology. [Google Scholar]
- Liu JQ, Wang CF, Qiu MH, Hu WX. Sterodial saponins from flowers of Hosta plantaginea and their antitumor activities. Chin Tradit Herb Drug. 2010;41(4):520–526. [Google Scholar]
- Lin JT, Yang DJ. Determination of steroidal saponins in different organs of yam (Dioscorea pseudojaponica Yamamoto) Food Chem. 2008;108:1068–1074. doi: 10.1016/j.foodchem.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Zhoua HY, Yan Q. Fingerprint analysis of Dioscorea nipponica by high-performance liquid chromatography with evaporative light scattering detection. Anal Chim Acta. 2007;582:61–68. doi: 10.1016/j.aca.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Li R, Zhou Y, Wu ZJ, Ding LS. ESI-QqTOF-MS/MS and APCI-IT-MS/MS analysis of steroid saponins from the rhizomes of Dioscorea panthaica. J Mass Spectrom. 2006;41:1–22. doi: 10.1002/jms.988. [DOI] [PubMed] [Google Scholar]
- Mabberley DJ. The Plant Book. 2. Cambridge University Press; New York, NY: 1997. [Google Scholar]
- Martin FW. Agricultural Handbook. 466. USDA; 1974. Tropical Yams and Their Potential. Part 2. Dioscorea bulbifera. [Google Scholar]
- Morgan B. Kirk-Othmer Encyclopedia of Chemical Technology. 4. John Wiley and Sons; New York, USA: 1997. Steroids. [Google Scholar]
- Munafo JP, Jr, Ramanathan A, Jimenez LS, Gianfagna TJ. Isolation and structural determination of steroidal glycosides from the bulbs of easter lily (Lilium longiflorum Thunb.) J Agric Food Chem. 2010;58:8806–8813. doi: 10.1021/jf101410d. [DOI] [PubMed] [Google Scholar]
- Morgan M. Herbs for the treatment of dysfunctional uterine bleeding. [Accessed online July 27th 2011];A Phytotherapist’s Perspective. 2011 45 (May):1–3. at http://www.mediherb.com/pdf/6080.pdf. [Google Scholar]
- Raz L. Flora of North America Editorial Committee, editor. Flora of North America. Oxford University Press; New York: 2003. Dioscoreaceae: R. Brown: Yam Family; pp. 479–485. [Google Scholar]
- Sautour M, Miyamoto T, Lacaille-Dubois MA. Steroidal saponins and flavan-3-ol glycosides from Dioscorea villosa. Biochem Syst Ecol. 2006;34:60–63. [Google Scholar]
- Sautour M, Mitaine-Offer A-C, Lacaille-Dubois M-A. The Dioscorea genus: a review of bioactive steroid saponins. J Nat Med. 2007;61(2):91–101. [Google Scholar]
- Silva BP, Bernardo RR, Parente JP. New furostanol glycosides from Costus spicatus. Fitoterapia. 1998;69:528–532. [Google Scholar]
- Shen PP, Wang SL, Liu XK, Yang CR, Cai B, Yao XS. A new steroidal saponin from Dioscorea deltoidea Wall var. Orbiculata. Chin Chem Lett. 2002;13:851–854. doi: 10.1080/10286020290024013. [DOI] [PubMed] [Google Scholar]
- Soffa VM. Alternatives to hormone replacement for menopause. Altern Ther Health med. 1996;2:34–39. [PubMed] [Google Scholar]
- Shao Y, Poobrasert O, Kennelly VC, Chin K, Ho CT, Huang MT, Garrison SA, Cordell GA. Steroidal saponins from Asparagus officinalis and their cytotoxic activity. Planta Med. 1997;63:258–262. doi: 10.1055/s-2006-957667. [DOI] [PubMed] [Google Scholar]
- Theerasin S, Baker AT. Analysis and identification of phenolic compounds in Dioscorea hispida Dennst. As J Food Ag-Ind. 2009;2:547–560. [Google Scholar]
- USDA, NRCS. The PLANTS Database, Version 3.5. National Plant Data Center; Baton Rouge, LA: 2002. http://plants.usda.gov. [Google Scholar]
- Wang CY, Hu ZJ, Pang DP, Xu C. Study on steroidal saponins from Dioscorea zingiberensis. Chin Tradit Herb Drugs. 2009;40:36–39. [Google Scholar]
- Wunderlin RP, Hansen BF. Guide to the Vascular Plants of Florida. 2. University Press of Florida; Gainesville, FL: 2003. p. 787. [Google Scholar]
- Yang D-J, Lu T-J, Lucy S-H. Simultaneous determination of Furostanol and Spirostanol glycosides in Taiwanese Yam (Dioscorea spp.) Cultivars by HPLC. J Food Drug Anal. 2003;2:271–276. [Google Scholar]
- Yoon K-D, Chin Y-W, Yang M-H, Choi J, Kim J. Application of High-speed Countercurrent Chromatography-Evaporative Light Scattering Detection for the Separation of Seven Steroidal Saponins from Dioscorea villosa. Phytochem Anal. 2012;23(5):462–468. doi: 10.1002/pca.2342. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Xu F, Morikawa T, Pongpiriyadacha Y, Nakamura S, Asao Y, Kumahara A, Matsuda H. Chem. Medicinal Flowers. XII.1) New Spirostane-Type Steroid Saponins with Antidiabetogenic Activity from Borassus flabellifer. Pharm Bull. 2007;55:308–316. doi: 10.1248/cpb.55.308. [DOI] [PubMed] [Google Scholar]
- Zheng Q-A, Zhang Y-J, Li H-Z, Yang C-R. Steroidal saponins from fresh stem of Dracaena cochinchinensis. Steroids. 2004;69:111–119. doi: 10.1016/j.steroids.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Zhua J, Guoa X, Fua S, Zhangb X, Liang X. Characterization of steroidal saponins in crude extracts from Dioscorea zingiberensis C. H. Wright by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2010;53:462–474. doi: 10.1016/j.jpba.2010.05.019. [DOI] [PubMed] [Google Scholar]