Abstract
Glutathione is the predominant endogenous cellular antioxidant, playing a critical role in the cellular defensive response to oxidative stress by neutralizing free radicals and reactive oxygen species. With cysteine as the rate-limiting substrate in glutathione biosynthesis, the cystine/glutamate transporter (system xc−) represents a potentially attractive PET biomarker to enable in vivo quantification of xc− activity in response to oxidative stress associated with disease. We have developed a system xc− substrate that incorporates characteristics of both natural substrates, l-cystine and l-glutamate (l-Glu). l-aminosuberic acid (l-ASu) has been identified as a more efficient system xc− substrate than l-Glu, leading to an assessment of a series of anionic amino acids as prospective PET tracers. Herein, we report the synthesis and in vitro and in vivo validation of a lead candidate, 18F-5-fluoro-aminosuberic acid (18F-FASu), as a PET tracer for functional imaging of a cellular response to oxidative stress with remarkable tumor uptake and retention.
Methods
18F-FASu was identified as a potential PET tracer based on an in vitro screening of compounds similar to l-cystine and l-Glu. Affinity toward system xc− was determined via in vitro uptake and inhibition studies using oxidative stress–induced EL4 and SKOV-3 cells. In vivo biodistribution and PET imaging studies were performed in mice bearing xenograft tumors (EL4 and SKOV-3).
Results
In vitro assay results determined that l-ASu inhibited system xc− as well as or better than l-Glu. The direct comparison of uptake of tritiated compounds demonstrated more efficient system xc− uptake of l-ASu than l-Glu. Radiosynthesis of 18F-FASu allowed the validation of uptake for the fluorine-bearing derivative in vitro. Evaluation in vivo demonstrated primarily renal clearance and uptake of approximately 8 percentage injected dose per gram in SKOV-3 tumors, with tumor-to-blood and tumor-to-muscle ratios of approximately 12 and approximately 28, respectively. 18F-FASu uptake was approximately 5 times greater than 18F-FDG uptake in SKOV-3 tumors. Dynamic PET imaging demonstrated uptake in EL4 tumor xenografts of approximately 6 percentage injected dose per gram and good tumor retention for at least 2 h after injection.
Conclusion
18F-FASu is a potentially useful metabolic tracer for PET imaging of a functional cellular response to oxidative stress. 18F-FASu may provide more sensitive detection than 18F-FDG in certain tumors.
Keywords: oxidative stress, PET, functional imaging, tumor imaging, 18F
Several 18F-labeled amino acid derivatives have been used as PET imaging agents to visualize tumors, including anti-1-amino-3-18F-fluorocyclobutyl-carboxylic acid, O-(2-18F-fluoroethyl)-tyrosine, 6-18F-fluoro-l-dopa, and 18F-(4R)-4-fluoro-l-glutamine (1–4). These agents rely primarily on the neutral amino acid transporter systems L (large neutral amino acid preferring) and ASC (alanine-serine-cysteine preferring) for tumor uptake (5). By comparison, the most commonly used PET tracer, 18F-FDG, uses an increase in glucose uptake that feeds increased tumor metabolism. Although the molecular mechanisms of glucose and amino acid uptake may differ, they both target augmentations to cellular transport systems that enable higher demands for energy production and protein synthesis required for unrestrained proliferation.
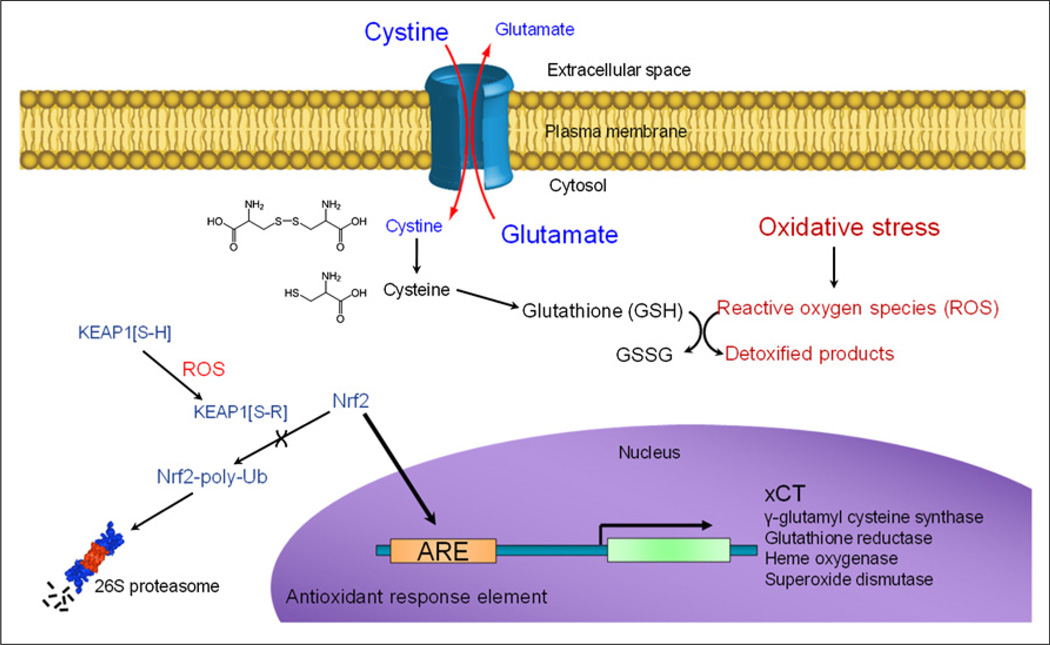
System xc− was first described by Bannai and Kitamura as a sodium-independent amino acid transport system with a very narrow natural substrate binding profile (6,7), especially when compared with other known transporters (8). This cystine/glutamate transporter is a dimeric cell surface transporter that consists of a heavy chain subunit (4F2hc) for membrane trafficking and xCT, the smaller subunit that imparts substrate specificity. The xCT subunit maintains very low basal expression in most normal tissues, with the exception of the brain, pancreas, spleen, and thymus, but is universally upregulated as cells respond to oxidative stress (9). The ubiquitin-ligase protein KEAP1 acts as a biosensor for oxidative stress through a reactive oxygen species–induced covalent modification of an active site cysteine residue. Modified KEAP1 can no longer facilitate the proteasomal degradation of the transcription factor Nrf2, resulting in the expression of several genes with antioxidant response element (ARE) promoters, including xCT (10–13) as shown in Figure 1. The resulting enhanced cystine influx, and intracellular reduction to cysteine, meets the increased demand for this rate-limiting precursor of glutathione synthesis. Therefore, system xc− imaging can provide functional information about the cellular response to oxidative stress in a tumor or organ, operating as an in vivo reporter for ARE activation.
FIGURE 1.
Cellular mechanism of xCT expression induced by oxidative stress and promoting increased glutathione synthesis.
Targeting system xc− for PET tracer development has potential advantages over other amino acid transporters. First, detection of oxidative stress distinguishes this biomarker from cell growth and proliferation-based imaging agents. Second, xCT is accessible at the cell surface and maintains a well-defined (and narrow) natural ligand complement, suggesting that PET tracers can be developed with much better selectivity for this class of transporter. Most amino acid–based PET agents use transport systems ASC or L, which transport 14 of the 20 standard amino acids (5), and many of these amino acids are also substrates for several other molecular transport systems. Of the standard amino acids, only l-glutamate (l-Glu) and l-cystine are substrates for system xc−, and far fewer additional transport systems use either of these substrates (9), most notably l-Glu as a substrate for the excitatory amino acid transporter of system X−AG (10,11). Finally, use of an accumulatory transport mechanism could also serve to improve PET signal contrast through enhanced tracer uptake, compared with saturable binding of an agent to a cell surface protein.
Two groups have reported radiolabeled l-Glu derivatives 18F-(4R)fluoro-l-glutamate (18F-FGLU) and (4S)-18F-fluoropropyl-l-glutamate (18F-FSPG) as PET agent substrates of the cystine/glutamate transporter for cancer imaging (3,14–16). Here, we report evaluation of a series of anionic amino acids of varying carbon chain lengths to identify the best inhibitor of system xc−, synthesis of the novel radiofluorinated derivative 18F-5-fluoro-l-aminosuberic acid (18F-FASu), and preclinical evaluation in tumor xenografts. Our efforts to develop a PET imaging substrate of system xc− focused on amino acid derivatives that show molecular similarities to the natural uptake substrate, l-cystine. We reasoned that the longer carbon backbone of l-aminosuberic acid (l-ASu) was more cystine-like and may be a better transporter substrate because l-cystine was reportedly more potent toward system xc− than l-Glu (9). In addition, cystine-like derivatives may be less likely to participate as substrates for other glutamate transporters or bind to glutamate receptors (17), potentially resulting in better specificity of 18F-FASu in peripheral tissues and the brain (18). The cystine/glutamate transporter is often active in oncology (9,10); using this transporter to image a cellular response to oxidative stress may provide functional information useful for clinical cancer care, such as detection, staging, patient stratification, monitoring of response to therapy, and development of new therapies.
MATERIALS AND METHODS
l-aspartic acid (l-Asp), d-aspartic acid (d-Asp), l-Glu, l-leucine (l-Leu), l-serine (l-Ser), suberate, sulfasalazine (SSZ), 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), and diethylmaleate were obtained from Sigma. l-aminoadipic acid, dl-aminopimelic acid, and l-aminosuberic acid (l-ASu) were obtained from Bachem. 3H-l-glutmate (3H-l-Glu) and 3H-l-Leucine (3H-l-Leu) were purchased from American Radiolabeled Chemicals. l-aminoazelaic (l-AAz), l-aminosebacic acid, and l-amino 4,5-3H-suberic acid (3H-l-ASu) were synthesized as described in the supplemental data (supplemental materials are available at http://jnm.snmjournals.org).
Radiosynthesis of 18F-FASu
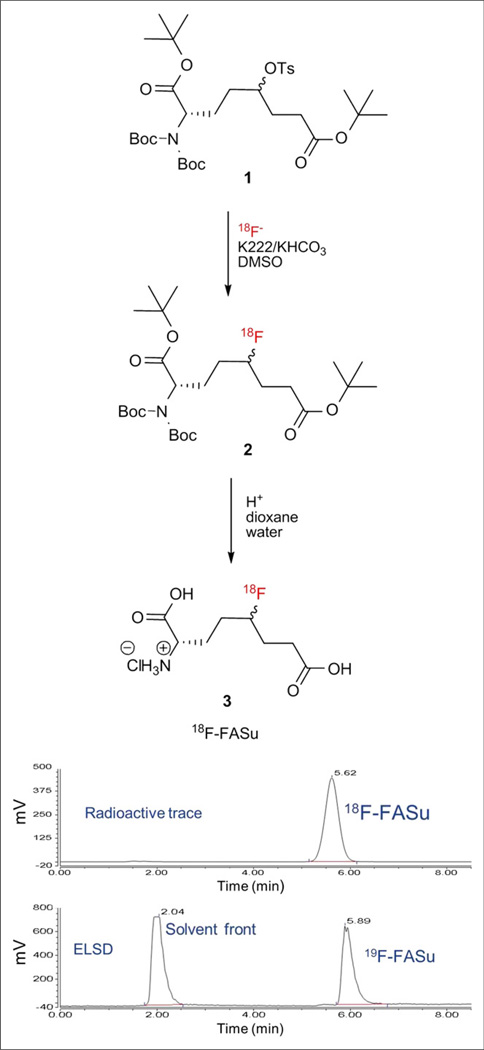
The synthesis of 18F-FASu was independently performed at both GE Global Research and TRIUMF, with efforts at GE Global Research focusing on a radiopharmaceutical preparation method involving high-performance liquid chromatography (HPLC). TRIUMF, along with the BC Cancer Agency, focused on a SepPak (Waters)-based purification method, avoiding the use of HPLC altogether. Detailed descriptions of each group’s experimental protocols are provided in the supplemental data. Briefly, 18F-FASu was prepared from the tosylate precursor using a 2-step anhydrous 18F− nucleophilic displacement strategy as shown in Figure 2.
FIGURE 2.
2-step radiosynthesis and identification of 18F-FASu. HPLC of final product with γ detector demonstrates more than 98% radiochemical purity. HPLC of 19F-FASu standard with evaporative light scattering detector (ELSD) shows that differences in retention time of peaks matches 0.27-min (16-s) delay between the 2 detectors.
Cell Culture Experiments
The human ovarian carcinoma cell line SKOV-3 and murine lymphoma cell line EL4 were obtained from the American Type Tissue Collection and cultured according to vendor recommendations. Cells were maintained in T-75 culture flasks in a humidified incubator at 37°C and 5% CO2 and routinely passaged at confluence. Tumorigenic cell lines were chosen for in vitro and in vivo studies. EL4 cells were chosen to provide large rapidly growing tumors with potential for assessment in immunocompetent animals (for future studies). SKOV-3 cells were chosen to provide slower-growing tumors with more control over tumor size and relevance to human disease.
SKOV-3 cells were subcultured into 12- or 96-well culture-treated plates and grown to confluence. Cells were incubated with 100 µM diethylmaleate 24 h before the uptake assay. Cells were washed with a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–buffered Hanks Balanced Salt solution (HBS). 3H-l-Glu, 3H-l-ASu, 3H-l-Leu, or 18F-FASu was added in the absence or presence of a 1 mM concentration of test compound. For 12-well plate assays, 0.74 kBq (20 nCi) per well was delivered in a 500-µL volume (or 0.037 MBq [1 µCi] per well for 18F-FASu). For 96-well plate assays, 7.4 kBq (200 nCi) per well were delivered in a 50-µL volume. Cells were washed 3 times after the uptake incubation period with HBS. For 96-well plate assays, the last wash was aspirated, 200 µL of Microscint 20 (Perkin Elmer) were added to each well, and the plate was analyzed with a TopCount microplate scintillation counter (PerkinElmer). For 12-well plate assays, cells were solubilized by the addition of 1 mL of 1M NaOH. For uptake in EL4 cells, equal numbers of diethylmaleate-treated cells were aliquoted into microcentrifuge tubes and washed 2 times with HBS by centrifugation. Cell pellets were resuspended in HBS containing 18F-FASu in the absence or presence of inhibitor compounds, incubated while rocking at room temperature for 30 min. Cells were washed 2 times with fresh HBS, and cell pellets were resuspended in 500 µL of 1M NaOH. Solubilized samples with tritium were transferred to a vial with 12 mL of scintillation cocktail and analyzed with a Tri-Carb scintillation counter (Perkin Elmer). For 18F-FASu assays, solubilized cells were added to vials and analyzed using a Wizard γ counter (Perkin Elmer).
Small-Animal PET and Biodistribution Studies
CD-1 nude mice were purchased from Charles River Laboratories, and Rag2-M mice were bred in-house at the BC Cancer Agency. Animals were housed under sterile conditions with food and water ad libitum and a 12-h light cycle. Mice were injected subcutaneously with 1 × 107 EL4 or SKOV-3 cells in phosphate-buffered saline or culture medium while under 2% isoflurane anesthesia, and tumors were allowed to grow for approximately 5–10 or 21 d, respectively. All animal studies were performed in accordance with federal and Institutional Animal Care and Use Committee guidelines using approved protocols.
For biodistribution studies, mice bearing EL4 tumor xenografts were injected via the tail vein with between 0.07 and 0.59 MBq (2–16 µCi) of 18F-FASu. Direct comparison of 18F-FASu and 18F-FDG involved a cohort of 10 nude mice bearing SKOV-3 tumors randomly separated into 2 groups of 5; each group was injected with approximately 0.44 MBq (12 µCi) of either 18F-FASu or 18F-FDG. Mice were euthanized by CO2 asphyxiation at the postinjection time points indicated, and then blood was collected by cardiac puncture and organs were excised, weighed, and counted on a γ counter. All urine was collected on filter paper and counted with the bladder. The unused tissues and remaining carcass were collected and counted. The percentage injected dose per gram (%ID/g) was calculated using the sum of the counts from all tissues, urine, and carcass as the injected dose value.
For PET imaging studies, CD-1 nude mice bearing SKOV-3 tumors were injected with 8.5–11.1 MBq (230–300 µCi) of 18F-FASu via the tail vein. PET imaging data were acquired with an eXplore VISTA preclinical PET scanner (GE Healthcare) under 2% isoflurane anesthesia. Static PET data conducted at 1 h (±5 min) after injection were acquired over 10 min (55–65 min after injection) using 2 bed positions. Mice were then immediately euthanized while under anesthesia by cervical dislocation, and an 18F biodistribution evaluation was performed as described above. Images were reconstructed, and region-of-interest analysis was performed on the tumor. The injected dose for imaging studies was determined with a dose calibrator (Capintec). % ID/g and standardized uptake value were calculated using a scaling factor determined by analysis of an equivalent acquisition of a dose-calibrated 18F-FASu standard.
Alternatively, Rag2-M mice bearing EL4 tumors were anesthetized (2% isoflurane), a 27.5-Ga catheter intravenous line containing saline was placed into the tail, and the rodent was positioned on the scanner imaging bed with integrated heating pad (Inveon; Siemens). Each PET scan was initiated using between 4.5 and 16.4 MBq (122–444 µCi) of 18F-FASu in no more than 125 µL of saline administered via the intravenous line. PET data were collected in list-mode for up to 2 h after injection of the tracer. Scan data were then reconstructed with correction from CT-based attenuation, and regions of interest were placed on the tumor and organs of interest to evaluate the time–activity curves for 18F-FASu in the tissues of interest.
RESULTS
In Vitro Cell Uptake Studies
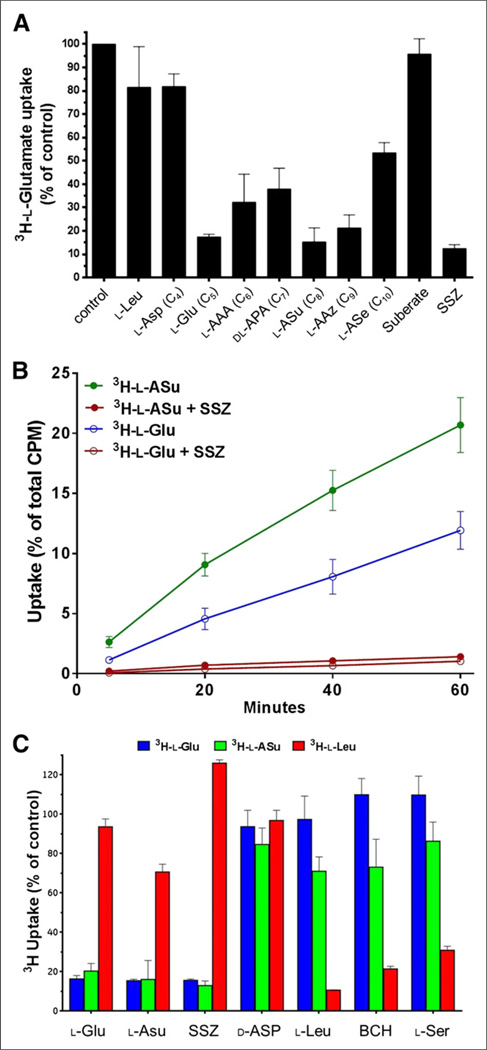
The inhibition of 3H-l-Glu uptake in SKOV-3 cells determined for anionic amino acids ranging from 4 carbons (C4) to 10 carbons (C10) is shown in Figure 3A. l-Leu and suberate were also examined as negative control compounds. A specific system xc− non-substrate inhibitor, sulfasalazine, was used as a positive control. The results show that the 5-carbon l-Glu inhibits 3H-l-Glu uptake via the xc− transporter by 83%. As the carbon chain is extended to 6-, 7-, or 10-carbon atoms in length, the inhibition of 3H-l-Glu uptake is reduced to between 40% and 70%. However, the 8-carbon compound l-ASu and the 9-carbon derivative l-AAz demonstrate inhibition of 3H-l-Glu uptake similar to l-Glu at 85% and 79%, respectively. The best inhibitors appear to have a distance between the 2 terminal carboxyl groups (in a fully extended conformation) that best approximates the distances in the natural substrates l-Glu (5.0 Å, C5) and l-cystine (9.8 Å, between C8 and C9). Negative controls suberate and l-Leu, as well as the 4-carbon l-Asp, showed less than 20% inhibition of 3H-l-Glu uptake.
FIGURE 3.
In vitro system xc− inhibition, uptake, and specificity studies in diethylmaleate-treated SKOV-3 cells. (A) Competitive inhibition of test agents on 3H-l-Glu uptake during 30-min incubation. l-Asp, l-Glu, l-aminoadipic acid, dl-aminopimelic acid, l-ASu, l-AAz, and l-aminosebacic acid are all anionic amino acids from 4 to 10 carbons (C4–C10) in length, respectively. Values are normalized to uptake of 3H-l-Glu in phosphate-buffered saline alone (mean ± SD, n = 3). (B) Comparison of direct cell uptake of 3H-l-ASu and 3H-l-Glu. 3H-l-ASu and 3H-l-Glu were matched for specific activity and radiation dose per sample. Uptake values are expressed as percentage of total activity used per well (mean ± SD, n = 3). (C) Comparison of 3H-l-Glu, 3H-l-ASu, and 3H-l-Leu uptake in presence of selected amino acid transporter inhibitors for 30 min. 3H-l-Glu, 3H-l-ASu, and 3H-l-Leu were matched for specific activity and radiation dose per sample. Values are normalized to uptake of each agent in phosphate-buffered saline alone (mean ± SD, n = 3). SSZ = sulfasalazine.
A comparison of direct uptake of 3H-l-ASu and 3H-l-Glu in SKOV-3 cells is shown in Figure 3B. 3H-l-ASu uptake was 2.3, 2.0, 1.9, and 1.7 times more than the uptake of 3H-l-Glu at 5, 20, 40, and 60 min, respectively. These results suggest that while competitive inhibition results were similar, l-ASu appears to be a more efficient transporter substrate, resulting in better in vitro uptake. Uptake of 3H-l-ASu and 3H-l-Glu was inhibited 92.6% and 92.1%, respectively, with 500 µM sulfasalazine, suggesting that most uptake of these agents can be attributed to the xc− transporter.
To evaluate specificity of l-ASu for the system xc− transporter, we measured the uptake of 3H-l-Glu, 3H-l-ASu, and 3H-l-Leu in the absence and presence of inhibitors (Fig. 3C). Inhibitors used included the system xc− inhibitor sulfasalazine; the excitatory amino acid transporter inhibitor d-Asp; transport systems L, B0, and B0+ inhibitor l-Leu; transport system L inhibitor BCH; and systems A, ASC, B0, and B0+ inhibitor l-Ser. Results demonstrate less than 20% uptake relative to controls for 3H-l-Glu and 3H-l-ASu in the presence of l-Glu, l-ASu, and sulfasalazine, with little, if any, effect on uptake in the presence of l-Leu, BCH, and l-Ser. An inverse relationship was noted for 3H-l-Leu, demonstrating more than 80% uptake in the presence of l-Glu, l-Asu, and sulfasalazine and pronounced inhibition with the latter 3 inhibitors. d-Asp had little inhibitory effect on all tracers.
Radiosynthesis and Uptake of 18F-FASu
Both GE Global Research and TRIUMF used a 2-step nucleophilic displacement strategy for the synthesis of 18F-FASu (Fig. 2) but proceeded to tracer formulation with and without HPLC purification, respectively. Decay-corrected yields were between 5% and 15%, with more than 98% radiochemical purity as determined by peak integration of the corresponding analytical HPLC traces, and were dependant on the experimental protocol. The radiochemical purity of the final product after isolation was more than 98%, regardless of the synthetic method used.
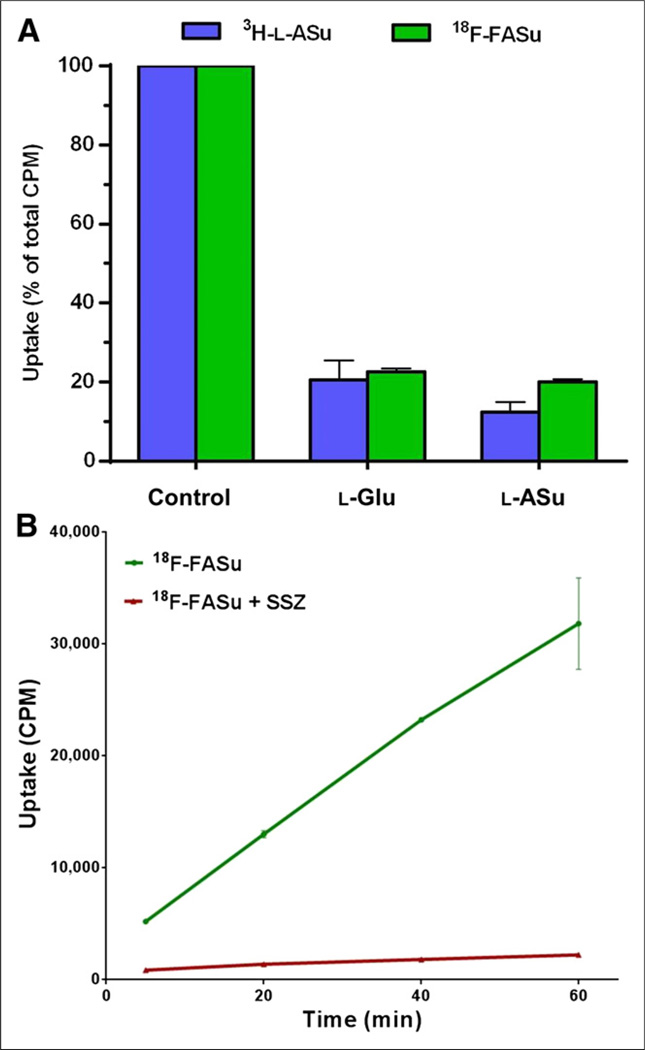
The uptake of 3H-l-ASu and 18F-FASu were both inhibited in EL4 cells by l-ASu and l-Glu (Fig. 4A), suggesting that 18F-FASu is also a substrate for the system xc− transporter. 18F-FASu was assayed for cell uptake in cultured SKOV-3 cells (Fig. 4B) and showed nonsaturable uptake over 1 h, which is potently inhibited to between 7% and 16% of control by sulfasalazine, consistent with uptake via system xc−. Because system xc− activity and 18F-FASu uptake were observed in these in vitro models, we decided to evaluate uptake and imaging in EL4 and SKOV-3 subcutaneous xenograft tumors in vivo.
FIGURE 4.
Uptake of HPLC-purified 18F-FASu in EL4 and SKOV-3 cells in vitro. (A) EL4 cells were evaluated for uptake of 3H-l-ASu and 18F-FASu in absence or presence of 1 mM l-Glu or l-ASu for 30 min. Uptake and inhibition profiles were similar for both radiolabeled compounds. Values are normalized to uptake of each agent in phosphate-buffered-saline-alone control (mean ± SD, n = 4). (B) 18F-FASu uptake in SKOV-3 cells in absence and presence of 0.5 mM sulfasalazine was evaluated with 5-, 20-, 40-, and 60-min incubations. Uptake values are raw CPM values per sample (mean ± SD, n = 3). SSZ = sulfasalazine.
18F-FASu In Vivo Biodistribution and Imaging Studies
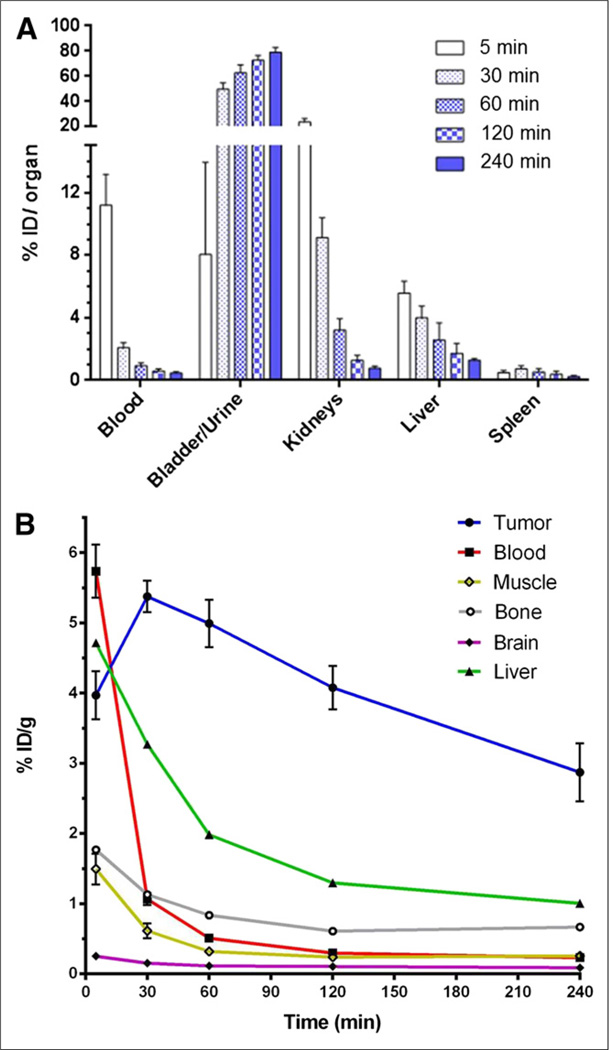
In vivo biodistribution studies were first performed in CD-1 nude mice bearing subcutaneous EL4 xenograft tumors. The clearance of 18F-FASu in mice is shown in Figure 5A. Renal clearance predominates because most activity is quickly partitioned to the kidney, bladder, and urine. Tracer continued to accumulate in the bladder and urine and to clear from blood, kidneys, liver, and spleen over the course of 4 h. Figure 5B shows that EL4 tumor uptake is significant and remains fairly constant up to 2 h after injection. Evaluation of 18F-FASu tissue distribution at 1 h after tail vein injection is shown in Table 1, with tumor-to-blood ratios of approximately 12 and tumor-to-muscle ratios of approximately 28. 18F-FASu also demonstrated prominent retention in the pancreas and significant retention in the spleen.
FIGURE 5.
In vivo biodistribution in CD-1 nude mice bearing EL4 xenograft tumors with HPLC-purified 18F-FASu. (A) 18F-FASu retention in clearance organs at 5, 30, 60, 120, and 240 min after injection are shown in%ID/organ. (B) 18F-FASu retention in tumor, blood, muscle, bone, brain, and liver. Data are %ID/g (mean ± SD, n ≥ 4).
TABLE 1.
Biodistribution of 18F-FASu and 18F-FDG in Mice Bearing Xenograft Tumors
| Organ or tissue | EL4 tumors, 18F-FASu | SKOV-3 tumors | |
|---|---|---|---|
| 18F-FDG | 18F-FASu | ||
| Blood | 0.49 ± 0.12 | 0.58 ± 0.30 | 0.72 ± 0.30 |
| Tumor | 5.81 ± 1.26 | 1.55 ± 0.96 | 8.08 ± 2.03 |
| Muscle | 0.25 ± 0.16 | 4.92 ± 2.26 | 0.35 ± 0.17 |
| Liver | 1.24 ± 0.18 | 0.74 ± 0.15 | 0.86 ± 0.08 |
| Kidney | 10.11 ± 2.58 | 1.04 ± 0.16 | 15.36 ± 5.63 |
| Spleen | 4.98 ± 1.28 | 1.73 ± 0.59 | 6.74 ± 1.02 |
| Heart | 0.33 ± 0.09 | 54.11 ± 29.00 | 0.32 ± 0.07 |
| Lungs | 2.00 ± 0.38 | 3.61 ± 1.68 | 1.95 ± 0.97 |
| Brain | 0.11 ± 0.03 | 7.76 ± 2.77 | 0.09 ± 0.02 |
| Bone | 0.85 ± 0.26 | 3.58 ± 1.74 | 0.85 ± 0.53 |
| Pancreas | 23.34 ± 5.73 | 1.49 ± 0.54 | 21.19 ± 6.61 |
| Fat | 0.29 ± 0.22 | 0.59 ± 0.36 | 0.62 ± 0.67 |
| Tumor-to-blood | 12.01 ± 1.58 | 2.61 ± 0.27 | 12.08 ± 0.4.39 |
| Tumor-to-muscle | 28.16 ± 10.39 | 0.33 ± 0.13 | 29.59 ± 19.44 |
| Tumor size | 1,140 ± 296 | 72.60 ± 37.65 | 71.60 ± 39.09 |
Uptake values are %ID/g of tissue at 1 h after tracer injection; tumor size is in mg, mean ± SD (n ≥ 5).
We conducted a side-by-side comparison of 18F-FASu and 18F-FDG in CD-1 nude mice bearing SKOV-3 tumors at 1 h after injection (Table 1); we chose the SKOV-3 cells for this study because their slow growth allowed for more control over tumor size. 18F-FASu tumor uptake at 1 h after injection reached 8.1 %ID/g, with tumor-to-blood and tumor-to-muscle ratios of approximately 12 and approximately 30, respectively. As expected, 18F-FASu also showed pronounced uptake in the pancreas and spleen. 18F-FDG tissue distribution is also shown in Table 1. Compared with 18F-FDG, 18F-FASu showed a 5.2-fold-greater tumor uptake and a 4.6-fold-greater tumor-to-blood ratio. 18F-FASu also demonstrated much lower background in muscle, heart, and brain.
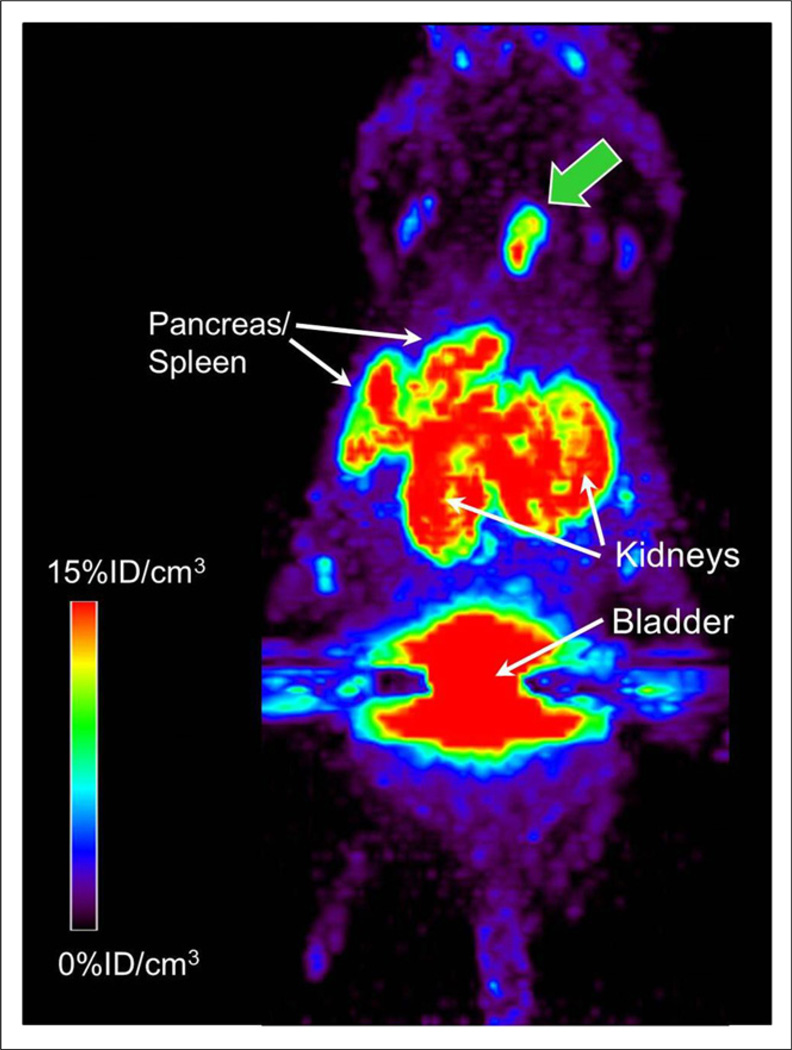
CD-1 nude mice bearing SKOV-3 tumor xenografts were injected with 18F-FASu by tail vein, and PET imaging was conducted 1 h after injection (Fig. 6). Tumor uptake obtained by necropsy biodistribution evaluation of the mouse immediately after imaging, 8.4 %ID/g, correlated well with the mean tumor uptake analysis from the PET image, 8.6 %ID per cubic centimeter (%ID/cm3). Maximum pixel intensity in the tumor was 16.7 %ID/cm3. The mean standardized uptake values of the tumor were 2.5 and 4.9, respectively. Imaging results were consistent with SKOV-3 biodistribution data shown in Table 1.
FIGURE 6.
18F-FASu PET imaging in CD-1 nude mice bearing SKOV-3 xenograft tumors 1 h after injection. Maximum-intensity-projection image of 1 of 2 SKOV-3 tumoRbearing nude mice imaged in this study; tumor is indicated by green arrow. 18F-FASu was HPLC-purified. On necropsy, it was determined that weight of this tumor was 31 mg.
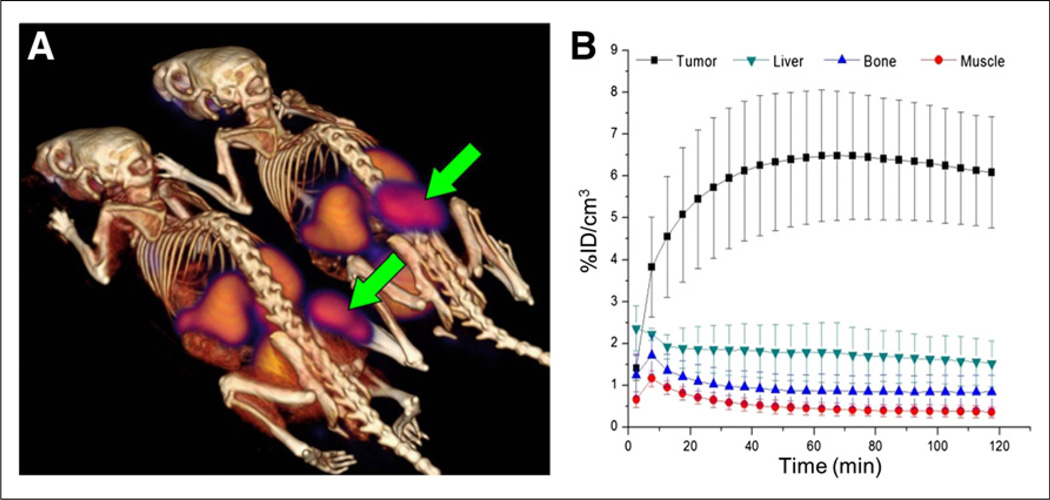
Dynamic PET images of EL4 tumors in Rag2-M mice (Fig. 7A) revealed a rapid and high initial tumor uptake exceeding 6 %ID/cm3 by 40 min after injection, staying close to this level for the 2-h duration of the scan (Fig. 7B). A high tumor-to-muscle ratio of approximately 13 at 120 min after injection was also indicative of a low overall background uptake of the tracer. Uptake in the kidneys and bladder was consistent with renal clearance. Liver retention was low relative to tumor, and the bone signal cleared to less than 1 %ID/cm3, indicating that defluorination is not a prevalent metabolic pathway for 18F-FASu.
FIGURE 7.
In vivo 18F-FASu dynamic PET imaging in Rag2 M mice bearing EL4 xenograft tumor. 18F-FASu was SepPak-purified. (A) PET/CT image summed over 110–120 min after injection. Tumors are indicated by arrows. (B) Region-of-interest analysis of 18F-FASu uptake in tumor, liver, bone, and muscle (mean ± SD, n = 4).
DISCUSSION
18F-FDG and many amino acid analog PET tracers enable detection and monitoring of tumors based on cellular metabolic changes required for sustaining cell growth and proliferation. Amino acids are needed by highly proliferative cells for anabolic protein and nucleotide synthesis and also contribute to energy metabolism by providing a carbon source for the tricarboxylic acid cycle (19). The use of amino acid analogs as PET tracers is useful, not because tumor cells increase their metabolism but rather because they enhance amino acid transport mechanisms to feed that metabolism. Here we use an l-cystine analog to target the narrow substrate profile of the cystine/glutamate transporter as a reporter of oxidative stress.
Two glutamate analogs have been reported as system xc− substrate PET agents, 18F-FGLU and 18F-FSPG (3,14). Our development strategy differed from previous reports in that we focused on a compound with similarity to the preferred uptake substrate l-cystine (9). Our lead compound, l-ASu, has an 8-atom backbone mirroring cystine while lacking a problematic disulfide bond and 1 amine to better maintain the anionic nature vital to system xc− substrates at physiologic pH. Indeed, we demonstrated that 3H-l-ASu was taken up more efficiently than 3H-l-Glu in our in vitro studies with SKOV-3 cells. Longer-chain anionic amino acids, such as l-ASu, have been shown to have drastically reduced affinity for metabotropic glutamate receptors (17,20); therefore, we proposed that cystine-like compounds may have a benefit of specificity over glutamate-like compounds, which are more likely to bind various glutamate receptors or use transport system X−AG. Koglin et al. reported 18F-FSPG uptake in SKOV-3 tumors at approximately 3 %ID/g, whereas we demonstrated 18F-FASu SKOV-3 tumor uptake of approximately 8 %ID/g at the same time after injection. In addition, reports of preclinical and human evaluation of 18F-FSPG have generally demonstrated tumor uptake similar to or less than 18F-FDG (14–16). We demonstrated here that 18F-FASu resulted in a 5-fold enhanced uptake over 18F-FDG in SKOV-3 tumor xenografts. Further studies comparing xc− selectivity; glutamate receptor binding; in vitro system xc− uptake; in vivo biodistribution; and imaging with 18F-FASu, 18F-FGLU, and 18F-FSPG will help evaluate differences in tracer performance. Additional comparisons with 18F-FDG may reveal specific indications in which 18F-FASu would be superior to or useful in addition to 18F-FDG.
Activation of the gene (SLC7A11) encoding the xCT subunit of the xc− transport system has been reported in several cancers and cancer cell lines (10), with several groups exploring the feasibility of using the xCT transporter as a viable target for cancer therapy (10,21,22) by inducing cysteine starvation. Inhibition of the transporter reportedly restricts the growth of lymphoma (23,24), glioma (25,26), head and neck (27), breast (22), prostate (28), lung (29), and pancreatic (21,30) cancer cells both in culture and in in vivo xenograft tumors. Increased xCT transporter activity is also associated with resistance of tumors to certain types of chemotherapy, and inhibition of the xCT transporter has been shown to increase the sensitization of cancer cells to the chemotherapeutic drugs cisplatin, doxorubicin, gemcitabine, celastrol, and etoposide (27,31). Recently, a role for xCT in tumor meta-static potential has been established (32). xCT has also been linked to an undifferentiated stem-like tumor cell phenotype through its interaction with CD44 (27,33). A limited clinical study showed some success with sulfasalazine in combination with melphalan in ovarian cancer (34), whereas more recently a clinical trial has started in Japan for treatment of advanced gastric cancer with sulfasalazine (Unique Trial Number UMIN000010254). Using this transporter to image a cellular response to oxidative stress may provide functional information useful for clinical cancer care, such as detection, staging, patient stratification, and monitoring of response to therapy. If system xc−-targeted therapies such as sulfasalazine prove efficacious in clinical trials, companion diagnostic xCT-targeted PET tracers, such as 18F-FASu, may allow stratification of patients who will respond to treatment.
Oxidative stress has also been implicated in a wide variety of pathologies other than oncology, including neurodegenerative diseases, atherosclerosis, ischemia, organ transplant rejection, autoimmune diseases, and inflammatory conditions. An xCT-targeted imaging agent such as 18F-FASu may provide functional information relevant to many pathologic conditions. For example, whereas xc− transporter activity in the brain will provide an antioxidant defense against oxidative stress, the same mechanism appears to be complicit in neurodegeneration resulting from excitotoxic stimulation of ionotropic glutamate receptors by glutamate effluxed via system xc− (9,35–38). 18F-FASu, 18F-FGLU, and 18F-FSPG are not expected to be permeable to an intact blood–brain barrier, and high basal expression of xCT is reported in normal brain. Despite this, others have reported uptake of 18F-FSPG in human brain tumors (39). However, the mechanism of uptake is not clearly tumor cell–specific, because normal brain tissue uptake might be expected in regions of blood–brain barrier perturbations correlating with tumor growth or other conditions. Future studies in our group will seek to evaluate the feasibility of xCT in the brain as a potential PET tracer target.
CONCLUSION
l-ASu is taken up into cells with system xc− activity more readily than the natural substrate l-Glu. We have developed a novel system xc− substrate and demonstrated feasibility of PET imaging in SKOV-3 and EL4 tumor xenografts. 18F-FASu essentially serves as a reporter for the activation of the ARE promoter, allowing functional PET imaging of a universal cellular response to oxidative stress. The mechanism of 18F-FASu uptake is orthogonal to increased glucose and general amino acid uptake that is common to highly proliferative cells. 18F-FASu may provide more sensitive detection than 18F-FDG for certain tumor types, as was seen in SKOV-3 xenografts. Oxidative stress imaging may also find utility in determining chemotherapy resistance and as a predictor of early response to therapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Francois Bénard for guidance, along with May Wong and the staff of the Animal Resource Centre at the BC Cancer Agency, and Andrew Torres at GE for their skillful technical assistance during the imaging and biodistribution studies. We also thank the TRIUMF cyclotron team, in particular Linda Graham, Wade English, and Cornelia Hoehr, for their assistance in the development of the automated synthesis process.
Research reported in this publication was supported by the NIBIB and NIH under award number R01EB014250. Jack M. Webster, Bruce F. Johnson, Michael J. Rishel, and Christine A. Morton are employed by General Electric.
Footnotes
DISCLOSURE
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Neels OC, Koopmans KP, Jager PL, et al. Manipulation of [11C]-5-hydroxytryptophan and 6-[18F]fluoro-3,4-dihydroxy-L-phenylalanine accumulation in neuroendocrine tumor cells. Cancer Res. 2008;68:7183–7190. doi: 10.1158/0008-5472.CAN-08-0095. [DOI] [PubMed] [Google Scholar]
- 2.Kasper BS, Struffert T, Kasper EM, et al. 18Fluoroethyl-L-tyrosine-PET in long-term epilepsy associated glioneuronal tumors. Epilepsia. 2011;52:35–44. doi: 10.1111/j.1528-1167.2010.02754.x. [DOI] [PubMed] [Google Scholar]
- 3.Ploessl K, Wang L, Lieberman BP, Qu W, Kung HF. Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J Nucl Med. 2012;53:1616–1624. doi: 10.2967/jnumed.111.101279. [DOI] [PubMed] [Google Scholar]
- 4.McConathy J, Yu W, Jarkas N, Seo W, Schuster DM, Goodman MM. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med Res Rev. 2012;32:868–905. doi: 10.1002/med.20250. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- 7.Okuno S, Sato H, Kuriyama-Matsumura K, et al. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br J Cancer. 2003;88:951–956. doi: 10.1038/sj.bjc.6600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdovinos-Flores C, Gonsebatt ME. The role of amino acid transporters in GSH synthesis in the blood-brain barrier and central nervous system. Neurochem Int. 2012;61:405–414. doi: 10.1016/j.neuint.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Lewerenz J, Hewett SJ, Huang Y, et al. The cystine/glutamate antiporter system xc− in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo M, Wang YZ, Gout PW. The xc− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 11.Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system xc−: cystine supplier and beyond. Amino Acids. 2012;42:231–246. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki H, Sato H, Kuriyama-Matsumura K, et al. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 14.Koglin N, Mueller A, Berndt M, et al. Specific PET imaging of xC− transporter activity using a 18F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin Cancer Res. 2011;17:6000–6011. doi: 10.1158/1078-0432.CCR-11-0687. [DOI] [PubMed] [Google Scholar]
- 15.Baek S, Choi CM, Ahn SH, et al. Exploratory clinical trial of (4S)-4-(3-[18F] fluoropropyl)-L-glutamate for imaging xC− transporter using positron emission tomography in patients with non-small cell lung or breast cancer. Clin Cancer Res. 2012;18:5427–5437. doi: 10.1158/1078-0432.CCR-12-0214. [DOI] [PubMed] [Google Scholar]
- 16.Baek S, Mueller A, Lim YS, et al. (4S)-4-(3-18F-fluoropropyl)-l-glutamate for imaging of xC− transporter activity in hepatocellular carcinoma using PET: preclinical and exploratory clinical studies. J Nucl Med. 2013;54:117–123. doi: 10.2967/jnumed.112.108704. [DOI] [PubMed] [Google Scholar]
- 17.Bräuner-Osborne H, Slok FA, Skjaerbaek N, et al. A new highly selective metabotropic excitatory amino acid agonist: 2-amino-4-(3-hydroxy-5-methylisoxazol-4-yl)butyric acid. J Med Chem. 1996;39:3188–3194. doi: 10.1021/jm9602569. [DOI] [PubMed] [Google Scholar]
- 18.Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011;63:35–58. doi: 10.1124/pr.110.004036. [DOI] [PubMed] [Google Scholar]
- 19.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadian H, Nielsen B, Brauner-Osborne H, et al. (S)-homo-AMPA, a specific agonist at the mGlu6 subtype of metabotropic glutamic acid receptors. J Med Chem. 1997;40:3700–3705. doi: 10.1021/jm9703597. [DOI] [PubMed] [Google Scholar]
- 21.Lo M, Ling V, Low C, Wang YZ, Gout PW. Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Curr Oncol. 2010;17:9–16. doi: 10.3747/co.v17i3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells. Anticancer Res. 2003;23:4571–4579. [PubMed] [Google Scholar]
- 23.Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 24.Gout PW, Simms CR, Robertson MC. In vitro studies on the lymphoma growth-inhibitory activity of sulfasalazine. Anticancer Drugs. 2003;14:21–29. doi: 10.1097/00001813-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Chung WJ, Lyons SA, Nelson GM, et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25:7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunrinu TA, Sontheimer H. Hypoxia increases the dependence of glioma cells on glutathione. J Biol Chem. 2010;285:37716–37724. doi: 10.1074/jbc.M110.161190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshikawa M, Tsuchihashi K, Ishimoto T, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–1866. doi: 10.1158/0008-5472.CAN-12-3609-T. [DOI] [PubMed] [Google Scholar]
- 28.Doxsee DW, Gout PW, Kurita T, et al. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67:162–171. doi: 10.1002/pros.20508. [DOI] [PubMed] [Google Scholar]
- 29.Guan J, Lo M, Dockery P, et al. The xc− cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol. 2009;64:463–472. doi: 10.1007/s00280-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 30.Lo M, Ling V, Wang YZ, Gout PW. The xc− cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer. 2008;99:464–472. doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müerköster S, Arlt A, Witt M, et al. Usage of the NF-kappaB inhibitor sulfasalazine as sensitizing agent in combined chemotherapy of pancreatic cancer. Int J Cancer. 2003;104:469–476. doi: 10.1002/ijc.10963. [DOI] [PubMed] [Google Scholar]
- 32.Chen RS, Song YM, Zhou ZY, et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene. 2009;28:599–609. doi: 10.1038/onc.2008.414. [DOI] [PubMed] [Google Scholar]
- 33.Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 34.Gupta V, Jani JP, Jacobs S, et al. Activity of melphalan in combination with the glutathione transferase inhibitor sulfasalazine. Cancer Chemother Pharmacol. 1995;36:13–19. doi: 10.1007/BF00685726. [DOI] [PubMed] [Google Scholar]
- 35.Qin S, Colin C, Hinners I, Gervais A, Cheret C, Mallat M. System Xc− and apolipoprotein E expressed by microglia have opposite effects on the neurotoxicity of amyloid-beta peptide 1–40. J Neurosci. 2006;26:3345–3356. doi: 10.1523/JNEUROSCI.5186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savaskan NE, Heckel A, Hahnen E, et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med. 2008;14:629–632. doi: 10.1038/nm1772. [DOI] [PubMed] [Google Scholar]
- 37.Domercq M, Sanchez-Gomez MV, Sherwin C, Etxebarria E, Fern R, Matute C. System xc− and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol. 2007;178:6549–6556. doi: 10.4049/jimmunol.178.10.6549. [DOI] [PubMed] [Google Scholar]
- 38.Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc− as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–382. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- 39.Kumar M, Mosci C, Keu KV, et al. Evaluation of the 18F-labeled L-glutamate derivative 18F-FSPG (BAY 94-9392) in brain and head and neck cancer patients [abstract] J Nucl Med. 2012;53(suppl):274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.