Abstract
The developmental programs that contribute to myogenic stem cell proliferation and muscle fiber differentiation control fiber numbers and twitch type. In this study, we describe the use of an experimental model system—androgen-regulated laryngeal muscle of juvenile clawed frogs, Xenopus laevis—to examine the contribution of proliferation by specific populations of myogenic stem cells to expression of the larynx-specific myosin heavy chain isoform, LM. Androgen treatment of juveniles (Stage PM0) resulted in up-regulation of an early (Myf-5) and a late (myogenin) myogenic regulatory factor; the time course of LM up-regulation tracked that of myogenin. Myogenic stem cells stimulated to proliferate by androgen include a population that expresses Pax-7, a marker for the satellite cell myogenic stem cell population. Since androgen can switch muscle fiber types from fast to slow even in denervated larynges, we developed an ex vivo culture system to explore the relation between proliferation and LM expression. Cultured whole larynges maintain sensitivity to androgen, increasing in size and LM expression. Blockade of cell proliferation with cis-platin prevents the switch from slow to fast twitch muscle fibers as assayed by ATPase activity. Blockade of cell proliferation in vivo also resulted in inhibition of LM expression. Thus, both in vivo and ex vivo, inhibition of myogenic stem cell proliferation blocks androgen-induced LM expression and fiber type switching in juveniles.
Keywords: satellite cell, myosin heavy chain, Pax7, myogenic stem cells, hypertrophy
INTRODUCTION
The development of muscles in vertebrates—their number and contractile properties—is subject to extrinsic influences that include embryonic signaling molecules, activity in the motor neurons that provide innervation, and circulating hormones. Muscles arise from the proliferation and fusion of mesodermally derived stem cells of the somites, prechordal mesoderm, and the paraxial head mesoderm (Buckingham et al., 2003; Punch et al., 2009; Relaix and Marcelle, 2009; Kang and Krauss, 2010). Signals derived from the neural tube such as BMPs and Wnts regulate the expression of myogenic regulatory factors (MRFs) that determine muscle cell fates. Neural activity can influence gene expression within innervated myofibers including the expression of myosin heavy chain (MHC) genes essential for the speed of muscle contraction and other enzymatic activities that contribute to fatigue resistance (Schiaffino and Serrano, 2002; Ophoff et al., 2009). Circulating hormones, particularly androgens, influence muscle mass and fiber type across developmental epochs (Pette and Staron, 2001). Muscles, even those of adults, can regenerate following injury due to a specific population of myogenic stem cells, the satellite cells (Biressi and Rando, 2010). These cells also contribute to muscle growth in juveniles and adults (Hawke and Garry, 2001).
Since muscle wasting contributes to disease- and age-related morbidity in human populations, understanding the interplay between extrinsic factors that maintain muscle mass and contractile properties and the activation of myogenic stem cells has evoked considerable interest (Ophoff et al., 2009). Rodent models used in examining this interplay have the complication that changes in muscle physiology occur over the course of several weeks (Nnodim, 2001). Physiology is controlled by both the muscle and the nerve, making it difficult to interpret the contribution of each (Bass et al., 1969). For androgen-sensitive muscles such as the levator ani, receptors are highly expressed in both muscle and motor neurons and can act at either or both loci (Rand and Breedlove, 1995). Androgens are known to promote muscle growth both through formation of new fibers and through fusion of satellite cells to parent muscle fibers with resultant increases in numbers of myonuclei (Ophoff et al., 2009). In addition, androgens bias muscle fiber types toward the fast-twitch type (Altuwaijri et al., 2004). An unexplored issue, addressed in this study, is whether the satellite cell proliferation and fusion that accompanies muscle growth may actually be required for fiber type switching from slow to fast.
We are developing a vertebrate model system for examining the role of androgenic steroids in muscle fiber number and the determination of twitch type: the intrinsic laryngeal muscles of the vocal organ in clawed frogs, Xenopus laevis. Sex differences in muscle fiber numbers emerge during juvenile (post-metamorphic) development due to an androgen-driven program of satellite cell proliferation and fusion (reviewed in Zornik and Kelley, 2011). As is the case for other vertebrates, skeletal muscles in this species are a mix of muscle fiber types: fast and slow, fatigable, and fatigue-resistant. Laryngeal muscle of both male and female juveniles is also composed of a mix of fiber types. Laryngeal muscle then undergoes fiber-type switching from this mixture to an all-fast muscle as part of a male-specific developmental program. Females maintain a mixed population of fiber types into adulthood while males gradually convert to an all-fast twitch phenotype. During juvenile development, male laryngeal muscle also enlarges in comparison to female muscle.
While these processes normally occur over several months of post-metamorphic development, treatment of juvenile Xenopus with exogenous testosterone yields rapid hypertrophy and complete fiber-type switching in less than 7 days. Fiber type in the Xenopus larynx is independent of innervation: transection of the laryngeal nerve neither alter fiber type nor the ability of androgen to induce the male-specific fast fiber type (Tobias et al., 1993). All mature male laryngeal muscle fibers are fast twitch and all express a laryngeal-specific isoform (LM) of the MHC gene family (Catz et al., 1992). Most laryngeal muscle fibers in adult females are slow twitch. Some of the fast twitch fibers express LM but most do not suggest expression of other members of the MHC gene family as is the case in X. tropicalis (Baur et al., 2008). Expression of LM in male larynges already exceeds that in female larynges at the end of metamorphosis, before males start to convert slow- to fast-twitch fibers. LM expression in females peaks at the end of metamorphosis and then declines over the next 9 months unless androgen is administered (Catz et al., 1995).
Xenopus laryngeal muscle thus has several characteristics that make it a promising model for studying control of muscle fiber type: lack of dependence on innervation, rapid and complete change in fiber-type, and the ease of experimental manipulation due to androgen control. In particular, the lack of dependence on innervation allows for experimental manipulation in the whole larynx cultured ex vivo, where the chemical environment can be controlled without disrupting cell–cell contacts in the muscle itself. In this report, we utilize manipulations both in vivo and in ex vivo whole organ culture to demonstrate that myogenesis is necessary for the change in muscle fiber type during androgen treatment in Xenopus laryngeal muscle.
METHODS
In Vivo Experiments: Androgen-Induced LM and MRF Expression, Pax7, and Cell Proliferation
Previous studies had established that treatment with the androgen dihydrotestosterone (DHT) induces cell proliferation and increases the expression of LM (Sassoon et al., 1986; Catz et al., 1995) in juvenile larynges. In this study, we characterize androgen-induced laryngeal muscle development at a single developmental stage: PM0 (within 3 weeks after metamorphosis is complete). We followed the expression of LM and MRFs, specifically Myf-5 and Myogenin, in cDNA from larynges taken from DHT-treated PM0 juveniles. There are two other known MRFs: MyoD and MRF4. In preliminary studies, MyoD was expressed at only very low levels. MRF4 expression was not responsive to androgen and is, in addition, regulated at the level of protein production and translocation to the nucleus (Sabourin and Rudnicki, 2000), and is thus not suitable for a study of mRNA levels.
Animals and Hormone Treatment
Juvenile X. laevis were obtained from commercial suppliers (NASCO, Xenopus I). Frogs were at stage PM0 at the beginning of the study [less than 2 weeks after full regression of the tail, less than 2 cm snout to vent length (Tobias et al., 1993)]. Animals were housed 20–30 in polycarbonate tanks with approximately 4 L of filtered tap water, were regularly fed frog pellets (NASCO) and water was changed twice per week.
Androgen was delivered as previously described (Edwards et al., 1999). Dihydrotestosterone powder (Sigma) was mixed at 10% with medical grade silicon, which was then extruded into 1 mm diameter tubing (Tygon). After hardening, the tubing was removed and the resulting silicon rope was cut into 2.5 mg pellets. Animals were immobilized by treatment with 0.1% MS-222 (ethyl m-amino benzoate, methane sulfonic acid; Sigma) and pellets were inserted into the dorsal lymph sac through a small incision.
Measurement of LM and MRFs
Animals were killed before androgen treatment, or 6 h, 2, 5, or 7 days after androgen treatment, and the amount of LM and MRF mRNA was quantified using real time PCR and normalized to the control gene elongation factor 1 (EF-1a). Reverse transcription was carried out using MMLV reverse transcriptase (Invitrogen) using approximately 100 ng of RNA isolated from larynges using Tri-Reagent (Sigma) following the manufacturer’s protocol. Real time measurements of fluorescence during polymerase chain reaction (qPCR) were carried out using a ABI Prism 7700 (Applied Bioscience) sequence detector and the Dynamo SYBR Green Kit (Bio-Rad) was used for PCR amplification of DNAse I treated cDNA. Primers used were as follows: LM—cttctga gacttcaggacctggtggaca and tgtccccagcttattgacttgagattca; Myf-5—cgatctacagacaagtttctcttcaaccaa and gtaggagacgggg tgatagagtctggaata; EF-1—tgacatgatcccaggaaagccaatgtgc and cttcttttccactgccttgatgactcctac; myogenin—gcaacatattca gaattaagcactctgcac; and ggttaataaatgcatatttgtctatgatgg.
RNA from three larynges was isolated individually and two replicates were carried out for each sample/probe combination. DNA amplification was ruled out by the inclusion of an RNA preparation that had not been reverse transcribed. Specificity of the resulting PCR product was determined either by electrophoresis or by melt-curve analysis following PCR amplification. Values for relative amounts of each probe tested were determined using the RQ software package (Applied Biosystems) using PM0 larynges as a calibrator and EF-1 as the endogenous control. Serial dilutions of reverse transcribed RNA were used to determine that PCR efficiency was within the parameters recommended by the manufacturer for all probes used. For statistical analysis, the two replicates were averaged for each gene tested, and the three independent larynges for each time point were included as variables for a single tailed Student’s t-test using the Bonferroni correction for multiple comparisons.
Androgen-Induced Proliferation and Pax7 Expression
Because the androgen-responsive myogenic stem cell has the ultrastructural characteristics of a satellite cell (Sassoon et al., 1986), we followed proliferation of this cell type specifically using expression of Pax-7, a well-established marker for satellite cells (Relaix and Marcelle, 2009) that is known to be expressed in the satellite cells responsible for the regeneration of the tadpole tail in X. laevis (Chen et al., 2006).
For whole mount immunocytochemistry, androgen-implanted (as described above) and untreated juveniles were injected intraperitoneally with 15 μL of a 10 mg/mL solution of BrDU in PBS; PBS alone served as a control. Animals were allowed to recover for 2 h after injection before being killed. Larynges were fixed in 4% paraformaldehyde for 2 h followed by 2 h in Dent’s fixative (4:1 methanol:DMSO) and then washed and permeabilized by overnight incubation in PBS with 1% Triton-X. Larynges were then washed and treated twice for 20 min in 2N HCl, which was then neutralized by immersion in sodium borate (pH = 8.5). To reduce autofluorescence, larynges were treated 10 times in 0.1% boroanhydride for 10 min after each incubation. After further washing in PBS, larynges were incubated overnight with a 1:100 dilution (in 1× PBS, 1% Triton-X, 5% Normal Goat Serum) of rabbit anti-BrDU (Immunology Consultant Laboratory) and a 1:4 dilution of supernatant containing a mouse monoclonal antibody against Pax-7 obtained from the University of Iowa Developmental Studies Hybridoma Bank. Following extensive washing, larynges were incubated overnight with 1:200 dilutions of goat anti-rabbit antibody labeled with AF488 (Invitrogen) and goat anti-mouse antibody labeled with AF568 (Invitrogen). After washes with PBS, larynges were dehydrated in methanol and cleared in BABB, then mounted for confocal imaging.
Measurements of bromodeoxyuridine (BrDU) incorporation showed an increase in cell proliferation between 2 and 4 days of androgen treatment; the 2-day duration was chosen. Larynges collected from the BrDU-treated animals were subjected to whole-mount immunocytochemistry using antibodies against BrDU and Pax-7 (Kawakami et al., 1997) and cells expressing Pax-7 and incorporating BrDU were counted in optical sections of laryngeal muscle.
Confocal imaging was carried out using an Olympus fluo-view microscope. For each larynx, three independent images, 256 μm × 256 μm (512 × 512 pixels) were obtained, from both laryngeal muscles. The two antibodies were detected sequentially to avoid bleed through and the voltage applied to the photomultiplier tubes was adjusted to best match the apparent brightness observed by epifluorescence.
Cell counting was automated using Image-J (NIH). For each image and each channel, a threshold was selected to include only the brightest 5% of pixels. Threshold exceeded 80 (luminosity on an 8 bit image) to exclude residual auto-fluorescence in images having few cells. Cells on the resulting image were than counted using the particle analysis function of ImageJ, counting objects of sizes between 100 and 300 square pixels. These parameters were determined empirically on test images to match manual counts. To obtain BrDU/Pax7 double expression, the two images were combined using the AND image logic function and counted as described above.
For each data point, the number of BrDU positive cells from the three counts for each of three larynges were then averaged. The number of positive cells counted in hormone-treated juveniles was compared with the number of BrDU positive cells from untreated juvenile animals using Student’s t-test (one tailed). A Bonferroni correction was applied to alpha by dividing the desired alpha by the number of comparisons, in this case, 5. To determine if the total number of Pax-7 expressing cells varied across samples, an ANOVA was performed using the Excel statistics package (Microsoft). The percentage of BrDU positive cells that were also Pax-7 positive was determined by counting the total number of BrDU positive cells as well as the total number of BrDU/Pax-7 positive cells across all three images from each larynx and dividing the number of double positive cells by the number of BrDU positive cells.
Ex Vivo Experiments: Laryngeal Explant Culture, Relation of Proliferation to Fiber Type, and LM Expression
To determine whether cell proliferation contributes to fiber type switching, we developed an ex vivo system that permits direct administration of androgens and antimitotic agents without compromising overall juvenile development. PM0 animals were anesthetized in MS-222, then surface sterilized in 100% ethanol and transferred to a sterile field. Larynges were dissected and cleaned, transferred to L-15 media, placed in a 12-well tissue culture plate on beds of 300 μL 1% Agarose and covered with 300 μL of L-15 media containing 10% fetal bovine serum (Invitrogen) with 1× PSN antibiotic mix (Invitrogen), 50 μg/mL gentamycin, and 50 ng/mL prolactin (Sigma). Cultures were maintained at 21°C; where androgen was added, dihydrotestosterone (Sigma) was prepared in 100% ethanol at 1000 fold the concentration to be used, and ethanol alone was used as vehicle control. DHT concentration was 1 μg/mL except as indicated. Larynges were harvested after 2, 3, 4, 6, 8, and 10 days in culture without replacement of media. For dose response, larynges were cultured for 10 days.
To test whether inhibition of cell proliferation inhibited upregulation of LM, larynges were cultured either in the absence of DHT, the presence of DHT, or DHT along with cis-platin. Cis-platin functions by covalently linking the two strands of DNA, resulting in DNA breaks at S-phase, resulting in apoptosis in most dividing cells (Schimke et al., 1994). In pilot experiments, inclusion of cis-platin at 50 μM concentrations resulted in a dramatic reduction in the incorporation of tritium-labeled thymidine (not shown), demonstrating that it has an anti-proliferative affect on the explant larynx. After treatment in culture, larynges were sectioned and examined for acid stable ATPase activity or LM mRNA expression as detected by in situ hybridization. For ATPase staining, larynges were frozen in dry ice in 60% OCT (O.C.T 4583, Tissue Tek) and transversely sectioned (30 μm) using a Bright cryostat at −20°C. Sections were allowed to dry for 4 h before being stored at −80°C. To distinguish between slow- and fast-twitch fibers, we used a modification of (Guth and Samaha, 1970). Slides were incubated for 15 min in acid buffer (50 mM potassium acetate, pH 4.5) and then for 30 min in ATPase solution (100 mM adenosine monophosphate, 2.7 mM ATP, 50 mM KCl, 18 mM CaCl2) at 30°C, washed with 1% CaCl2, placed for 3 min in cobalt chloride (2% in water), washed in water, and finally placed for 3 min in an ammonium sulfide solution (2% in water) before being washed and coverslipped in Crystal Mount (Biomeda). Using this procedure, slow-twitch fibers stain more intensely due to a black precipitate, while fast-twitch fibers stain less intensely (Sassoon et al., 1987).
In situ hybridization was performed using the same probe as in Catz et al. (1992); no labeling was apparent using sense probes. Probe was labeled with digoxygenin using T7 RNA polymerase following manufacturers protocol (Roche), labeled probes were incubated with frozen tissue sections as described previously (Catz et al., 1992), and detection was carried out using a fluorescent-labeled anti-digoxygenin antibody (Roche).
Cell Proliferation and LM Expression: In Vivo Confirmation
To insure that the ex vivo results were due to the anti-proliferative effects of cis-platin and not to non-specific effects of the drug, or interactions of the drug with culture components, a different anti-proliferative drug was used in vivo to inhibit proliferation in response to androgen. The mechanisms of action of the drug used, cytosine arabinofuranoside (Ara-C), is distinct from cis-platin: incorporation into DNA results in interference with topoisomerase activity and DNA fragmentation, leading to cell-cycle arrest or apoptosis (Gmeiner et al., 2003). Ara-C was prepared as a 100 μg/mL solution in PBS. A total of 100 μL of Ara-C or PBS alone was administered by intraperitoneal injection Ara-C every other day after androgen implantation, and larynges were collected at the end of a 7-day period. BRDU incorporation was measured as described above for in vivo experiments.
Preparation of Antibodies to LM
To examine MHC expression in laryngeal muscle, LM protein expression was assayed in Western blots. The high degree of amino acid sequence similarity among MHC molecules makes generation of isoform specific antibodies difficult. As no antibody specific to laryngeal myosin was available, an antibody against LM was raised in chickens; preabsorption with myofibrillary protein enhanced its specificity for LM over other MHC isoforms. As an antigen, we used a peptide from a region of the X. laevis LM, where several amino acids differ in comparison to other MHC isoforms in Xenopus tropicalis (amino acids 1921–1934). The synthetic peptide (CZ DEQ ANA HIS HFR KNQ HEL EE) was injected into chickens. Antibody isolated from eggs from one chicken (#4808), when incubated with paraffin-sectioned laryngeal muscle from PM1 juveniles, showed muscle fiber-specific staining that was much higher in androgen-treated juvenile laryngeal muscle than in muscle from untreated juveniles; no muscle fiber-specific signal was detected from preimmune extract from eggs of the same chicken before peptide injection.
Some signal from this antibody was detected in the untreated juvenile and appeared to be restricted to a subpopulation of fibers (it also recognized an antigen in dot blots from thigh muscle). To remove antibodies that might bind to conserved regions of MHC within the peptide used as an antigen, the polyclonal chicken antibody was incubated with myofibrillar protein (including MHC from thigh muscle) in high potassium, in which myofibrillar proteins will not be water soluble. After removal of the myofibrillar protein pellet, binding to dot-blots was again compared. While binding to myofibrillar proteins from thigh muscle was greatly reduced, binding to myofibrillar protein from laryngeal muscle was not, suggesting that the procedure increased specificity for the LM protein.
Myofibril protein extracts from juvenile animals that were either untreated or implanted with androgen pellets for 7 days were separated by electrophoresis using a modified SDS-PAGE gel. MyHC was then detected using an antibody that binds to all MHC isoforms (MF-20 monoclonal antibody obtained from University of Iowa Developmental Studies Hybridoma Bank). These results yield partial separation of three bands in the untreated juvenile. Comparison with myofibrillar protein of larynges from DHT-treated animals revealed the presence of an additional band. The blot was sequentially probed with the preadsorbed LM antibody and showed much higher binding to myofibrillar protein from androgen-treated laryngeal muscle than from muscle-untreated animals. The strong band detected by the LM antibody corresponds with the band detected by the MHC antibody in protein from androgen-treated larynges. This androgen-regulated band, which binds to an antibody generated against an LM peptide, therefore is likely to be LM protein.
Electrophoresis and Western Blotting
MHC isoforms were separated using an electrophoretic modification of a previously published protocol (Talmadge and Roy, 1993). Myofibrillar protein was isolated by homogenizing larynges in 500 μL myofibril dissociation buffer (250 mM sucrose, 100 mM KCl, 5 mM EDTA, and 20 mM Tris, pH 6.81), followed by 10 s of centrifugation. Pellets were washed briefly in buffer (175 mM KCl, 2 mM EDTA, 20 mM Tris pH = 6.8, 0.5% Triton-X100). Following centrifugation, pellets were dissolved in sample buffer (100 mM Tris, pH = 6.7, 40% glycerol, 4% SDS, 5% 2-mercaptoethanol) and boiled. Samples were run through a stacking gel (30% glycerol, 4% acrylamide, 70 mM Tris, pH = 6.7, 0.4% SDS) and a 7.5% acrylamide gel (7.5% acrylamide, 0.1 M glycine, 0.4% SDS, 40% glycerol, 20 mM Tris pH = 8.8). The gel was run for 26 h at 120 V at 40°C.
Following electrophoresis, proteins were transferred to nitrocellulose paper overnight. Blots were blocked for 30 min in 1× PBS, with 5% powdered milk, then incubated with a 1:2000 dilution of a preadsorbed anti-LM antibody, and 1:500 dilution of MF-20 monoclonal antibody (obtained from University of Iowa Developmental Studies Hybridoma Bank) for 2 h in 1× PBS, 5% powdered milk, and 0.3% Tween-20. Following several washes, blots were incubated with 1:10,000 dilutions of AF-647 labeled anti-chicken (Invitrogen), or IRDye800 labeled anti-mouse antibody (Li-Cor) for 2 h. After further washing, blots were dried and imaged using an Odyssey Infrared Imaging System (Li-Cor).
RESULTS
In Vivo Experiments: Androgen-Induced LM and MRF Expression, Pax7, and Cell Proliferation
Androgen-Induced LM and MRF Expression
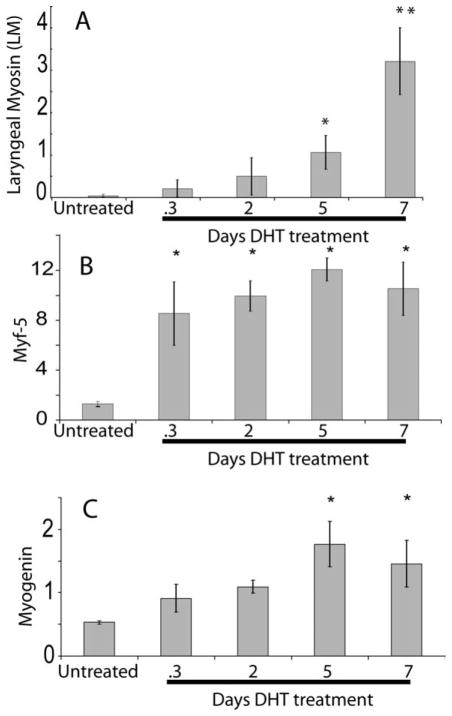
Using quantitative PCR, we followed androgen-induced expression of LM in relation to expression of MRFs in larynges from PM0 juveniles (Fig. 1). Expression levels were normalized to EF-1α and compared with values from untreated juveniles. While LM mRNA levels increased during the 7 days of treatment, levels were significantly different from untreated controls only after 5 (corrected p-value <0.05) and 7 (corrected p-value <0.01) days of treatment [Fig. 1(A)]. As previously reported (Sassoon and Kelley, 1986), larynges also enlarged after 5 days of treatment and further increased in size by 7 days (not shown).
Figure 1.
Increase in LM (A), Myf-5 (B), and myogenin (C) gene expression measured by quantitative PCR over 7 days of androgen treatment in juvenile Xenopus larynges. (A) After normalization of LM mRNA levels to EF-1, a statistically significant increase in LM mRNA expression is detected five (to p < 0.05) and seven (p < 0.01) days after androgen implantation. (B) Myf-5 is rapidly (by 6 h; p < 0.05 after Bonferroni correction) upregulated after androgen implantation and levels are elevated throughout 7 days of treatment. (C) Myogenin is upregulated more slowly in response to androgen treatment; 5 and 7 days values differ significantly from control (0 Days) levels (C, p < 0.05 after Bonferroni correction). Error bars show SEM, *p < 0.05 indicates a statistically significant increase and **p < 0.01 indicates a statistically significant increase when compared with the untreated mRNA level (0 days) after Bonferroni correction. N = 3 for all samples.
Myf-5 responds to androgen more rapidly than LM. Myf-5 expression increases relative to control values within 6 h of androgen treatment, and remains high throughout the androgen response [Fig. 1(B)]. Myogenin, in contrast, attains statistically significant increases in expression over control values only at 5 and 7 after androgen implantation [Fig. 1(C)], a pattern that parallels the time course of androgen-induction of LM [Fig. 1(A)]. The sequential upregulation of an early (Myf-5) and a late (myogenin) MRF by androgen confirms our previous observations (see below) on the role of this hormone in laryngeal myogenesis in X. laevis and provides additional molecular markers for following masculinization.
Pax7 and Cell Proliferation
Androgen treatment has been previously shown to increase the rate of incorporation of labeled nucleotides into Xenopus larynges (Sassoon et al., 1986; Fischer et al., 1993). An increase in cell proliferation in both the muscle and cartilage components was demonstrated and electron microscopy suggested that the proliferating cells in muscle are muscle satellite cells. To explore this issue, we collected larynges from androgen-treated juveniles and examined cell proliferation with BrDU and expression of a satellite cell marker gene, Pax-7.
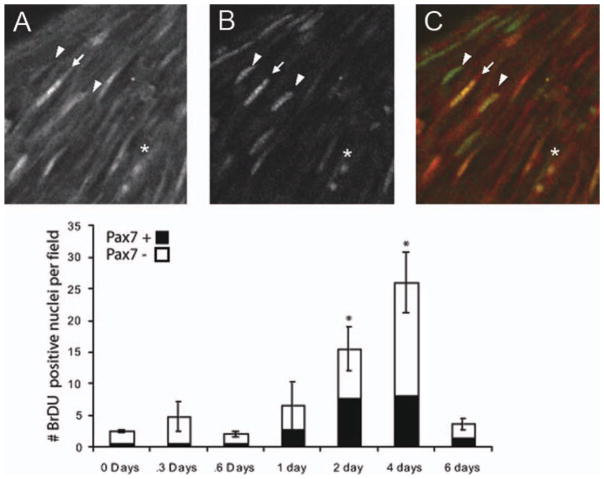
Nuclei of proliferating cells appeared as either a pair of small round nuclei (asterisk) indicative of the end of mitosis or as a distinct elongated morphology [arrows and arrowheads; Fig. 2(A–C)]. Following androgen treatment, the overall number of Pax-7 expressing cells per field remained unchanged (not shown, ANOVA, p = 0.054) although the density of cell labeling was slightly higher than in larynges of untreated animals (2 days of androgen treatment: 22 cells/field in untreated vs. 34 cells/field in treated; p-value = 0.050). Both before (not shown) and after androgen treatment, some proliferating cells were found to express Pax-7 [Fig 2(B)]. Cells that were found to incorporate BrDU, but not express Pax-7, had nuclear morphologies similar to Pax-7 expressing cells. Compared with untreated controls, where 26% of BrDU incorporating cells were Pax-7+ (6/23 cells), a somewhat larger percentage of proliferating cells expressed Pax-7 after at least 1 day of treatment with 42% (25/59 cells), 49% (69/140 cells), 31% (73/234 cells), and 42%(14/33 cells) of BrDU labeled cells also expressing Pax-7 at 1, 2, 4, and 6 days, respectively [Fig. 2(D)].
Figure 2.
Pax-7 expression and BrDU incorporation during androgen treatment of newly metamorphosed animals. (A) After 2 days of androgen treatment, BrDU has accumulated in nuclei shown in confocal image taken parallel to muscle fibers of whole mount larynges. Nuclei are elongated and are similar to the morphology of Pax-7 immunoreactivity (B) in the same location. Pax-7 and BrDU immunoreactivity overlap (A–C, arrow), but not all BrDU positive cells are Pax-7 positive (A–C, arrowhead), and not all Pax-7 cells are BrDU positive as is shown in an overlay of Pax-7 (red) and BrDU (green) labeling (C). (D) Counts of cells show that the number of BrDU positive cells increases during androgen treatment (error bars show SEM, * denotes statistically significant difference from day 0 of p < 0.05 for Student’s t-test after Bonferroni correction for multiple comparisons). Throughout androgen treatment, BrDU accumulating cells include cells that are Pax-7 positive, as well as those that are Pax-7 negative (black bars show proportion of BrDU positive cells that were also positive for Pax-7.
That the percentage of Pax-7 expressing cells doubles by 2 days after androgen treatment is begun, suggests that this population is sensitive to DHT. Cells that incorporate BrDU but do not express Pax-7 could be satellite cells that have downregulated Pax-7 expression during differentiation. Laryngeal muscle may also contain myogenic populations that do not express Pax-7, androgen-sensitive or not.
Ex Vivo Experiments: Laryngeal Explant Culture, Relation of Proliferation to Fiber Type and LM Expression
Larynges removed from early postmetamorphic frogs survived in explant cultures for periods of at least 2 weeks and maintained expression of housekeeping genes, persistence of muscle ATPase activity, and the absence of changes in nuclear morphology normally associated with apoptotic or necrotic tissue. During this period, larynges remained sensitive to androgen. Supplementing the culture media with androgen increased LM mRNA expression [Fig. 3(B)] and produced an increase in the overall size of the larynx [Fig. 3(A)]. Without androgen supplementation, the relatively small amount of LM already expressed at this stage declined [Fig. 3(B), untreated].
Figure 3.
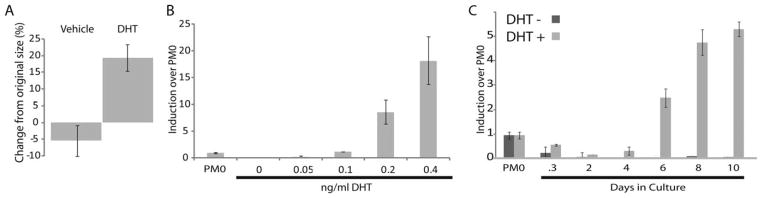
An ex vivo model of muscle fiber type switching. Larynges were dissected from PM0 X. laevis and cultured in the presence or absence of DHT. (A) Larynges were measured across the widest part of the larynx. In the absence of DHT, laryngeal size was reduced by 5%. Larynges cultured in DHT increased in size by 18%. (B) During 10 days of ex vivo culture, LM expression, as measured by qPCR and normalized to EF1, maintained sensitivity to DHT. At DHT concentrations lower than 0.1 ng/mL, LM expression dropped below levels found in PM0 larynges, while at 0.2 ng/mL or higher, LM expression increased. (C) LM expression in ex vivo culture decreased over the first 4 days in culture. However, in the presence of DHT, LM expression levels after 4 or 8 days increased over levels of expression in untreated PM0 larynges.
The response of LM mRNA expression to androgen was dose dependent [Fig. 3(B)]. Without addition of androgen, LM mRNA levels fell to one-tenth the levels found in larynges taken directly from animals of the same stage without any period of explant culture [Fig. 3(B)]. Supplementation of the media with 0.1 ng/mL DHT was sufficient to maintain LM mRNA levels typical of larynges from PM0 juveniles. At higher concentrations of DHT (0.2 ng/and 0.4 ng/mL), LM mRNA levels were 10 and 20 times those characteristic of larynges from PM0 frogs [Fig. 3(B)].
The time-course of LM expression is also similar to that of the in vivo larynx. In ex vivo culture, regardless of the presence of DHT, there is a decrease in LM mRNA expression, however, in the presence of DHT, LM levels rise above initial values by day 6, while they continue to fall in cultures not treated with DHT [Fig. 3(C)].
Myogenesis, Satellite Cells, and Fiber Type Switching
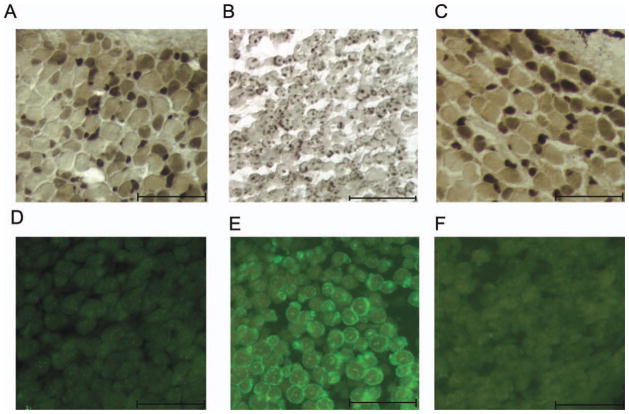
To test whether inhibition of cell proliferation inhibited androgen-induced upregulation of LM, larynges were cultured either in the absence of DHT, the presence of DHT, or DHT together with cis-platin. In explant culture, the juvenile larynx maintains the mix of fiber types characteristic of juvenile larynges at this stage in vivo: small fibers that stain intensely for ATPase, medium-sized fibers that exhibit moderate staining and large fibers that stain lightly [Fig. 4(A)]. Treatment with DHT resulted in a loss of acid-stable ATPase activity in muscle fibers [Fig. 4(B)] and conversion to a staining pattern and more uniform fiber size characteristic of the fast-twitch fibers of the adult male [Fig. 4(B)]. Inclusion of cis-platin along with DHT in the explant culture media inhibited this response to androgen, resulting in retention of the juvenile staining pattern [Fig. 4(C)]. Treatment of explant larynges with DHT induced LM in muscle [compare Fig. 4(E) to Fig. 4(D)] while laryngeal explants cultured with DHT and cis-platin did not have detectable LM mRNA in muscle [Fig. 4(F)]. Thus results from ex vivo cultures suggest that the conversion of juvenile muscle fibers from fast to slow twitch requires androgen-evoked cell proliferation.
Figure 4.
The effects of DHT with and without an antimitotic agent (cis-platin) on LM expression and muscle fiber type in laryngeal explant cultures 10 days in vitro. (A) Explanted larynges cultured in the absence of androgen did not express LM. (B) Ten days of androgen treatment increased LM mRNA expression. (C) However, inclusion of cis-platin along with DHT in the media prevented the increase in LM mRNA. (D) Explanted larynges cultured in the absence of androgen maintain typical juvenile patterns of acid stable ATPase activity in muscle fibers. (E) Androgen treatment for 10 days changed the ATPase staining pattern: fibers were uniformly light and small dark fibers were not present. (F) In the presence of cis-platin and DHT, fiber type ATPase staining resembled the untreated control (D).
In Vivo Experiments: Confirmation of the Relation of Proliferation to Fiber Type and LM Expression
To confirm that the inhibition of LM upregulation was due to the anti-proliferative effects of cis-platin and not due to nonspecific effects of the drug, or interactions of the drug with culture components, another anti-proliferative drug was used in vivo. Animals given 10 μg Ara-C after 3 days of androgen treatment had greatly reduced amounts of cell proliferation as measured by BrDU incorporation [Fig. 5, compare (B) to (A)].
Figure 5.
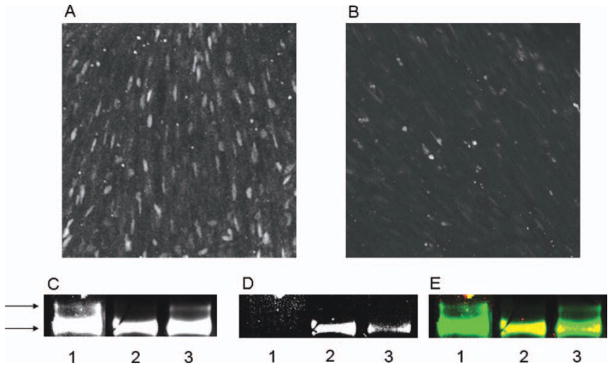
Treatment with the antimitotic agent, Ara C, blocks cell proliferation, and reduces LM expression in vivo. (A) Androgen-evoked BrDU labeling in juvenile laryngeal muscle. (B) Treatment with Ara-C reduces the number of BRDU positive cells. (C–E) Western blot of myofibrillar protein using an antibody that recognizes all isoforms of MHC (MF-20) to visualize MHC resolves two bands in untreated juveniles [(C), lane 1], but only one in androgen-treated juveniles [(C), lane 2]. When androgen treatment was accompanied by Ara-C treatment, MHC isoforms in myofibrillar protein separated into two bands [(C), lane 3]. The same blot showed recognition of the LM antigen in the androgen treated sample [(D), lane 2], but not in myofibrillar protein from untreated larynges [(D), lane 1]. Ara-C administered during androgen treatment led to a diminished reactivity of myofibrillar protein with the LM antibody [(D) lane 3]. The overlay of MF-20 immunoreactivity (green) and LM immunoreactivity (red) in yellow indicates that that LM immunoreactivity runs at the same location as the lower band detected by the MF-20 antibody (E).
To assess the prevalence of different MHC iso-forms, a specialized system of poly-acrylamide gel electrophoresis (MHC-PAGE) was used along with an antibody that had been raised against LM. MHC iso-forms from untreated juvenile Xenopus separated into two bands as indicated by arrows [Fig. 5(C)], neither of which reacted with the LM antibody [Fig. 5(D), lane 1]. After 7 days of DHT treatment, MHC from larynges resolved into only a single band [Fig. 5(C), lane 2], which did react with the LM antibody [Fig. 5(D), lane 2]. The LM immunoreactivity maps to the lower band of MHC reactivity [Fig. 5(E)]. Larynges from animals receiving Ara-C throughout the 7 days of androgen treatment retained the upper band [Fig. 5(C), lane 3], normally only found in the untreated larynx, and the reaction of the lower band to the LM antibody was greatly reduced [Fig. 5(D) and (E), lane 3]. Thus, both in vivo and ex vivo, inhibition of cell proliferation results in a reduction in the induction of LM and fiber type switching in juvenile X. laevis.
DISCUSSION
Explant Laryngeal Culture as a Model of MyHC Gene Expression
The lack of requirement for innervation during changes in MHC gene expression seen in X. laevis laryngeal muscle is very unusual for vertebrates. We have taken advantage of this characteristic to generate an explant culture that undergoes androgen-induced changes in MHC gene expression that are similar to those in the intact animal. We have shown that this explant culture retains sensitivity to androgen as evinced by cell proliferation, growth in size, and upregulation of LM. The induction of LM is dependent on proliferating cells as it is in vivo. This system allows for the manipulation of the extracellular environment, while maintaining the three-dimensional structure of the muscle, and all intercellular interactions, intact.
Using this explant culture, we were able to show a dose-dependent response of the larynx to androgen. At 0.1 ng/mL DHT, levels similar to those recorded in males before the changes in MHC gene expression occur (Kang et al., 1995), cultured larynges expressed LM at levels that were very similar to those found in larynges taken directly from juvenile animals. Our results are consistent with the previous finding (Catz et al., 1995) that the small level of LM expressed in juveniles was responsive to go-nadectomy resulting in the removal of androgen. This initial, androgen sensitive, phase of LM induction may be due to expression of LM in myogenic stem cells before fusion, after which LM expression may be maintained independently of androgen (but see Zornik and Kelley, 2011).
Studies of circulating androgen levels during post-metamorphic development have shown that a statistical difference between males and females does not appear until well after conversion of the muscle to a male phenotype (Kang et al., 1995). Average circulating androgen levels are ~ 0. 2 to 0.5 ng/mL during the time that masculinization takes place. Our dose response study shows that LM induction can occur at levels less than 0.3 ng/mL DHT, and is thus consistent with androgen levels during post-metamorphic development. Brief pulses of slightly increased androgen secretion may be sufficient to promote masculinization of laryngeal muscle.
Androgen levels present in adult females (0.8 ng/mL; Kang et al., 1995), might be expected to be sufficient for induction of LM in laryngeal muscle. However, levels of LM mRNA drop over the course of female development and are not increased when females begin producing hormones at maturation (Catz et al., 1992). One explanation for this apparent contradiction might be that androgen-sensitive myogenic stem cells are either lost during female development, by differentiating into other tissue or apoptosis, or lose the ability to expand clonally in response to hormone stimulation. A trophic effect of androgen on muscle cells has also been observed in another well-studied sexually dimorphic muscle, the rodent levator ani (Nnodim, 2001).
Cell Proliferation is Required for LM Expression, MHC Isoform Expression, and Fiber Type Switching in Response to Androgen
Muscle is a complex tissue composed primarily of syncytial muscle fibers, the axons of motor neurons, and muscle stem cells. Both the motor neurons and the muscle stem cells are in close contact with the muscle fiber and are important for the maintenance, repair, and physiological regulation of the muscle fibers. Previous studies have shown that the changes in MHC gene expression that occur due to androgen treatment in X. laevis are independent of innervation (Tobias et al., 1993), indicating that muscle tissue itself is capable of directly responding to androgen. Responses include increases in cell proliferation, and with the exception of a small number of fibroblasts, the proliferating cells of the laryngeal muscle have morphologies and positions consistent with those of satellite cells. Laryngeal satellite cells were observed to eventually differentiate into new muscle fibers or to fuse with existing muscle fibers (Sassoon et al., 1986).
In the present study, the time course of MRF induction supports previous observations on the eventual differentiation of satellite cells in response to androgen. The earliest response to androgen treatment is an upregulation of Myf-5 detectable as soon as 3 h after the onset of treatment. Androgen-induced LM expression is concurrent with muscle differentiation as reflected in myogenin expression. Together these observations suggest a link between androgen-induced myogenesis and androgen-induced changes in MyHC gene expression. A similar coupling between myogenesis and MHC expression in Xenopus occurs when larval muscle tissue degenerates and is replaced by adult muscle (Shimizu-Nishikawa et al., 2002). In rodents, muscle regeneration due to activation of satellite cells is linked to expression of slow-twitch myosins especially under conditions of chronic, low frequency electrical stimulation (Pette et al., 2002).
During laryngeal muscle development in male X. laevis, new muscle fibers are added at a rapid rate, when the adult complement is reached the process of muscle fiber conversion from slow to fast twitch begins (reviewed in Zornik and Kelley, 2011). During this time, there is no morphological evidence for the degeneration of existing muscle fibers (Robertson et al., 1994) suggesting that the male-typical complement of entirely fast-twitch fibers is due to both the addition of this type and conversion of slow-twitch fibers to fast. Several studies, including some in Xenopus laryngeal muscle (Sassoon et al., 1986), have shown that when satellite cells are activated, they can either differentiate into new myo-tubes or fuse with pre-existing muscle fibers, thus donating nuclei to those muscle fibers (Schultz and McCormick, 1994). Together these observations support the hypothesis that laryngeal fast twitch muscle fibers arise from satellite cells nuclei that direct LM expression either upon differentiation of new fibers or after fusion with existing fibers.
The developmental origin of the myogenic stem cells fated to express LM has been unclear. Although a previous study using EM had identified all but a few of the proliferating cells as satellite cells (Sassoon et al., 1986), in this study only 30–50% of proliferating cells expressed Pax-7, the most common marker of satellite cells. This result could point to distinct populations of androgen-sensitive myogenic stem cells, each of which might have different fates upon differentiation. Indeed, myogenic stem cells isolated from juvenile Xenopus laryngeal muscle were found to be heterogeneous in their expression of LM, both as dividing myogenic stem cells as well as after myogenic stem cell fusion (Kelley, unpublished observations).
Myogenic stem cell populations that differ in MHC expression fates, or in response to hormone stimulation, have been previously reported in Xenopus. For example, during metamorphosis, specific myogenic stem cell populations respond differently to thyroxine, the controlling hormone (Shimizu-Nishikawa et al., 2002). One population undergoes apoptosis, while thyroxine acts as a trophic factor for the other, facilitating conversion of larval MyHC isoforms to adult MyHC isoforms by muscle death and replacement. In mammals, myogenic stem cell colonies with propensities for differentiation into fast or slow MyHC expressing myotubes have been isolated (Robson and Hughes, 1999).
An even more striking example is the difference in primary and secondary myogenesis during embryonic and fetal development of mammals (Chanoine and Hardy, 2003). During embryonic development, initial colonization of the limb buds by myogenic stem cells is followed by differentiation into primary myotubes. Myogenic stem cells isolated from limb buds during this time give rise to short fibers, containing few nuclei and are insensitive to TGF-β̃. The fibers formed by these myogenic stem cells in vivo mostly express the slow type MHC isoform. During fetal development, another population of myogenic stem cells expands within the limb bud, fusing with each other to form new myotubes along the length of the primary myofibers and also fusing with the primary myofibers. These secondary myotubes mostly express fast type MyHC isoforms initially, but may later adopt either fast or slow MyHC isoforms expression. In culture, these secondary myogenic stem cells form long myotubes, containing 12–15 nuclei, and reduce their rate of proliferation in response to TGF-β̃
Thus, the developing muscles of vertebrates contain multiple populations of myogenic stem cells, sometimes with very different properties, these different populations mix and sometimes fuse with each other. In this study, we have showed that in Xenopus laryngeal muscle (both in vivo and ex vivo) this process is accompanied by changes in gene and protein expression, as seen in the increase in LM expression, the decrease in acid stable ATPase activity and changes in MHC isoform expression, which is eliminated when the dividing cells are removed through drug treatment. While the proliferating cells eliminated in these experiments might support changes in MHC expression through secreted factors or other forms of intercellular interaction, it is more likely that the mechanism for these changes involves fusion of myogenic stem cells fated to express LM to pre-existing muscle fibers and to each other. Such a mechanism would imply communication between the new nuclei and the pre-existing nuclei either due to the transport of transcription factors made by the new nuclei that regulate MyHC gene expression or to the activation of pre-existing signaling mechanisms within the fiber by the incoming nuclei, such as the release of calcium stores in the vicinity of the newly fused myogenic stem cell, the introduction of an activated kinase, or the generation of cAMP or other signaling molecule by the fusing myogenic stem cell (Horsley and Pavlath, 2004).
Acknowledgments
Supported by a Ruth L. Kirschstein pre-doctoral NRSA fellowship DC007567-03 to Brian T Nasipak and NS23684 to Darcy B. Kelley.
References
- Altuwaijri S, Lee DK, Chuang KH, Ting HJ, Yang Z, Xu Q, Tsai MY, Yeh S, Hanchett LA, Chang HC, Chang C. Androgen receptor regulates expression of skeletal muscle-specific proteins and muscle cell types. Endocrine. 2004;25:27–32. doi: 10.1385/endo:25:1:27. [DOI] [PubMed] [Google Scholar]
- Bass A, Gutmann E, Hanzlikova V, Hajek I, Syrovy I. The effect of castration and denervation upon the contraction properties and metabolism of the levator ani muscle of the rat. Physiol Bohemoslov. 1969;18:177–194. [PubMed] [Google Scholar]
- Baur LA, Nasipak BT, Kelley DB. Sexually differentiated, androgen-regulated, larynx-specific myosin heavy-chain isoforms in Xenopus tropicalis; comparison to Xenopus laevis. Dev Genes Evol. 2008;218:371–379. doi: 10.1007/s00427-008-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S, Rando TA. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21:845–854. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. The formation of skeletal muscle: From somite to limb. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz DS, Fischer LM, Kelley DB. Androgen regulation of a laryngeal-specific myosin heavy chain mRNA isoform whose expression is sexually differentiated. Dev Biol. 1995;171:448–457. doi: 10.1006/dbio.1995.1295. [DOI] [PubMed] [Google Scholar]
- Catz DS, Fischer LM, Moschella MC, Tobias ML, Kelley DB. Sexually dimorphic expression of a laryngeal-specific, androgen-regulated myosin heavy chain gene during Xenopus laevis development. Dev Biol. 1992;154:366–376. doi: 10.1016/0012-1606(92)90075-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanoine C, Hardy S. Xenopus muscle development: From primary to secondary myogenesis. Dev Dyn. 2003;226:12–23. doi: 10.1002/dvdy.10206. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin G, Slack JM. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development. 2006;133:2303–2313. doi: 10.1242/dev.02397. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Yamamoto K, Kikuyama S, Kelley DB. Prolactin opens the sensitive period for androgen regulation of a larynx-specific myosin heavy chain gene. J Neurobiol. 1999;41:443–451. [PubMed] [Google Scholar]
- Fischer L, Catz D, Kelley D. An androgen receptor mRNA isoform associated with hormone-induced cell proliferation. Proc Natl Acad Sci U S A. 1993;90:8254–8258. doi: 10.1073/pnas.90.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner WH, Yu S, Pon RT, Pourquier P, Pommier Y. Structural basis for topoisomerase I inhibition by nucleoside analogs. Nucleosides Nucleotides Nucleic Acids. 2003;22:653–658. doi: 10.1081/NCN-120022604. [DOI] [PubMed] [Google Scholar]
- Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970;28:365–367. [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myogenic stem cell fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- Kang J, Krauss RS. Muscle stem cells in developmental and regenerative myogenesis. Curr Opin Clin Nutr Metab Care. 2010;3:343–348. doi: 10.1097/MCO.0b013e328336ea98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Marin M, Kelley D. Androgen biosynthesis and secretion in developing Xenopus laevis. Gen Comp Endocrinol. 1995;100:293–307. doi: 10.1006/gcen.1995.1160. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H. Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66:119–130. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Nnodim JO. Testosterone mediates satellite cell activation in denervated rat levator ani muscle. Anat Rec. 2001;263:19–24. doi: 10.1002/ar.1072. [DOI] [PubMed] [Google Scholar]
- Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P, Vanderschueren D. Androgen signaling in myo-cytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150:3558–3566. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- Pette D, Sketelj J, Skorjanc D, Leisner E, Traub I, Bajrovic F. Partial fast-to-slow conversion of regenerating rat fast-twitch muscle by chronic low-frequency stimulation. J Muscle Res Cell Motil. 2002;23:215–221. doi: 10.1023/a:1020974710389. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- Punch VG, Jones AE, Rudnicki MA. Transcriptional networks that regulate muscle stem cell function. Wiley Interdiscip Rev Syst Biol Med. 2009;1:128–140. doi: 10.1002/wsbm.11. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Marcelle C. Muscle stem cells. Curr Opin Cell Biol. 2009;21:748–753. doi: 10.1016/j.ceb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Robertson JC, Watson JT, Kelley DB. Androgen directs sexual differentiation of laryngeal innervation in developing Xenopus laevis. J Neurobiol. 1994;25:1625–1636. doi: 10.1002/neu.480251213. [DOI] [PubMed] [Google Scholar]
- Robson LG, Hughes SM. Local signals in the chick limb bud can override myogenic stem cell lineage commitment: induction of slow myosin heavy chain in fast myogenic stem cells. Mech Dev. 1999;85:59–71. doi: 10.1016/s0925-4773(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Kelley DB. The sexually dimorphic larynx of Xenopus laevis: Development and androgen regulation. Am J Anat. 1986;177:457–472. doi: 10.1002/aja.1001770404. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Segil N, Kelley D. Androgen-induced myogenesis and chondrogenesis in the larynx of Xenopus laevis. Dev Biol. 1986;113:135–140. doi: 10.1016/0012-1606(86)90115-6. [DOI] [PubMed] [Google Scholar]
- Sassoon DA, Gray GE, Kelley DB. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci. 1987;7:3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23:569–575. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- Schimke RT, Kung A, Sherwood SS, Sheridan J, Sharma R. Life, death and genomic change in perturbed cell cycles. Philos Trans R Soc Lond B Biol Sci. 1994;345:311–317. doi: 10.1098/rstb.1994.0111. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Shimizu-Nishikawa K, Shibota Y, Takei A, Kuroda M, Nishikawa A. Regulation of specific developmental fates of larval- and adult-type muscles during metamorphosis of the frog Xenopus. Dev Biol. 2002;251:91–104. doi: 10.1006/dbio.2002.0800. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Marin ML, Kelley DB. The roles of sex, innervation, and androgen in laryngeal muscle of Xenopus laevis. J Neurosci. 1993;13:324–333. doi: 10.1523/JNEUROSCI.13-01-00324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front Neuroendocrinol. 2011;32:353–366. doi: 10.1016/j.yfrne.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]