Abstract
Purpose
To determine whether the use of a polyurethane-cuffed endotracheal tube would result in a decrease in ventilator-associated pneumonia rate.
Materials and Methods
We replaced conventional endotracheal tube with a polyurethane-cuff endotracheal tube (Microcuff, Kimberly-Clark Corporation, Rosewell, Georgia) in all adult mechanically ventilated patients throughout our large academic hospital from July 2007–June 2008. We retrospectively compared the rates of ventilator-associated pneumonia before, during, and after the intervention year by interrupted time-series analysis.
Results
Ventilator-associated pneumonia rates decreased from 5.3 per 1000 ventilator days prior to the use of the polyurethane-cuffed endotracheal tube to 2.8 per 1000 ventilator days during the intervention year (p = 0.0138). During the first three months after return to conventional tubes, the rate of ventilator-associated pneumonia was 3.5/1000 ventilator days. Use of the polyurethane-cuffed endotracheal tube was associated with an incidence risk ratio of ventilator-associated pneumonia of 0.572 (95% CI 0.340–0.963). In statistical regression analysis controlling for other possible alterations in the hospital environment, as measured by rate of tracheostomy-ventilator-associated pneumonia, the incidence rate ratio of ventilator-associated pneumonia in patients intubated with polyurethane-cuffed endotracheal tube was 0.565 (p=0.032, 95% CI 0.335–0.953).
Conclusions
Use of a polyurethane-cuffed endotracheal tube was associated with a significant decrease in the rate of ventilator-associated pneumonia in our study.
Keywords: Pneumonia, Ventilator-associated, Nosocomial Infections, Ventilators, Mechanical, Endotracheal tube
Ventilator-associated pneumonia (VAP) is a significant cause of increased morbidity and cost in mechanically ventilated patients. VAP has been associated with a greater duration of mechanical ventilation, ICU and hospital length of stay, and estimated costs of $10,000–$40,000 per episode1–4. VAP increases mortality in medical intensive care patients5, in patients whose onset of VAP is after five days of mechanical ventilation6, and in patients with VAP due to Pseudomonas aeruginosa7. Despite substantial efforts made to prevent ventilator-associated pneumonia, 8–10, the incidence and impact of VAP remains significant.3, 11
One proposed mechanism for the development of ventilator-associated pneumonia is leakage of colonized oropharyngeal secretions beyond the endotracheal tube (ETT) cuff into the lower respiratory tract.12–14 Conventional endotracheal tubes use a high-volume, low-pressure polyvinyl cuff which was developed to decrease mucosal damage and tracheal necrosis. In order to distribute cuff pressure over a large surface area, the diameter of the cuff is greater than tracheal diameter. However, this leads to the formation of channels created by folds in the cuff. At the recommended inflation pressure of 20–30 cm H20,15 oropharyngeal secretions easily leak through these channels into the lower airway.14
Several endotracheal tubes have been designed to prevent passage of upper airway oropharyngeal secretions into the lower respiratory tract. 16–20 Subglottic secretion drainage endotracheal tubes have been shown to be effective in decreasing VAP in patients requiring mechanical ventilation >72 hours18, 21–24, however they have had limited utilization (SDD-ETT),25 ostensibly due to difficulty in accurately predicting appropriate patients for use, more intensive nursing needs associated with maintenance of the suction port, and higher expense.25, 26 Additionally, a study in sheep showed significant mucosal damage associated with the use of the SSD-ETT.27
Polyurethane cuffed endotracheal tubes (PUC-ETT) have been shown to decrease leakage of oropharyngeal secretions in vitro.12, 16 This endotracheal tube cuff has a 7 micron wall thickness, thinner than the usual 50 micron thickness seen in polyvinyl chloride cuffed tubes. PUC-ETT result in a tighter tracheal seal at cuff inflation pressures less than 30 cm H2O by minimizing the development of large cuff channels.12 PUC-ETT has been shown to decrease the incidence of VAP in small, single-unit studies with high baseline rates of VAP,16, 17 but its effect on VAP when utilized in unselected patients, including emergently intubated patients, in routine care is not known. We studied the effect of replacing the conventional polyvinyl chloride cuffed endotracheal tube (PVC-ETT) with the PUC-ETT on the incidence of VAP in patients throughout our adult intensive care units. We used two different control groups to overcome the weakness of pre-post studies. First, we compared VAP rates with PUC-ETT to those of patients with PVC-ETT both prior to and after the PUC-ETT period. Second, we also used VAP rates among tracheostomy patients at the same time as the PUC-ETT period to control for synchronous other changes in ventilator care or hospital microbiology.
Materials and Methods
Study Location and Patients
This study was conducted at a single study site, the University of Michigan, University Hospital in Ann Arbor, MI, a university-affiliated teaching institution. During a 27-month period, July 2006–September 2008, adult patients in intensive care units intubated for mechanical ventilation with an endotracheal tube were eligible for this investigation. Data on patients with tracheostomy tubes receiving mechanical ventilation were also collected to serve as an internal control population. The participating intensive care units were the Trauma-Burn Intensive Care Unit (TBICU, 10 beds), the Cardiac Intensive Care Unit (CICU, 10 beds), the Critical Care Medicine Unit (CCMU, 20 beds), the Neuro Intensive Care Unit (NICU, 10 beds, increased to 15 beds during the study period) and the Surgical Intensive Care Unit (SICU, 20 beds). During the intervention year, one intensive care unit, the Thoracic Intensive Care Unit moved into a building of new construction and more than doubled in size, from 10 beds to 24 beds. Due to major changes in staffing, staff education, and logistics, patients in this intensive care unit were excluded from analysis. The characteristics of each included intensive care unit are summarized in Table 1.
Table 1.
Unit Characteristics
| Type of patients | July 2006 – June 2007 | July 2007 – June 2008 | July 2008 – Sept 2008 | ||||
|---|---|---|---|---|---|---|---|
| # beds | Ventilator utilization % | #beds | Ventilator utilization % | #beds | Ventilator utilization % | ||
| TBICU | General surgery, trauma, burn | 10 | 40.5 | 10 | 42.8 | 10 | 34.3 |
| CICU | Medical cardiac care | 10 | 24.1 | 10 | 33.7 | 10 | 22.9 |
| CCMU | Medical intensive care | 20 | 56.0 | 20 | 63.1 | 20 | 60.9 |
| NICU | Neurology and neurosurgery | 10 | 19.3 | 15 | 21.3 | 15 | 21.1 |
| SICU | Surgery | 20 | 39.3 | 20 | 41.7 | 20 | 37.7 |
| Total | 70 | 39.1 | 75 | 43.1 | 75 | 38.2 | |
Ventilator utilization calculated as a number of ventilator days per number of patient days ×100%
This study was reviewed by the University of Michigan Institutional Review Board, as a retrospectively analyzed quality improvement project after a change in equipment.
Intervention
In July 2007, we replaced our conventional polyvinyl chloride cuffed endotracheal tube with a polyurethane-cuffed endotracheal tube (Microcuff, Kimberly Clark Corporation, Rosewell, GA) throughout our adult academic institution for one year (July 2007–June 2008). These tubes were provided free of charge from the company. In July 2008, our institution returned to the use of conventional polyvinyl chloride cuffed endotracheal tubes in anticipation of internal internal analysis of effectiveness and cost.
Data Collection and Definitions
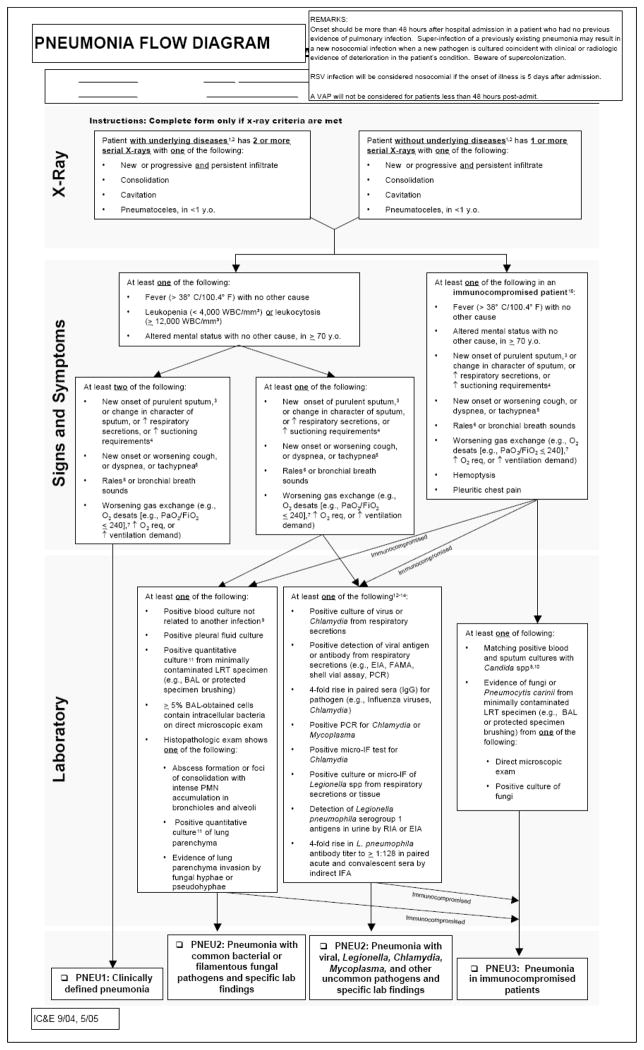
Data regarding hospital-acquired infections are routinely prospectively collected by professionals in the Department of Infection Control and Epidemiology on all patients within our institution for internal quality assurance. A diagnosis of VAP is made if the patient has been intubated at least 48 hours and meets our institution’s clinical or microbiologic criteria, based on the standard National Healthcare Safety Network (NHSN) definition (see APPENDIX figure 1). In our study, only the first diagnosis of VAP was included; patient days subsequent to first diagnosis of VAP were excluded from consideration. Additionally, for the purposes of this study, in cases where a patient underwent tracheostomy and was subsequently diagnosed with VAP, these episodes were included in the analysis if the VAP diagnosis occurred within 48 hours of tracheostomy, thereby attributing the pneumonia to the endotracheal tube (ETT). Rates of VAP were calculated per unit per 1000 ventilator days at risk. This data was routinely prospectively collected and diagnoses were made during the study period independently from our investigation. We retrospectively evaluated data for the year prior to the intervention, designated as the baseline year (July 2006–June 2007); the intervention year (July 2007–June 2008); and the first quarter after the intervention (July 2008–September 2008). In the absence of a randomly assigned control group, we also collected data on mechanically ventilated tracheostomy patients as a potential reflection of hospital acquired infection rates and infection control practices throughout the hospital during the study period.
APPENDIX Figure 1.
Our institution participates in the Michigan Health and Hospital Association (MHA) Keystone ICU initiative which involves multiple institutions throughout the state. This collaboration is an ongoing patient safety and quality improvement initiative aimed at decreasing nosocomial infections and improving outcomes through the use of multidisciplinary-bundled interventions.28 The bundle for mechanically ventilated patients includes elevation of the head of the bed, daily interruption of sedation, stress ulcer prophylaxis, and deep venous thrombosis prophylaxis. The bundle was implemented institution-wide early in 2006. No new recommendations for the care of mechanically ventilated patients to decrease the rate of VAP were made by MHA Keystone ICU during the entire observation period. Data collected internally for quality assurance purposes shows similar high rates of bundle compliance before, during and after the study period.(data not shown) Because of the addition of an oral care intervention to the mechanical ventilation bundle in several ICUs beginning in October 2008, we were unable to extend the period of observation beyond September 2008.
Statistical Analysis
Using interrupted time-series methodology, we compared rates of ventilator-associated pneumonia for the year before intervention, the year of intervention, and the first quarter after return to the conventional ETT. The rate of tracheostomy-associated VAP was calculated and compared for the year before intervention, the year of intervention, and the first quarter after the year of intervention.
Unadjusted analyses were calculated as incidence rate ratios, taking into account differences between units using Mantel-Haenszel weights. In order to adjust for temporal trends in other infection control processes throughout the hospital, we also adjusted for contemporaneous rates of VAP among patients with a tracheostomy using Poisson regression with a unit-level random effect. We tested for, and found no evidence of, overdispersion that would require use of negative binomial regression. As a sensitivity test, we also compared the effectiveness of the PUC-ETT using a linear time trend, and found efficacy estimates substantively identical to those obtained with the tracheostomy-VAP control. (data not shown)
All statistical analyses were done using Stata 9.2 (StataCorp LP, College Station, TX).
Results
Between July 2006 and September 2008, there were 3,207 patients intubated for mechanical ventilation within our five intensive care units for a total of 16,223 ventilator days. During the same time period, there were 674 patients receiving mechanical ventilation via a tracheostomy tube for a total of 8,869 ventilator days.
During the intervention year, there were 50 patients intubated with a conventional PVC-ETT for a total of 313 ventilator days. These PVC-ETTs were associated with 2 episodes of VAP, an incidence of 4% and a rate of 6.3 per 1000 ventilator days. The use of conventional ETT during the intervention year was most often due to patient transfer from an outside institution, although occasionally it was a result of the presence of old PVC-ETT stock in airway intubation kits within the study hospital. These patients were included in our calculations as an intention to treat analysis.
During the baseline year, there were 37 episodes of VAP among patients with endotracheal tubes, for a rate of 5.3/1000 ventilator days. During the intervention year, there were 21 episodes of ETT-associated VAP in 7,545 ventilator days, a rate of 2.8/1000 ventilator days (p = 0.0138). After return to the use of the conventional ETT, there were 6 episodes of VAP associated with ETTs in 1,712 ventilator days for a rate of 3.5/1000 ventilator days for the three month interval.
For unadjusted analyses, we combined the baseline year and the three months after return to the conventional PVC-ETT as there was no statistically significant difference in rates of VAP (p=0.3343). The incidence risk ratio of VAP during the intervention year was 0.572 (95% CI 0.340–0.963), equivalent to a relative risk reduction of 42.8% (95% CI 3.7%–66%). The results are summarized in Table 2.
Table 2.
ETT-associated VAP
| Non-PUC-ETT | PUC-ETT | IRR | CI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| #VAP | #ventilator days | VAP rate (per 1000 vent days) | #VAP | #ventilator days | VAP rate (per 1000 vent days) | ||||
| TBICU | 12 | 1050 | 11.4 | 6 | 925 | 6.5 | .568 | (.175–1.634 | |
| CICU | 3 | 968 | 3.1 | 2 | 1025 | 2.0 | .629 | (.052–5.496) | |
| CCMU | 18 | 3595 | 5.0 | 9 | 2782 | 3.2 | .646 | (.255–1.515) | p=ns |
| NICU | 3 | 693 | 4.3 | 1 | 722 | 1.4 | .320 | (.006–3.985) | |
| SICU | 7 | 2396 | 2.9 | 3 | 2091 | 1.4 | .491 | (.082–2.151) | |
| Total | 43 | 8678 | 5.0 | 21 | 7545 | 2.8 | .572 | (.340–.962) | p=0.027 |
To adjust for any unmeasured variations in clinical care which may have occurred during the study period, such as better hand washing, which might have confounded these results, we compared the rate of VAP associated with tracheostomy during the same time periods. During the baseline year, there were 27 episodes of VAP in 3,462 tracheostomy/ventilator days, a rate of 7.8/1000 ventilator days. During the intervention year, there were 26 episodes of VAP in 4,395 ventilator days, a rate of 5.9/1000 ventilator days. In the three months following the intervention year, 4 episodes of VAP were observed in 1,012 ventilator days, an incidence of 4.5% and a rate of 4.0/1000 ventilator days. There were no changes in the tracheostomy tubes or ventilator care protocols used by our institution during the study time period; however, there were several initiatives promoting hand washing which were ongoing during this time. When controlling for the incidence of VAP in tracheostomy patients and controlling for unit level effects in a Poisson regression, the incidence rate ratio of VAP in patients intubated with PUC-ETT was 0.565 (p=0.032, 95% CI 0.335–0.953), a 43.5% reduction in the incidence of VAP compared to the combined baseline year and three month post-intervention period when returning to PVC-ETT use.
Discussion
We found that the use of the PUC-ETT was associated with a clinically and statistically significant decreased rate of VAP when compared to a period when PVC-ETT were used at our institution, utilizing a pre-/post- analysis. The decrease in VAP was present in two distinct analyses: a control/treat/control time series, and relative to time-matched tracheostomy controls. The decrease was both clinically and statistically significant, and occurred at an institution with already-established VAP-prevention policies.
Two earlier studies evaluating the clinical use of the PUC-ETT16, 17 showed a significant decrease in the rate of VAP with the use of the PUC-ETT. Although both studies were randomized control trials, they were both single-unit studies with a high baseline VAP rate. One study17, conducted in a post-surgical cardiac unit, had a control group with a 42% VAP rate. A second trial using a PUC-ETT 16 found a rate of VAP in the control group of 22.1%. In addition, the ETT tube used in this second study also included subglottic secretion drainage, therefore the observation of VAP reduction may not necessarily be attributable to the PUC component. The presence of high background occurrence rates of VAP in a small sample size may limit generalizability of these studies.[29]
Although VAP rates vary between institutions and units, large multicenter studies have estimated the incidence of VAP to be 9–10%.3, 11 Additionally, the National Healthcare Safety Network System (NHSN) in its data summary for 2006 reported a composite VAP rate of 4.3/1000 ventilator days.29 The NHSN rate may be lower than the true national rate since participating hospitals likely represent a cohort of hospitals with increased motivation to decrease nosocomial infections. Our baseline rate of VAP during the study period (5.3/1000 ventilator days) is similar to the NHSN rate at the time and likely reflects infection control practices at least as effective as the national average.
Although the pre-/post-intervention approach utilized by this study lacks the benefits of a randomized controlled trial, our unselected patient population is a more general reflection of critically ill patients than subjects enrolled in randomized controlled trials. In particular, existing randomized controlled trials of PUC-ETT may have introduced a selection bias as they required individual-level informed consent prior to intubation, potentially excluding many emergent intubations.30 Patients undergoing emergent intubation have been shown to have a greater risk of subsequent pulmonary infection31, and represent a patient population not usually studied in VAP prevention trials involving novel endotracheal tubes.
One weakness of an observational study design such as ours is the possibility that some other change in clinical practice accounted for or contributed to the decrease seen in VAP during the intervention period. The introduction of PUC-ETT in our hospital was a quality-improvement effort that we retrospectively analyzed to produce generalizable knowledge. Although VAP rates in the first quarter after return to the PVC-ETT did not differ significantly from the initial baseline VAP rates, there was a point-estimate with lower rates in this final quarter. We suspect that this decrease is related to an institution-wide campaign to improve handwashing technique and compliance which spanned the study period. In an attempt to control for the possibility of changes in the care of mechanically ventilated patients by nurses or respiratory therapists accounting for the decrease seen in VAP during the intervention period, we calculated the rate of VAP in mechanically ventilated tracheostomy patients during these same time periods. We did note a steady decrease in VAP rates in tracheostomy patients during the study period. However, in comparison between the two groups, the decrease in the likelihood of VAP in patients intubated with the PUC-ETT remained significant. While we cannot exclude the possibility that the decrease in VAP among the PUC-ETT population was attributable to some other unrecognized variable, such a variable would have to have been uniquely applicable to PUC-ETT patients and not the tracheostomy patients. In addition, this variable would have to have been both introduced and removed at the same time as the PUC-ETT.
Our study has other limitations. We were unable to examine any difference in tube failure rates between the PVC-ETT and PUC-ETT due to limitations in our data collection. As reintubation increases the risk of VAP32, we cannot exclude the possibility that the differences seen in VAP rate were accounted for by a lower rate of tube failure in the PUC-ETT group, or simply a lower rate of reintubation during the intervention period. This appears unlikely as an earlier study16 documented no difference in reintubation with the PUC-ETT compared to the control PVC-ETT, and our clinical practice regarding weaning and extubation did not change during the intervention period. Additionally, although our study was performed in a single center, we believe our results are generalizable as it was conducted in an array of ICUs with a varied patient population. As must be considered in the adoption of any infection control measure, comparative cost-effectiveness of widespread use of the PUC-ETT must be weighed against costs and effectiveness of other preventative measures on a hospital-by-hospital basis.
Conclusions
VAP remains a significant cause of morbidity and increased health care costs. The PUC-ETT represents a simple intervention which can be rapidly introduced hospital-wise with no increase in personnel requirements and appears to be associated with a significantly decreased rate of VAP.
Acknowledgments
The authors would like to thank Dr. Benrong Chen for his assistance with the statistical analysis.
Funding/Support:
This study was partially funded by Kimberly-Clark Corporation, Rosewell, Georgia, which manufactures Microcuff. Kimberly-Clark Corporation supplied the Microcuff polyurethane-cuffed endotracheal tubes for the intervention year free of charge. Kimberly-Clark Corporation employees were not involved in the concept, design, or conduct of the study, nor were they involved in the analysis of data or review of the manuscript prior to submission.
Dr. Robert Hyzy has received a consulting fee of $5800 in 2008 and a $6,000 unrestricted educational grant in 2007 from the Kimberly-Clark Corporation.
References
- 1.Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am J Respir Crit Care Med. 1996;153:343–349. doi: 10.1164/ajrccm.153.1.8542141. [DOI] [PubMed] [Google Scholar]
- 2.Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Int Med. 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 4.Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respiratory Care. 2005;50:725–739. discussion 739. [PubMed] [Google Scholar]
- 5.Heyland DK, Cook DJ, Griffith L, et al. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159:1249–1256. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 6.Vallés J, Pobo A, García-Esquirol O, et al. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33:1363–1368. doi: 10.1007/s00134-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 7.Crouch Brewer S, Wunderink RG, Jones CB, et al. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Int Med. 2003;138:494–501. doi: 10.7326/0003-4819-138-6-200303180-00015. [DOI] [PubMed] [Google Scholar]
- 9.Lorente L, Blot S, Rello J. Evidence on measures for the prevention of ventilator-associated pneumonia. Eur Respir J. 2007;30:1193–1207. doi: 10.1183/09031936.00048507. [DOI] [PubMed] [Google Scholar]
- 10.Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23:126–137. doi: 10.1016/j.jcrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Chastre J, Fagon J-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 12.Dullenkopf A, Gerber A, Weiss M. Fluid leakage past tracheal tube cuffs: evaluation of the new Microcuff endotracheal tube. Intensive Care Med. 2003;29:1849–1853. doi: 10.1007/s00134-003-1933-6. [DOI] [PubMed] [Google Scholar]
- 13.Oikkonen M, Aromaa U. Leakage of fluid around low-pressure tracheal tube cuffs. Anaesthesia. 1997;52:567–569. doi: 10.1111/j.1365-2044.1997.149-az0153.x. [DOI] [PubMed] [Google Scholar]
- 14.Young PJ, Rollinson M, Downward G, et al. Leakage of fluid past the tracheal tube cuff in a benchtop model. Br J Anaesth. 1997;78:557–562. doi: 10.1093/bja/78.5.557. [DOI] [PubMed] [Google Scholar]
- 15.Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. BMJ (Clinical research ed 1981) 1984;288:965–968. doi: 10.1136/bmj.288.6422.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorente L, Lecuona M, Jiménez A, et al. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med. 2007;176:1079–1083. doi: 10.1164/rccm.200705-761OC. [DOI] [PubMed] [Google Scholar]
- 17.Poelaert J, Depuydt P, De Wolf A, et al. Polyurethane cuffed endotracheal tubes to prevent early postoperative pneumonia after cardiac surgery: a pilot study. J Thorac Cardiovasc Surg. 2008;135:771–776. doi: 10.1016/j.jtcvs.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Vallés J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Int Med. 1995;122:179–186. doi: 10.7326/0003-4819-122-3-199502010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Young PJ, Ridley SA, Downward G. Evaluation of a new design of tracheal tube cuff to prevent leakage of fluid to the lungs. Br J Anaesth. 1998;80:796–799. doi: 10.1093/bja/80.6.796. [DOI] [PubMed] [Google Scholar]
- 20.Zanella A, Cressoni M, Epp M, et al. A double-layer tracheal tube cuff designed to prevent leakage: a bench-top study. Intensive Care Med. 2008;34:1145–1149. doi: 10.1007/s00134-008-1016-9. [DOI] [PubMed] [Google Scholar]
- 21.Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118:11–18. doi: 10.1016/j.amjmed.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 22.Kollef MH, Skubas NJ, Sundt TM. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest. 1999;116:1339–1346. doi: 10.1378/chest.116.5.1339. [DOI] [PubMed] [Google Scholar]
- 23.Mahul P, Auboyer C, Jospe R, et al. Prevention of nosocomial pneumonia in intubated patients: respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med. 1992;18:20–25. doi: 10.1007/BF01706421. [DOI] [PubMed] [Google Scholar]
- 24.Smulders K, van der Hoeven H, Weers-Pothoff I, et al. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–862. doi: 10.1378/chest.121.3.858. [DOI] [PubMed] [Google Scholar]
- 25.Krein SL, Kowalski CP, Damschroder L, et al. Preventing ventilator-associated pneumonia in the United States: a multicenter mixed-methods study. Infect Control Hosp Epidemiol. 2008;29:933–940. doi: 10.1086/591455. [DOI] [PubMed] [Google Scholar]
- 26.Girou E, Buu-Hoi A, Stephan F, et al. Airway colonisation in long-term mechanically ventilated patients. Effect of semi-recumbent position and continuous subglottic suctioning. Intensive Care Med. 2004;30:225–233. doi: 10.1007/s00134-003-2077-4. [DOI] [PubMed] [Google Scholar]
- 27.Berra L, De Marchi L, Panigada M, et al. Evaluation of continuous aspiration of subglottic secretion in an in vivo study. Crit Care Med. 2004;32:2071–2078. doi: 10.1097/01.ccm.0000142575.86468.9b. [DOI] [PubMed] [Google Scholar]
- 28.Pronovost P, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008;23:207–221. doi: 10.1016/j.jcrc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Edwards JR, Peterson KD, Andrus ML, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805–813. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 31.Croce MA, Tolley EA, Fabian TC. A formula for prediction of posttraumatic pneumonia based on early anatomic and physiologic parameters. J Trauma. 2003;54:724–729. doi: 10.1097/01.TA.0000054643.54218.C5. discussion 729. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim EH, Tracy L, Hill C, et al. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120:555–561. doi: 10.1378/chest.120.2.555. [DOI] [PubMed] [Google Scholar]