Abstract
Working memory depends on communication between the hippocampus and the prefrontal cortex (PFC); however, the neural circuitry that mediates interactions between these brain areas has not been well characterized. Two candidate structures are the thalamic reuniens (RE) and rhomboid (Rh) nuclei, which are reciprocally connected with both the hippocampus and PFC. These known anatomical connections suggest that RE/Rh may be involved in mediating hippocampal-prefrontal communication, and therefore may be critical for working memory processing. To test the hypothesis that RE/Rh are necessary for working memory, we trained separate groups of rats to perform one of two tasks in a T-maze. The first task was a working memory-dependent conditional discrimination (CDWM) task, and the second task was a non-working memory-dependent conditional discrimination (CD) task. These tasks took place in the same maze, featured the same number of trials, and utilized the same cue (a tactile-visual maze insert). After rats had learned either task, RE/Rh were transiently inactivated with the GABAA receptor agonist muscimol, and performance was assessed. RE/Rh inactivation caused performance deficits on the CDWM task, but not the CD task. This result suggests that RE/Rh are a necessary component of working memory task performance, which is also thought to depend on the hippocampal-prefrontal circuit. RE/Rh inactivation did not cause a performance deficit on the CD task, suggesting that RE/Rh have dissociable contributions to working memory-dependent and non-working memory-dependent tasks, independently of the known contributions of these two thalamic nuclei to the sensorimotor and attention-related aspects of other memory tasks.
Keywords: Nucleus reuniens, rhomboid nucleus, working memory, conditional discrimination, hippocampal-prefrontal circuit
Introduction
The rodent medial prefrontal cortex (mPFC) is implicated in an array of cognitive functions (see Euston et al. 2012). Lesions of the rat mPFC produce behavioral deficits in spatial delayed response tasks (Kolb et al. 1974; Eichenbaum et al 1983), and mPFC single units exhibit firing correlates in working memory tasks (Jung et al. 1998). These results suggest that the rodent mPFC is important for retaining trial-specific information that can be used to guide goal-directed behavior. The rodent mPFC receives fibers from the hippocampus (Swanson 1984; Jay and Witter 1991), a brain structure that is implicated in spatial cognition (O’Keefe and Nadel 1978; Morris et al. 1982) and declarative learning and memory (see Squire 1992; Tulving and Markowitsch 1998). Lesions and transient inactivation of the rat dorsal hippocampus (dHC) produce deficits in spatial delayed alternation tasks (Ainge et al. 2007; Czerniawski et al. 2009; Hallock et al. 2013). Hippocampal neurons also exhibit trial-unique firing rate variability during the delay period of spatial delayed alternation tasks (Ainge et al. 2007; Pastalkova et al. 2008; Hallock and Griffin 2013). These data suggest that the dHC and mPFC might comprise a neural circuit that is important for working memory; supporting this view, theta oscillations in the dHC and mPFC are coherent during the choice phase of a spatial working memory task (Jones and Wilson 2005), and the entrainment of mPFC single units to dHC theta oscillations correlates with performance in working memory-dependent tasks in both rats (Jones and Wilson 2005; Hyman et al. 2010) and mice (Sigurdsson et al. 2010).
Although synchronous activity is observed between dHC and the mPFC during working memory-dependent tasks, the rodent mPFC receives projections from pyramidal neurons in the ventral/intermediate hippocampus (Ferino et al. 1987). Disconnection of the intermediate hippocampus from the mPFC impairs the performance of a win-shift radial arm maze task following long (5 min.), but not brief (10 sec.) delay periods (Churchwell and Kesner 2011); however, the anatomical connections that mediate neural communication between dHC and the mPFC remain poorly understood. The ventral midline thalamic nucleus reuniens (RE) and rhomboid nucleus (Rh) are two brain structures that are well-positioned to gate the flow of information between dHC and the mPFC, as they are reciprocally connected with both brain areas (Vertes et al. 2006; Vertes et al. 2007). These data suggest that RE/Rh participate in tasks that require the integrity of both dHC and the mPFC; in accordance with this hypothesis, RE/Rh lesions produce performance deficits in a working memory-dependent win-shift radial arm maze task (Hembrook and Mair 2011).
Although RE/Rh inactivation produces deficits in some delayed response tasks, the performance of other delayed response tasks is unaffected following muscimol (a GABAA receptor agonist) infusions into the thalamic nuclei. Hembrook et al. (2012) found that the performance of a delayed non-match to position (DNMTP) task was disrupted following RE/Rh inactivation, but that muscimol infusions into RE/Rh do not impair the performance of a delayed non-match to place task (VC-DNM) in a radial arm maze. This finding has been interpreted as evidence that RE/Rh are necessary for the performance of tasks that are sensitive to both hippocampal and mPFC damage, as performance of the DNMTP task is impaired following both hippocampal and mPFC lesions, but VC-DNM task performance is only disrupted following lesions of the hippocampus (Porter et al. 2000). However, these tasks differ among a number of other variables, such as the motor pattern used to complete each task, the spatial cues available to the rat during testing, sensory information available to the rat between tasks, and the number of trials given. These task differences, coupled with findings that RE/Rh also participate in non-mnemonic aspects of memory-guided tasks (Dolleman-van der Weel et al. 2009; Prasad et al. 2013), highlight the need for a comparison between tasks that differ only in their memory demand before a conclusion can be drawn about the relationship between RE/Rh function and working memory.
In order to delineate the role of RE/Rh in the performance of working memory-dependent tasks, we trained rats on one of two conditional discrimination tasks in a T-maze, and temporarily inactivated RE/Rh with muscimol after rats had reached asymptotic performance levels. The first task was a non-working memory-dependent conditional discrimination (CD) task, in which the rat learned to associate the appearance and texture of a floor insert with the location of a food reward. During this task, floor insert cues are continuously present while the rat makes a choice. Performance of the CD task is sensitive to striatal inactivation, but not inactivation of either dHC or the mPFC (Shaw et al. 2013; Hallock et al. 2013). The second task was a working memory-dependent version of the conditional discrimination (CDWM) task, in which the floor insert cue is present prior to the choice point, but is not available while the rat is making a choice, necessitating the retention of the cue’s identity during the decision making process. This experimental design provided the advantage of comparing performance across two tasks that took place in the same testing room and behavioral apparatus, and contained the same cues. The utilization of the CD and CDWM tasks allowed us to test the hypothesis that RE/Rh inactivation would cause a selective performance impairment in the CDWM task, lending further evidence to the claim that RE/Rh play a specific role in the memory-guided components of tasks that rely on working memory.
Materials and Methods
Subjects
13 adult male Long-Evans hooded rats were individually housed in a temperature-controlled colony room on a 12 h light/dark cycle. During the handling, pre-training, and testing phases of the experiment, rats were kept at 80-90% of their free-feeding body weight and given ad libitum access to water. All procedures were carried out in accordance with the University of Delaware Institutional Animal Care and Use Committee.
Apparatus
Tasks were performed on a wooden T-maze, which consisted of a central stem (116 × 10 cm), two return arms (112 × 10 cm each), and two goal arms (56.5 × 10 cm each) and was surrounded by 6 cm high wooden walls. Plastic cups were located at the end of each goal arm for delivery of the chocolate sprinkle reward. Between trials, animals waited on a pedestal located at the base of the maze that was blocked off from the maze by a large, removable wooden barrier.
Handling, Pre-Training, and Behavioral Training
The handling and pre-training procedures were identical to those described previously by our laboratory (see Hallock and Griffin 2013; Hallock et al. 2013). After completing pre-training, rats learned one of two tasks on the T-maze. The first task was a non-working memory-dependent tactile-visual conditional discrimination (CD) task, which has been used in our laboratory previously (Griffin et al. 2012; Shaw et al. 2013; Hallock and Griffin 2013; Hallock et al. 2013). During this task, floor inserts were placed in the stem and goal arms of the T-maze. One side of each floor insert was covered with black mesh, and the other side was bare wood. Rats learned to associate the floor insert with the location of the chocolate sprinkle reward (half of the rats were trained to associate black mesh with the right goal arm, and bare wood with the left goal arm; the other half of the rats learned the opposite rule). Each training session consisted of 24 trials (12 wood and 12 mesh), with the texture of the floor insert on each trial being presented according to a pseudorandom sequence (Fellows, 1967). Between trials, rats waited on the pedestal for 20 seconds while the experimenter flipped the floor inserts in preparation for the next trial. Floor inserts were always flipped on consecutive trials even if the same texture was presented so that the rat could not use auditory cues to solve the task. The pseudorandom presentation of floor insert texture ensured that the rat could not use a working memory strategy to successfully perform the task. Rats were given one session daily of CD until they could perform the task at a predetermined criterion level (>80% correct choices on two consecutive sessions).
The second task was a working-memory dependent version of the CD task (CDWM). This task was the same as the CD task, except that the floor insert only covered the first half of the maze stem. The portion of the stem closest to the goal arms, as well as the goal arms themselves, were left bare. During this task, the rat had to use the same information that was available during the CD task (the texture and appearance of the floor insert predicted the location of the chocolate sprinkle reward), but, unlike the CD task, the tactile-visual cue was not available throughout the entire maze stem and goal arms, which required that rats retain that information until reaching the T-intersection of the maze (i.e. “choice point”). Rats were trained on the CDWM task (24 trials per session, one session daily) until choice accuracy was >80% on two consecutive sessions.
Surgical Procedures
After rats had reached performance criterion on either the CDWM or CD task, guide cannulae targeting the nucleus reuniens (RE) and rhomboid nucleus (Rh) of the thalamus were surgically implanted according to published procedures (Shaw et al., 2013; Hallock et al., 2013). Briefly, rats were anesthetized with isoflurane, a scalp incision was made and bone screws were secured to the skull with dental acrylic (Lang Dental). A hole was then drilled 1.8 mm posterior to bregma and 2.0 mm lateral to the midline. An 8.0 mm guide cannula (PlasticsOne) was lowered 6.5 mm ventral to the surface of the brain at a 15° angle. The cannula was cemented to the skull and the bone screws with dental acrylic, and a dummy cannula made to fit the guide cannula with a 1.0 mm projection was inserted. Banamine (2.5 mg/kg) was injected subcutaneously one hour prior to the end of surgery for pain relief. Following surgery, children’s Ibuprofen (20 mg/ml) was mixed into each rat’s drinking water for two days.
Infusion Protocol
Dummy cannulae were removed, and internal cannulae made to fit the guide cannulae with a 1.0 mm projection were inserted. Internal cannulae were attached to a tube that contained either PBS or muscimol, which was attached to a microinfusion syringe (Hamilton), placed into an automated infusion pump (World Precision Instruments) that controlled infusion rate and volume (0.25 μl/min and 0.5 μl, respectively). Position of the infusate was monitored by marking an air bubble that separated the infusate from distilled H2O within the tubing. Internal cannulae sat in the brain for 2 minutes post-infusion. Behavioral testing took place 20 minutes after infusions were given.
Behavioral Testing
Following a 5 day post-surgery recovery period, rats were re-trained on either the CDWM or CD task until they reached a criterion of >80% correct choices for three consecutive sessions. Prior to the following session, 0.5 μl of a vehicle (phosphate-buffered saline (PBS)) was infused, and task performance was measured. Prior to the next session, 0.5 μl of the GABAA receptor agonist muscimol (dissolved in PBS) was infused and task performance was measured. Muscimol was given in three concentrations, in the same order for all animals across both tasks: 0.5 μg/μl, 0.25 μg/μl, and 0.125 μg/μl. To guard against muscimol dose order effects, rats were given as many infusion-free sessions between muscimol infusions as needed to reach 80% choice accuracy.
Histology
After behavioral testing, rats were given an infusion (0.5 μl volume) of a fluorophore-conjugated BODIPY TMR-X muscimol (Life Technologies, Carlsbad, CA) in order to visualize the spread of the muscimol in brain (Allen et al. 2008). The fluorescent muscimol was diluted to a concentration of 0.25 μg/μl by placing the powder into a solution made of half PBS and half DMSO in order to aid in dissolution. 20 minutes following infusion of the fluorescent muscimol, rats were transcardially perfused with 0.9% saline followed by 10% buffered formalin. Brains were removed and placed in 10% buffered formalin for 2 days, and were then transferred to a 30% sucrose solution (30 mg sucrose/100 ml PBS). After sinking, the brains were sectioned (40 μm) with a cryostat and mounted on slides. Half of the slides were stained with cresyl violet (0.5%) and photographed using a camera mounted to a microscope. The other half of the slides were counter-stained with ProLong Gold with DAPI (Life Technologies, Carlsbad, CA), and visualized with a confocal microscope. Cannula placement and spread of the fluorescent muscimol were characterized by placing digital plates from the Paxinos and Watson (2005) rat brain atlas over pictures of the cresyl-stained and DAPI-stained brain slices using Adobe Illustrator.
Data Analysis
For learning rate, number of sessions needed to reach pre-surgery performance criterion (>80% correct choices for two consecutive sessions) was compared between rats performing the CD and CDWM tasks with an independent samples t-test. A 2 (task) × 4 (session) mixed-factor ANOVA was used to compare behavioral performance on saline and muscimol infusion sessions between the CDWM and CD tasks. If a significant interaction was found, post-hoc t-tests with Bonferroni corrections were used for within-subject pairwise comparisons. An alpha level of 0.05 was used for all statistical tests.
Results
Histology
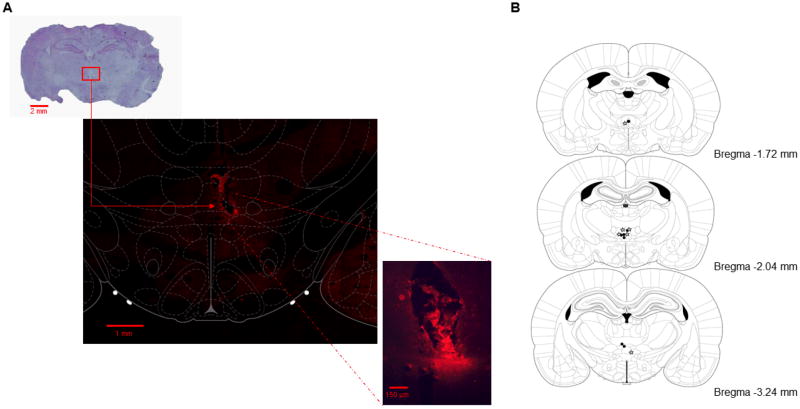
17 rats were implanted with guide cannulae, infused with muscimol, and tested on either the CDWM or CD task. Of these, 13 rats had infusion cannulae tracks that terminated in RE/Rh (see Figure 1B). 7 of the 13 rats were tested on the CDWM task, while the other 6 rats were tested on the CD task. Prior to perfusion, 9 (3 CDWM rats, 6 CD rats) of the 13 rats were given an infusion of fluorophore-conjugated muscimol. Fluorescence was observed in RE/Rh in the brains of all 9 animals, as well as in the dorsal hypothalamic area (3 animals), submedius thalamic nucleus (2 animals), and the paraxiphoid thalamic nucleus (3 animals). The largest area of florescence observed was ~150 μm ventral, and ~150 μm medial and lateral to the tip of the internal cannula (see Figure 1A)
Figure 1.
A) Representative photographs detailing cannula placements. The top panel shows a coronal slice stained with cresyl violet, with a red box surrounding the location of the cannula tip The middle panel shows a coronal slice from the same ratvisualized with a confocal microscope with a digital plate from the Paxinos and Watson (2005) rat brain atlas overlaying it. This plate details the boundaries of the thalamic nuclei in relation to the observed spread of the fluorescent muscimol, which remained largely localized to the ventral reunions nucleus (vRE) in this slice. The bottom panel shows the same coronal slice as visualized with a fluorescent microscope. B) Coronal plates showing the placements of the injector cannulae in rats that were trained on the CDWM task (black dots) and rats that were trained on the CD task (stars). All plates are re-printed with permission from The Rat Brain in Stereotaxic Coordinates: 5th Edition, pages 87-100, by G. Paxinos & C Watson, 2005, Burlington, MA: Elsevier Academic Press. Copyright 2005 by G. Paxinos & C. Watson
Behavior
Rats took a greater number of sessions to learn the CDWM task than the CD task (t(11) = 3.211, p = 0.008; see Figure 2B), indicating that the retention interval during the CDWM task made learning the association between the cue and the goal arm more difficult. For testing sessions, a 2 (task) × 4 (session) mixed-factor ANOVA revealed a significant task by session interaction, F(3,33) = 13.798, p < 0.001, a significant within-subjects main effect of session, F(3,33) = 6.063, p = 0.002, and a significant between-subjects main effect of task, F(1,11) = 56.85, p < 0.001. Planned comparisons revealed significant between-task performance differences on the 0.5 μg/μl muscimol session, t(1) = 4.37, p = 0.001, the 0.25 μg/μl muscimol session, t(1) = 10.08, p < 0.001, and the 0.125 μg/μl muscimol session, t(1) = 5.1, p <0.001, but no significant performance differences between tasks on the saline session, t(1) = 0.6, p = 0.56. Within-subject pairwise comparisons revealed that performance of rats performing the CDWM task on the 0.5 μg/μl muscimol session, performance on the 0.25 μg/μl muscimol session, and performance on the 0.125 μg/μl muscimol session all significantly differed from performance on the saline session (p < 0.01 in all cases), but that performance between the three muscimol sessions did not significantly differ (p > 0.05 in all cases). Performance of rats performing the CD task did not significantly differ between any of the four sessions (p > 0.1 in all cases) (see Figure 2C).
Figure 2.
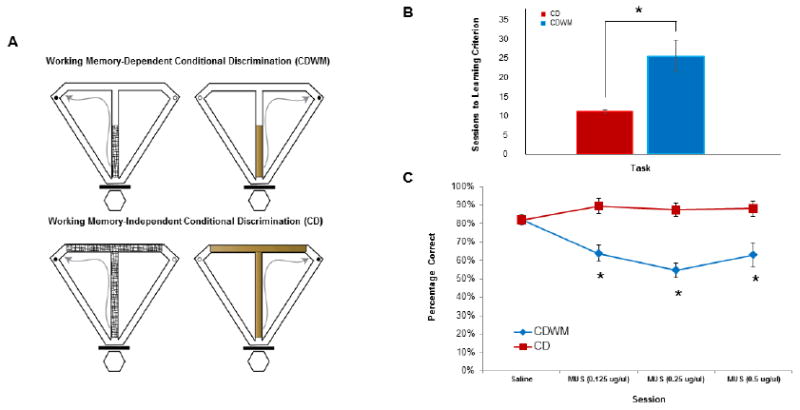
A) Schematic of the CDWM (top) and CD (bottom) tasks. During each task, rats were required to run up the central stem, choose a goal arm, consume the chocolate sprinkle reward (black dots on figures), and return to the start box via the return arms where they were confined until the next trial. Rats were required to choose the goal arm that contained reward based on the texture and appearance of a floor insert (e.g., left on mesh, right on wood). For the CD task (bottom), the floor insert covers the stem and goal arms. For the CDWM task (top), the floor inserts only cover the first half of the maze stem. B) Number of sessions needed to reach learning criterion (>80% correct for two consecutive sessions) between the CD (red) and CDWM (blue) tasks. C) Choice accuracy for the group that learned the CD task (red) and the group that learned the CDWM task (blue) in response to microinfusions of PBS (control) and 3 concentrations of muscimol. For all 3 muscimol concentrations, RE/Rh inactivation impaired choice accuracy on the CDWM task, but not the CD task. Error bars represent standard error of the mean (SEM), *p < 0.01
Discussion
Inactivation of the thalamic nucleus reuniens (RE) and rhomboid nucleus (Rh) impaired the performance of a working memory-dependent conditional discrimination task (CDWM), while leaving the performance of a non-working memory-dependent conditional discrimination task (CD) intact. The CD and CDWM tasks take place in the same T-maze, have the same number of trials per session, require the same motor pattern to be used on each trial, and feature the same cue (tactile-visual maze inserts). These findings are consistent with previous experiments that have demonstrated a performance impairment in working memory-dependent tasks following either lesions or temporary inactivation of RE/Rh, and extend these results by establishing a working memory-specific role for these two thalamic nuclei (Hembrook and Mair 2011; Hembrook et al. 2012), independently of their contributions to non-mnemonic aspects of other memory tasks (Dolleman van-der Weel et al. 2009; Prasad et al. 2013). Although muscimol concentrations were not counterbalanced across animals, the lack of performance deficits on the CD task following muscimol infusions suggests that the behavioral impairments observed during the CDWM task were not a result of mechanical damage induced by multiple drug infusions. In order to guard against dose-order effects, rats were re-trained to a performance criterion of >80% correct choices between muscimol infusions, making it likely that the effects of each muscimol infusion were independent of the effects of the other muscimol infusions employed throughout the testing sessions.
The use of a fluorophore-conjugated muscimol allowed us to confirm that the drug spread to RE/Rh in the brains of animals that performed the CD and CDWM tasks. Although fluorescence was also seen in the dorsal hypothalamic area, submedius thalamic nucleus, and paraxiphoid thalamic nucleus in the brains of some animals, fluorescence in RE/Rh was detected in the brains of all animals that were infused with fluorophore-conjugated muscimol. Lesions of RE/Rh have previously been shown to affect working memory-specific behavior (Hembrook and Mair 2011), and muscimol infusions in RE/Rh, but not in anatomical control sites within the thalamus, produce behavioral deficits in working memory-dependent tasks (Hembrook et al. 2012). These results, along with the finding in the current study that muscimol infusions produced behavioral deficits in the CDWM, but not the CD, task suggest that RE/Rh inactivation is the most likely cause of the observed performance impairments. Previous studies using a combination of muscimol infusions and in vivo extracellular recordings have shown that muscimol disrupts theta oscillations in the entorhinal cortex for 3-6 hours, allowing us to be confident that the effects of the muscimol employed in the current study persisted throughout the entire behavioral session (Brandon et al. 2011).
RE/Rh may contribute to working memory tasks by gating the flow of communication between the dHC and mPFC, two brain regions which are also thought to be important for working memory (see Colgin 2011; Gordon 2011). The current study contributes to this theory by demonstrating that RE/Rh inactivation did not cause performance deficits on a CD task that requires a habit-like memory system (Hallock et al. 2013), identifying RE/Rh function as a component of a hippocampal-prefrontal circuit that supports declarative-like memory and working memory (Squire 2004). Previous studies have shown that inactivation of the direct pathway from the ventral/intermediate hippocampus (vHC) to the mPFC causes working memory-specific performance deficits over long, but not short, inter-trial delay periods (Churchwell and Kesner 2011); this raises the possibility that direct unidirectional afferents from vHC to the mPFC and bidirectional connections from RE/Rh to the mPFC and hippocampus are functionally dissociable, with RE/Rh being critical for the maintenance of trial-specific information during shorter retention intervals, and the vHC-mPFC circuit being important for the maintenance of trial-specific information during longer retention intervals. Further research should attempt to link RE/Rh and hippocampal-prefrontal function by inactivating RE/Rh and the vHC-mPFC circuit prior to performance of the CDWM task while varying the length of the retention interval. The results of the current study lay the groundwork for this future line of research by characterizing the relationship between RE/Rh function and spatial working memory-task performance between two tasks that rely on disparate memory systems.
Acknowledgments
The authors would like to acknowledge Monica Patel, Sarah Amos, Brendan Farrell, and Dylan Layfield for assisting with rat training, as well as David Martin, Anna Klintsova, Ouyang Liangqi, Chin-Chen Kuo, and Karen Boschen for assistance with fluorescence and confocal microscopy.
References
- Ainge AA, van der Meer MAA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in alternation behavior. Hippocampus. 2007;17:988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171:30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behavioural Brain Research. 2011;225:389–395. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Morris RGM, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Structure and Function. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Experimental Neurology. 1983;79:434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows BJ. Change stimulus sequences for discrimination tasks. Psychological Bulletin. 1967;67:87–92. doi: 10.1037/h0024098. [DOI] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon’s horn to the medial prefrontal cortex in the rat. Experimental Brain Research. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, Owens CB, Peters GJ, Adelman PC, Cline KM. Spatial representations in dorsal hippocampal neurons during a tactile-visual conditional discrimination task. Hippocampus. 2012;22:299–308. doi: 10.1002/hipo.20898. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Griffin AL. Dynamic coding of dorsal hippocampal neurons between tasks that differ in structure and memory demand. Hippocampus. 2013;23:169–186. doi: 10.1002/hipo.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, Griffin AL. Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiology of Learning and Memory. 2013;100:108–116. doi: 10.1016/j.nlm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22:853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: Evidence for a direct thalamo-hippocampal pathway in the rat. Journal of Comparative Neurology. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions, but not with prefrontal neuron firing rates. Frontiers in Integrative Neuroscience. 2010 doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Research. 1989;505:337–40. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal–prefrontal interactions in a spatial memory task. PLoS Biology. 2005 doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cerebral Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. Journal of Comparative Physiological Psychology. 1974;87:772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; 1978. [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos J, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier; 2005. [Google Scholar]
- Porter MC, Burk JA, Mair RG. A comparison of the effects of hippocampal or prefrontal cortical lesions on three versions of delayed non-matching-to-sample based on positional or spatial cues. Behavioural Brain Research. 2000;109:69–81. doi: 10.1016/s0166-4328(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Structure and Function. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Shaw CL, Watson GDR, Hallock HL, Cline KM, Griffin AL. The role of the medial prefrontal cortex in the acquisition, retention, and reversal of a tactile visuospatial conditional discrimination task. Behavioural Brain Research. 2013;236:94–101. doi: 10.1016/j.bbr.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis of findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Research. 1981;217:150–54. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Cristina do Valle A, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. Journal of Comparative Neurology. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck L, Leranth C. Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Research Bulletin. 2007;71:601–09. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]