Carbonaceous nanoparticles (CNP) are promising in a wide range of biomedical applications including CNP-based vaccine adjuvants, drug carriers, and contrast agents.[1–3] As the most potent antigen-presenting cells, dendritic cells (DCs), are actively engaged in shaping immune responses whereby they are capable of inducing and maintaining both T cell-mediated immunity and immune tolerance.[4] Thus, the use of nanomaterials for targeting DC’s functions is an encouraging approach to novel therapies and vaccine development for different immune-mediated diseases. However, studies on effects of nanomaterials on DC functions are very limited. Recently, we have reported the modulation of DC functions by single walled carbon nanotubes.[5] In the current study, we compared the regulation of key DC functions by three CNPs with distinctive geometries and surface properties–planar and negatively-charged graphene oxide (GO), non-charged “spherical” C60-fullerenes, and negatively-charged “spherical” C60-TRIS fullerenes. We demonstrated the opposite regulatory effects of the DC’s ability to prime antigen-specific T lymphocytes—suppression or activation—by GO and C60-fullerenes, respectively. The inhibition of antigen presentation in GO-treated DCs was not mediated by alterations of antigen engulfment or MHCI/peptide - TCR interaction but was associated with down-regulation of intracellular levels of subunit LMP7 of immunoproteasome responsible for antigens processing in DCs. In silico modeling indicated that LMP7 preferentially binds to the basal plane of GO, leading to a strong electrostatic association as compared to weaker hydrophobic modes of binding of C60-fullerenes. The demonstrated strong dependence of functional and phenotypic modulation of DCs by CNP with differing surface properties should be considered when enhancement of the immunostimulatory or tolerogenic functions of DCs are required for clinical utilization.
The CNP used in the present study were carefully characterized as described in the Supporting Information (Figure S1, S2). The zeta potential was −13.6, −26.1, and −32.4 mV for C60 fullerenes, C60-TRIS fullerenes, and GO, respectively. The average particle sizes obtained by TEM were 45.2 ± 25.3 nm for C60 fullerenes and 45.6 ± 18.8 nm for C60-TRIS (Figure S2). GO was characterized as previously described.[6]
The key function of conventional DCs is the activation of antigen-specific T cells, which relies on the efficient antigen capture, intracellular processing of protein antigens and presentation of antigenic peptides within MHC/peptide complex on DC surface to antigen-specific T lymphocytes recognizing antigenic peptides by T cell receptors (TCR). Depending on the presence of additional co-stimulatory and cytokine-mediated stimuli provided by DCs and the micro-environmental elements, T cell-mediated immunity, unresponsiveness or tolerance may be induced and maintained. Furthermore, inefficient DC/T cell interaction and antigen presentation may results in a weak immune response or T cell suppression.[7] Therefore successful employment of nanomaterials to improve DC loading with antigens or targeting other features of therapeutic DC vaccines should consider the ability of CNP to affect the key function of DCs since both immunogenic or tolerogenic properties of DCs may be unintentionally affected.
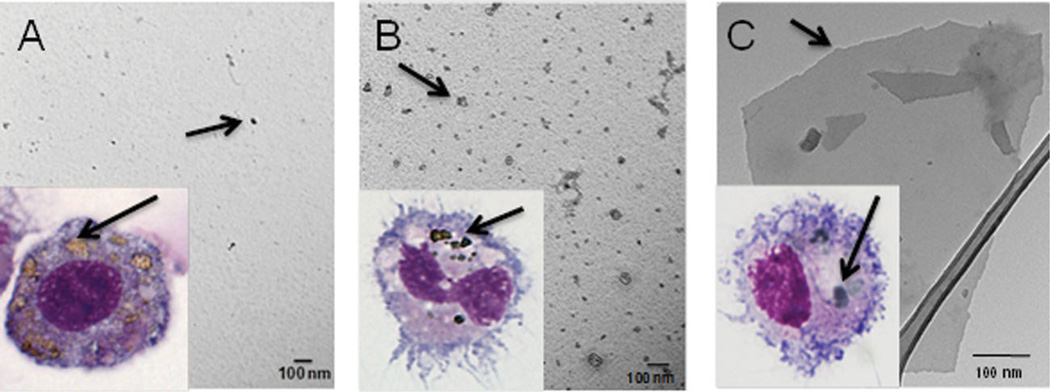
To determine how CNP may alter antigen-presenting activity of DCs in vitro, we first tested whether nanomaterials could directly interact with DCs. Co-incubation of bone marrow-derived DCs with GO, C60 and C60-TRIS fullerenes resulted in efficient incorporation of CNP into the cells, as determined by visualization of aggregates of nanomaterials in DC cytoplasm (Figure 1, inset). Next we examined the ability of different CNP to modify the key function of DCs: presentation of antigens and activation of antigen-specific T cells.
Figure 1.
Scanning electron microscope (SEM)/transmission electron microscope (TEM) micrographs for C60 (A), C60-TRIS (B) and GO (C) as seen in phosphate buffered saline (PBS) suspensions. Light microscopy images of cultured DCs engulfing CNP (A, B, C inset). Notably, DCs were capable of effective CNP uptake.
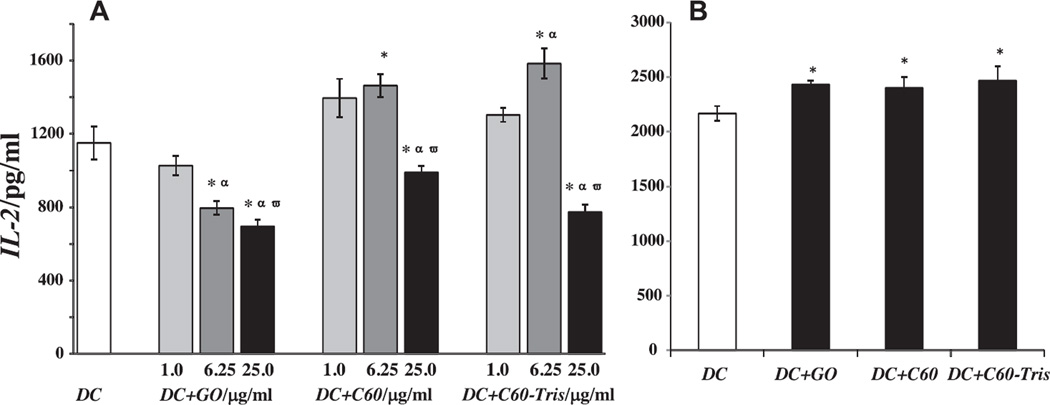
To this end, we tested the capacity of control and CNP-treated DCs to present an antigen to syngeneic T cells utilizing ovalbumin (OVA) as a model antigen. The CTL epitope and Th epitope of this antigen have been well characterized and the model is well established in our lab.[8] DCs were exposed to GO and fullerenes prior to OVA-loading and then mixed with B3Z86/90.14 (B3Z) CD8+ T cells specific for the H-2Kb-restricted anti-mouse OVA257–264 (SIINFEKL) peptide. Production of IL-2 by T cells reflected T cell activation upon recognition of the OVA epitope 257–264 in the context of the H-2Kb molecules (MHC class I). The results showed that pre-treatment of DCs with 6.25 µg/mL of C60 or C60-TRIS fullerenes prior to antigen loading promoted the ability of DCs to activate OVA-specific T cells. Higher dose of fullerenes (25 µg/mL), however, negatively affected stimulatory capacity of DCs (Figure 2A). In contrast, pre-exposure of DCs to 6.25 and 25 µg/mL of GO resulted in significantly impaired stimulatory potential of DCs (Figure 2A). These results suggest that structural features of CNP may be responsible for their differential effects on the ability of DCs to present antigens to T cells. They also demonstrate that, in spite of the general belief that nanomaterials activate antigen presentation by DCs, CNP, e.g., GO, may efficiently inhibit this key function of DCs. These important results open a new opportunity to use DC vaccines not only for induction of immune responses, as required, e.g., in cancer, but also to utilize DCs for the treatment of autoimmune disorders where inhibition of antigen-specific immune responses is desirable.
Figure 2.
(A) IL-2 production by B3Z T cells stimulated by OVA-loaded DCs pre-exposed to CNP in vitro. GO exposure decreased the ability of DCs to stimulate T cells, while fullerene exposure facilitated T cell responses. Data are shown as mean values ± SEM (n = 3); *p < 0.05 vs. control, αp < 0.05 vs. exposure to 1.0 µg/mL, ωp < 0.05 vs. exposure to 6.25 µg/mL. (B) IL-2 production by B3Z T cells stimulated by SIINFEKL (0.01 µM) peptide-loaded DCs pre-exposed to CNP (6.25 µg/mL) in vitro. Notably, GO did not interfere with the cross-presentation of readily available antigenic peptide in DCs. Data are shown as mean values ± SEM (n = 3); *p < 0.05 vs. control.
It is important to note that exposure of DCs to CNP after loading with protein antigen did not significantly affect the ability of DCs to stimulate antigen-specific B3Z cells (data not shown). This suggests that tested CNP affected the processes associated with antigen uptake, preparation or presentation and were ineffective after these steps were finalized. In fact, presentation of antigenic peptides delivered from exogenous proteins, such as OVA, in the context of MHC class I molecules (cross-presentation) requires endocytosis of the antigen and its subsequent processing by the multicatalytic proteases within the immunoproteasomes.[9] The resulting short peptides (8–12 residues long) are translocated into the endoplasmic reticulum and form complexes with MHC class I molecules. These complexes are further transported to the cell surface to be presented to TCR on T cells.[9] Our results revealed that antigen presenting potential of DCs was up- or down-regulated by C60 fullerenes and GO, respectively, when cells were pre-treated with CNP prior to addition of OVA. Thus, it is conceivable that CNP may interfere with either antigen uptake by DCs (i.e., pinocytosis of soluble protein) or antigen processing by MHC class I antigen-processing machinery (APM) components in DCs or presentation of antigenic peptides within MHCI/peptide complex on DC surface to TCR on T cells. To better understand the mechanisms of modulation of antigen-presenting function of DCs by CNP, we experimentally tested each of these three pathways.
We evaluated whether CNP could alter the final step of antigen presentation, i.e., the presentation of an OVA-related antigenic peptide, which does not require intracellular proteasomal degradation processing and is readily presented by DCs after binding with MHC class I molecules. Because DCs process OVA protein to a number of peptides, including an octapeptide SIINFEKL, which is presented by H-2Kb (MHC class I) complexes, we used SIINFEKL for loading into DCs pre-exposed to CNP. DCs were then co-incubated with SIINFEKL-specific T cells as above to assess their ability to present the antigen. The results revealed that GO did not suppress the antigen-presenting ability of DCs loaded with OVA257–264 peptide (Figure 2B), in contrast to DCs loaded with the whole OVA protein (as in Figure 2A). All tested CNP slightly, but significantly, elevated presentation of SIINFEKL peptide by DCs, suggesting that the intracellular transport of the peptide and its binding with MHC class I molecules in EPR or on the cell surface are not the targets for CNP in DCs. Therefore this pathway, i.e., the final step of antigen presentation during MHCI/peptide -TCR interaction, cannot explain the differential effects of GO and fullerenes on DC ability to present antigens to T cells.
The next potentially targeted by CNP pathway in DCs is their endocytic activity. To test whether exposure of DCs to GO could disrupt uptake of OVA in DCs, CNP-treated cells were co-incubated with FITC-labeled OVA or Lucifer Yellow (LE) (as a control). Analysis of incorporation of FITC-OVA and LY into DCs, which reflects the pinocytic activity of cells, demonstrated that the amount of FITC-OVA and LY engulfed by DCs pre-treated with CNP was similar to that seen in control DCs (p > 0.1, n = 3). For instance, uptake of FITC-OVA, reflected by alteration of MFI was 7030 ± 786, 6723 ± 916, 6962 ± 889 and 6658 ± 779 MFI in DCs treated with medium, C60, C60-TRIS and GO, respectively. Similar results were obtained for incorporation of LE in control and CNP-treated DCs. Therefore, GO induced down-regulation of the antigen-presenting potential of DCs cannot be explained by the GO effect on endocytic property of DCs.
Because GO did not alter antigen uptake and did not suppress presentation of an antigenic peptide by DCs, the next potential mechanism of its inhibitory influence on DCs could be the MHC class I antigen-processing machinery (APM). Processing of protein antigens in DCs involves the cascade of protein fragmentation facilitated by immunoproteasomes. It has been previously demonstrated that the activity of immunoproteasomes incorporating three proteolytic proteasome subunits (LMP2, LMP7 and MECL-1) is essential for the generation of T cell epitopes.[10] Immunoproteasome-derived peptides are further transported to the endoplasmic reticulum by the Transporters Associated with Antigen Processing (TAP1/2) and loaded into MHC-I complexes. The loading of peptide into MHC I involves association of MHC I heavy chain with calnexin and subsequent incorporation into peptide-loading complex involving TAP, tapasin, calreticulin and ERp57. Binding of a peptide to MHC class I molecule promotes its dissociation from the peptide-loading complex.[11, 12]
To determine whether CNPs interfere with MHC class I APM components in DCs, we measured the expression of APM molecules in DCs after CNP treatment. The results revealed that the exposure of DCs to GO resulted in significantly decreased levels of one of the critical immunoproteosome subunits - LMP7. In contrast, incubation of DCs with C60 and C60-TRIS fullerenes did not result in significant changes in the APM components (Table 1). These results identified a potential pathway and a protein targeted by GO in DCs as mediators of the GO-induced inhibition of antigen-presenting potential of DCs. They also raised a question about possible mechanisms of GO interactions with LMP7 that were not realized upon treatment of DCs with C60 and C60-TRIS fullerenes.
Table 1.
Expression on the major components of MCH class I antigen processing machinery by DCs after exposure to CNP in vitro.a)
| APM components | Control DC | C60 | C60-Tris | Graphene Oxide |
|---|---|---|---|---|
| LMP2 | 183 ± 4 | 177 ± 3 | 179 ± 5 | 178 ± 10 |
| LMP7 | 1348 ± 34 | 1380 ± 60 | 1514 ± 39 | 551 ± 16* |
| LMP10 (MECL-1) | 467 ± 32 | 495 ± 16 | 503 ± 8 | 461 ± 19 |
| ERp57 | 265 ± 3 | 289 ± 4 | 252 ± 14 | 261 ± 15 |
| Calreticulin | 785 ± 24 | 803 ± 15 | 826 ± 47 | 738 ± 76 |
| TAP-1 | 1120 ± 54 | 1136 ± 39 | 1091 ± 25 | 1034 ± 51 |
| Calnexin | 1380 ± 23 | 1414 ± 27 | 1312 ± 27 | 1302 ± 13 |
Data are shown as mean of fluorescence intensity (MFI) ± SEM (n = 3).
p < 0.05 vs. control non-treated DCs.
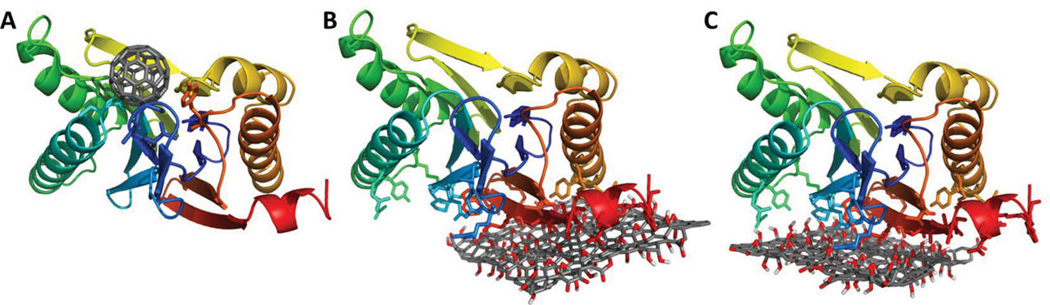
To get a better insight into mechanisms of interactions between LMP7 and CNP, we employed molecular modeling of 3D structures corresponding to C60 fullerenes and GO sites for docking to LMP7 using Autodock Vina procedure.[13] The predicted binding mode of C60 fullerene and GO on LMP7 is shown in Figure 3. All the predicted binding poses of C60 fullerene on LMP7 were identical and localized in close proximity (with in 5 Å) to residues T93, A94, G95, G119, C120, S168, S202, and Y241 (Figure 3a). In contrast to this, the predicted binding site of LMP7 on GO was localized to a different region composed of two overlapping binding sites (Figure 3b,c). The predicted binding sites were confined to residues R102, N104, E108, R126, R129, Y139, R145, E216, P221, E222, Y225, R229, K252, D254, G255, W256, V257, K258, V259, S261, D263, S265, D266, Y269 and K270. While the predicted binding site in the case of C60 included hydrophobic and aromatic residues, the GO binding site was mostly composed of charged residues. This indicates that the GO interaction with LMP7 is due to a strong electrostatic contact between the negatively-charged oxidized groups of GO and positively-charged residues on LMP7 molecules. Thus, the interaction between GO and LMP7 molecules was preferentially driven by charged residues, while C60 fullerene occupied a different site on LMP7 with dominating hydrophobic interactions (Figure 3). The electrostatic interaction of LMP7 with GO may lead to a strong adsorption of LMP7 on GO, which cannot be easily disrupted, thus resulting in the low levels of free intracellular LMP7 detected in GO-treated DCs. This strong GO/LMP7 interaction likely prevented efficient processing of protein antigen by immunoproteosomes in DCs, leading to a diminished antigen cross-presentation and T cell response.
Figure 3.
Predicted interaction sites of C 60 and GO on LMP7. (A) The predicted binding site of C 60 on LMP7. The predicted interaction (B) site 1 and (C) site 2 of GO on LMP7. LMP7 is represented as cartoon and is colored in blue-red from N-C terminus. The residues that are predicted to be within close proximity (5Å) to either C60 or GO are represented as sticks. The structures of C60 and GO are represented as sticks and colored in dark grey, red and white to highlight the C, O and H atoms.
Our results indicated that GO, in contrast to C60 fullerenes, suppressed antigen processing in DCs and thus down-regulated their ability to activate antigen-specific T lymphocytes. However, several reports have showed that CNP are potent inducers of DC maturation, the process commonly associated with increased ability of DCs to stimulate clonal expansion of T cells. For instance, exposure of DCs to carbon black facilitated DC maturation in vitro.[14] Fullerene derivative [Gd@C(82)(OH)(22)](n) has been reported to induce phenotypic and functional maturation of DCs, including increased production of IL-12.[15] Therefore, an important question was whether GO shared similar properties with other CNP structures or was unable to affect the maturation status of DCs: if GO does not up-regulate DC maturation, it might probably block this process, which could also contribute in the GO ability to suppress antigen presentation by DCs.
Consequently, we next evaluated expression of MHC and co-stimulatory molecules on DCs exposed to C60, C60-TRIS and GO. Noteworthy, all CNP preparations were first examined for the endotoxin content known as a potent inducer of DC maturation. We found that endotoxin levels in CNPs were below the detection limit of chromogenic Limulus amebocyte lysate (LAL) assay (0.01 EU/mL).[16] In these experiments, LPS served as a positive control. As Table 2 shows, both GO and fullerenes induced increased expression of co-stimulatory CD86 and CD80, as well as MHC molecules on DCs, suggesting that all tested CNP share similar ability to cause DC maturation in vitro. In spite of this capacity, the overall effect of GO on the ability of DC to activate antigen-specific T cells was negative (Figure 2). However, the stimulatory potential of C60 fullerenes on DCs may, at least in part, be explained by their ability to up-regulate expression of MHC and co-stimulatory molecules.
Table 2.
DC phenotype following CNP exposure in vitro.a)
| Vehicle | GO | C60 | C60-Tris | LPS | |
|---|---|---|---|---|---|
| CD80 | 50.7 ± 4.2 | 68.3 ± 5.1* | 58.5 ± 3.8 | 55.4 ± 4.6 | 122 ± 5.9* |
| CD86 | 61 ± 2.9 | 71.7 ± 3.7 | 74 ± 4.2* | 79.1 ± 3.6* | 139 ± 7.2* |
| CD40 | 3.9 ± 0.3 | 4.2 ± 0.7 | 3.5 ± 0.5 | 3.7 ± 0.9 | 16.5 ± 1.2 |
| MHC Class I | 71.3 ± 3.1 | 95 ± 5.6* | 84.7 ± 4.3* | 84.8 ± 3.5* | 151 ± 9.9* |
| MHC Class II | 35.4 ± 3.4 | 47.8 ± 2.8* | 35.8 ± 2.1 | 39.9 ± 4.3 | 71.4 ± 5.1* |
Data are shown as mean of fluorescence intensity (MFI) ± SEM (n = 3).
p < 0.05 vs. vehicle-treated DCs.
While OVA-loaded DCs pre-exposed to GO were more phenotypically mature than control OVA-loaded DCs, they failed to effectively prime OVA-specific MHC I-restricted antigen-specific T cells. This indicates that GO-mediated regulation of APM represents the dominant pathway of DC inhibition by GO. This is also supported by the fact that exposure of DCs to CNP after OVA loading did not significantly affect the ability of DCs to stimulate antigen-specific T cells. Overall, our data suggest that structural differences between CNP molecules determine their ability to interfere with antigen processing in DCs and therefore control DC’s ability to induce antigen-specific immune response. Our data also suggest that evaluations of CNPs effects on phenotypic maturation of DCs cannot serve as a definitive test for the immunogenic potential of DC vaccines. Indeed, in spite of commonly observed induction of DC maturation, some CNP, e.g., graphene oxide, may cause the immunosuppressive effect on the key functions of DCs, such as antigen presentation.
The results of our studies demonstrate that both C60 fullerenes and graphene capable of modulating the antigen-specific T cell responses due to their ability to directly affect the functional activity of DCs, the most potent antigen-presenting cells. Specifically, C60 fullerenes fostered the ability of DCs to stimulate T cells, in part by promoting phenotypic maturation of DCs assessed as increased expression of MHC and co-stimulatory molecules on DC surface. In contrast, GO disrupted the protein antigen processing in DCs by down-regulating the levels of immunoproteasome LMP7 and, thus, diminishing the ability of DCs to induce activation of antigen-specific T lymphocytes. This immunosuppressive effect of GO dominated the ability of GO to potentiate maturation of DCs. Although our molecular modeling experiments revealed strong electrostatic binding sites as defining GO/LPM7 interactions, further studies should fully explore the detailed mechanisms of GO-induced effects on LMP7 expression in DCs and other cell. Moreover, our findings may provide new opportunities for targeting components of antigen-processing machinery and thus controlling the development of autoimmune and other immune-mediated diseases. Our results also emphasize the importance of careful and extensive assessments of immunomodulatory effects of nanoparticles considered for diverse biomedical applications. Conversely, if appropriately controlled, the immunoregulatory properties of CNPs may be exploited for both immunostimulatory and immunosuppressive therapeutic gains.
Experimental Section
Experimental procedures are provided in the Supporting Information.
Supplementary Material
Acknowledgements
This work was supported by NIOSH OH008282, NORA 0HELD015, NIH HL70755, ES019304, ES020693, ES021068, U19AI068021, NORA 927ZJQP
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Contributor Information
Alexey V. Tkach, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA
Naveena Yanamala, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA.
Shyla Stanley, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA; West Virginia University, Robert C. Byrd Health Science Center, Morgantown, WV, USA.
Michael R. Shurin, University of Pittsburgh, 100 Technology Drive, Pittsburgh, PA 15219, USA
Galina V. Shurin, University of Pittsburgh, 100 Technology Drive, Pittsburgh, PA 15219, USA
Elena R. Kisin, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA
Ashley R. Murray, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA
Samantha Pareso, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA; West Virginia University, Robert C. Byrd Health Science Center, Morgantown, WV, USA.
Timur Khaliullin, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA.
Gregg P. Kotchey, University of Pittsburgh, 100 Technology Drive, Pittsburgh, PA 15219, USA
Vincent Castranova, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA.
Sanjay Mathur, University of Cologne, Albertus-Magnus-Platz 1, 50931 Cologne, Germany.
Bengt Fadeel, Karolinska Institutet, Nobels Vag 13, S-17177 Stockholm, Sweden.
Alexander Star, University of Pittsburgh, 100 Technology Drive, Pittsburgh, PA 15219, USA.
Valerian E. Kagan, University of Pittsburgh, 100 Technology Drive, Pittsburgh, PA 15219, USA
Anna A. Shvedova, Email: ats1@cdc.gov, Health Effects Laboratory Division, NIOSH, 1095 Willowdale Road, Morgantown, WV 26505, USA; West Virginia University, Robert C. Byrd Health Science Center, Morgantown, WV, USA.
References
- 1.Wang T, Zou M, Jiang H, Ji Z, Gao P, Cheng G. Eur. J Pharm. Sci. 2011;44:653. doi: 10.1016/j.ejps.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Li J, Li W, Zhang Y, Yang X, Chen N, Sun Y, Zhao Y, Fan C, Huang Q. Theranostics. 2012;3:302. doi: 10.7150/thno.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khandare JJ, Jalota-Badhwar A, Satavalekar SD, Bhansali SG, Aher ND, Kharas F, Banerjee SS. Nanoscale. 2012;4:837. doi: 10.1039/c1nr11540e. [DOI] [PubMed] [Google Scholar]
- 4.Swiecki M, Colonna M. Immunol. Rev. 2010;234:142. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tkach AV, Shurin GV, Shurin MR, Kisin ER, Murray AR, Young SH, Star A, Fadeel B, Kagan VE, Shvedova AA. ACS Nano. 2011;5:5755. doi: 10.1021/nn2014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, Klein-Seetharaman J, Kagan VE, Star A. ACS Nano. 2011;5:2098. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinski P, Lebre MC, Kramer D, de Jong EC, Van Schijndel JW, Kapsenberg ML. Allergy. 2003;58:648. doi: 10.1034/j.1398-9995.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 8.Shurin CV, Tourkova IL, Chatta GS, Schmidt G, Wei S, Djeu JY, Shurin MR. J. Immunol. 2005;174:3394. doi: 10.4049/jimmunol.174.6.3394. [DOI] [PubMed] [Google Scholar]
- 9.Heemels MT, Ploegh H. Annu. Rev. Biochem. 1995;64:463. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 10.Palmowski MJ, Gileadi U, Salio M, Gallimore A, Millrain M, James E, Addey C, Scott D, Dyson J, Simpson E, Cerundolo V. J. Immunol. 2006;177:983. doi: 10.4049/jimmunol.177.2.983. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman AL, Cresswell P. J. Immunol. 2003;170:4178. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 12.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Immunol. Rev. 2005;207:145. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 13.Trott O, Olson AJ. J. Comput. Chem. 2010;31:455. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike E, Takano H, Inoue K, Yanagisawa R, Kobayashi T. Chemosphere. 2008;73:371. doi: 10.1016/j.chemosphere.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, Zhao Y, Guo H, Li Y, Tewary P, Xing G, Hou W, Oppenheim JJ, Zhang N. ACS Nano. 2010;4:1178. doi: 10.1021/nn901478z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. Nano Lett. 2006;6:1682. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.