Abstract
Background:
Despite advances in acne therapy in recent years, treatment failure is common. Isotretinoin is the only drug that affects almost all factors in acne pathogenesis, but side-effects are common at the doses reported in published studies in the literature. The aim of this study was to investigate the efficacy of low daily dose isotretinoin in moderate to severe acne patients. The secondary objective was to measure the rate of relapse 5 years after the completion of therapy.
Materials and Methods:
In this retrospective, noncomparative study, 146 patients with moderate to severe scare prone acne. Treatment regimen consisted of isotretinoin, fixed 20 mg daily, and duration of treatment-based on the weight of patient, until total cumulative dose of 120 mg/kg of body weight is achieved. No topical or other systemic therapy was allowed during the trial. Liver function tests (serum glutamic-oxalocetic transaminase, serum glutamate pyruvate transaminase, direct and total bilirubin), and lipid profiles (total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride) were evaluated for all patients, before the initiation of treatment and again after the 2nd month of treatment. All data analyzed by Microsoft Office Excel 2007; in descriptive statics frequency and SPSS.18 software.
Results:
At the end of treatment course, (96.4%) demonstrated complete clearing of their acne, defined as no acne or occasional isolated lesions. In 5-year follow-up, relapse accrued in 11 (7.9%) of patients. All adverse effects were mild, and discontinuation of treatment was not necessary.
Conclusion:
Low dose isotretinoin was found to be a safe and effective choice for patients with moderate to severe scar prone acne vulgaris.
Keywords: Acne, isotretinoin, low dose
INTRODUCTION
Acne is a very common skin disease in young people, aged 12-24 years old, but it can occur in whole life.[1] This disease is a multifaceted disorder of the sebaceous glands.[2] Over the last 3 decades, scientific understanding of the pathogenesis of acne has increased considerably. Acne vulgaris is traditionally managed with a variety of topical and systemic medications. Isotretinoin has been used more commonly in recent years, especially for treatment of nodular and nodulocystic acne.[3,4] Without doubt, oral isotretinoin is the single most effective agent for treatment of acne.[5] On the other hand, this agent is the only available drug that affects all four of the major pathogenic processes in acne.[6] Peck, et al. recommended a 6-12 month course of isotretinohn to reach a total cumulative dose of 150 mg/kg for most cases of severe acne.[4] The downside of this drug is its toxicity. Conventional dose of isotretinoin causes many side-effects such as teratogenicity, mucocutaneous events, increasing serum lipids and liver enzymes, gastrointestinal, ocular, and psychological side-effects.[7,8,9] Hence, most clinicians try to keep the dose as low as possible.[10,11,12] For these reasons, we also tried low dose isotretinoin in acne patients. In this study, we have investigated a new method to tretinoin prescription in order to decrease the side-effects and improve the tolerability. The prolonged use time make no problem because of the daily dosage would be lowered and although the total dose increased, but it has been considered ethically. This kind of application time seems necessary to reach a total standard dose.
MATERIALS AND METHODS
In this retrospective, noncomparative study 140 patients (89 females and 51 males) with moderate (moderate disease that is visible at 50 cm or greater and is not easily covered with make-up or the normal shadow of a shaved beard hair). Stretching the skin can flatten the scar. Examples include more significant rolling scars, shallow boxcar scars, and mild to moderate hypertrophic scars) to severe scar (severe disease as in Grade 3 but scarring is not flattened by stretching the skin. Examples include severe boxcar scars, deep divots, ice pick scars, and hypertrophic/keloid scarring) prone acne localized to face, back, chest, and arm, were included in this clinical trial, from December 2010 to 2011 in dermatology clinic of Hazrat-e-Rasoul-Akram Hospital. Adolescent and postadolescent patients, with ages between 18 and 40 years (mean, 23.78 an. 54 years) were included. All patients had chronic moderate to severe acne, unresponsive to long-term antibiotic therapy, with a tendency to cause scarring and leading to negative psychological effects. Before treatment was initiated; age, weight, and anatomic location of lesions were recorded. Treatment regimen consisted of isotretinoin, fixed 20 mg daily, and duration of treatment-based on the weight of patient, until total cumulative dose of 120 mg/kg of body weight is achieved. No topical or other systemic therapy was allowed during the trial. Liver function tests (serum glutamic-oxalocetic transaminase, serum glutamate pyruvate transaminase, direct and total bilirubin), and lipid profiles (total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride) were evaluated for all patients, before the initiation of treatment and again after the 2nd month of treatment. Thereafter, 6 monthly testing is indicated only if an abnormal value is not found on previous tests.
Female patients underwent pregnancy tests and were advised to use two reliable simultaneous forms of contraception, during treatment and for 3 months after the termination of treatment. The evaluations were done at 1-month intervals, and each patient was assessed by two dermatologists and a resident of dermatology each time. Determination of response was made on the basis of physician examination. After termination of treatment protocols, all patients were followed-up for another 5 years period. Primary efficacy parameter was total inflammatory lesional (papules, pustules, and nodules) complete clearing or occasional isolated lesions. Exclusion criteria included pregnant and lactating women and sexually active women without using reliable contraception.
Ethics
This study was performed on human subjects; thus, all patients were aware of the presence of the study and they were fully informed about the drug and its side-effects. The study was approved by medical ethics committee of Tehran University of Medical Sciences.
Statistics
All data analyzed by Microsoft Office Excel 2007; in descriptive statics frequency (for qualitative data), mean, range, and standard deviation (for quantitative data), were used to demonstrate the results. t-test and Chi-square test were used to compare data in SPSS.18 software. P < 0.05 was considered to be significant.
RESULTS
In this study, 146 patients with acne enrolled the study but 6 patients excluded because of severe complications. At all 140 patients (51 male [36.4%] and 89 female [63.6%]) enrolled the study. The age range of patients was between 18 and 40 (mean, 23.78 ± 8.54). Forty-four (31.4%) patients were younger than 21 years old and 96 (69.6%) patients were 22 years or older.
Acne clearing rate had not any relation with the age of patients. No statistical significant correlations were found between patients age, sex, duration of disease, location, and type of acne.
Family history of acne was positive in 56 patients (40%).
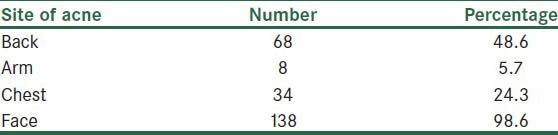
Most common site of involvement was face (138 patients (98.6%)), then back (68 patients (48.6%)) [Table 1]. Acne clearing rate had not any relation with anatomical location of lesions.
Table 1.
Sites of acne in patients
At the end of treatment course, 135 patients (96.4%) demonstrated complete clearing of their acne, defined as no acne or occasional isolated lesions. Mean time of complete clearing after therapy was 4.53 (SDpy. 17) months. Acne clearing rate had not any relation with gender of patients.
An acne flare-up occurred during the first 2 months of treatment in only 14 patients (10%). This flare up (17.6% vs. 5.6%) was significantly higher in male patients (P = 0.025).
The longest duration of treatment was 22 months and the shortest duration was 10 months. Patients weight ranged from 50 to 110 kg (mean, 65.89 ane. 02). Fifty-eight patients were below 60 kg and 82 were above 60 kg, relapse were seen in 6.9% of patients below 60 kg and 8.5% of patients above 60 kg, and there were no statistically difference between two groups (P = 0.492). In 5-year follow-up, relapse occurred in 11 (7.9%) of patients. Mean time of relapse after treatment termination was 17.27 (SDna. 56) months. Mean age of patients with relapse was 23.91 (SDse. 19), and mean age of the remainder was 23.77 (SDnd. 41) years (P = 0.935).
5.9% of male patients and 8.9% of female patients had relapsed. There was no relation between gender, anatomical location, and clearance or relapse in patients (P = 0.380).
The side-effects observed during the study are presented in Table 2. The most common side-effects were mild cheilitis in 93 patients (66.4%), mild xerosis in 12 patients (8.5%), epistaxis in 4 (2.8%), and arthralgia in 5 patients (4.2%), respectively. Six patients excluded the study because of complications such as depression (in 3 patients), Melena, severe arthralgia and severe headache. Adverse effects were higher in male patients (30.3%) versus 17.6% in females, but there was not statistically significant difference (0.071).
Table 2.
Side-effects during the study

In only twenty patients, slight elevation of liver enzymes and serum lipids was detected. In no patient was the treatment discontinued due to the abnormality in laboratory values.
DISCUSSION
Isotretinoin is a synthetic isomer of all-trans retinoic acid with proven long-term effectiveness in treatment-resistant nodular and nodulocystic acne.[13] It is currently the most effective acne treatment available, with reported long-term remission rate up to 70-89%.[3,14,15,16]
In order to improve the tolerability of isotretinoin therapy in acne patient, we decided to use a modified treatment regimen of fixed 20 mg daily isotretinoin. The longest duration of treatment was 22 months and the shortest duration was 10 months. Improvement of acne lesions generally started during the 6th week, and was considered excellent at the end of treatment by all patients. Marked clinical improvement in inflammation, flattening of papulopustular lesions, started 1 or 2 months after initiation of treatment. Pustules tend to clear more rapidly than papules or nodules, and the lesions on the face, and upper arms, responded more quickly than trunk lesions.
Maximum clearance of 90% was observed at 6 months. The remainder of lesions (100%) gradually cleared until the total course of treatment period termination.
There are different opinions about the dose of isotretinoin for treatment of acne. The suggested standard dose is 0.5-1 mg/kg/day for 20-24 weeks, with a maximum cumulative dose of 120-150 mg/kg.[3,17,18] This regimen is known to produce good results; but it causes several dose dependent side-effects. Low dose isotretinoin to treat acne, has been tried previously.[10,12,19,20,21] Hermes et al. administered isotretinoin for duration of 8.3 months in an initial dosage of 10 mg/day up to 0.43 mg/kg and reported very good results in 62.8% and good results in 31.9% of the patients.[12] In Sundström et al. study, after treating patients with 0.3 to 0.4 mg/kg/day dosage for 6 months, complete remission of 94.4% achieved in patients.[8] Complete clearing in our study (96.4%) was higher than that of Amichai et al., and Hermes group. The higher values (96.4%) in the present study are most likely due to the longer treatment duration with healing of all lesions.
Hermes et al. by using moderate dose of Isotretinoin revealed that 27% of patients had initial worsening of disease.[12] An acne flare-up (10%) occurred during the first 2 months of treatment in our study was lower than that of Hermes et al. during the 3rd and 4th month, there was significant improvement of these aggravated lesions with addition of an oral antibiotic such as cotrimoxazole for a limited period of time, to the treatment protocol. None stopped treatment.
Relapse is reported more frequently following treatment with a lower dose.[14,22] In a 10-year follow-up, patients treated using isotretinoin with a dosage of 1 mg/kg/day, have shown a post treatment recurrence rate of 22-30%, while the rate was 39-82% for those treated with a dosage of 0.5 mg/kg/day.[3] In another study,[15,23] with a dosage of 1mg/kg body weight (22%), and 0.5 mg/kg body weight after short (38%) or long-term follow-up (41%) relapse reported.[15,23] In another study, Hermes et al. using moderate dose of isotretinoin revealed that 33% of patients had relapse.[12] In another study by Sardana et al., alternate day fixed 20 mg isotretinoin for only 6 months, resulted in very good response in (68.2%), good response in (19.34%). Relapse occurred in (16.39%) of patients. Relapses were more common in females with polycystic ovarian disease.[24]
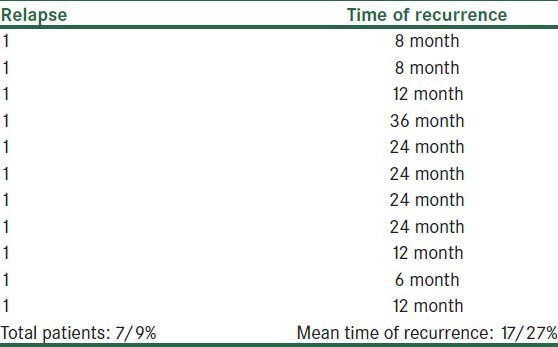
In 5-year follow-up, relapse occurred in only 11 (7.9%) of our patients, and mean time of recurrence after treatment was17.27 (SD = 9.56) months [Table 3]. These relapses controlled with topical therapy alone or combined with oral antibiotics. A second course of isotretinoin is needed in approximately 10% of these patients. The relapse rate observed by us was lower than that of above mentioned studies.
Table 3.
Relapse and time of recurrence
In a study by Amichai et al., with a follow-up of 4 years, observed a low relapse rate (3.9-5.9%) with daily 20 mg isotretinoin.[10] In Katsambas et al. study, relapse is reported in 15 port of patients. Patients at increased risk for early relapse included younger patients with acne of relatively recent onset, those with truncal acne, and women with polycystic ovary syndrome. In our study, relapse rate had not any relation with patient's weight, age, and anatomical location of lesions.
Safety was assessed during the study by the reporting of adverse events. The most common side-effects in our study were mild cheilitis in 93 patients (66.4%), mild xerosis in 12 patients (8.5%), epistaxis in 4 (2.8%), and arthralgia in 5 patients (4.2%), respectively. The observed side-effects were mild, and only 6 patients discontinued study medication because of severe adverse events.
Isotretinoin can cause variant side-effects; mucocutaneous side-effects are the most common, experienced by virtually all patients. Dryness of the lips (100%), skin (50%), nasal passages (30 sages, and eyes (20%) can result in dermatitis, cheilitis, epistaxis, and conjunctivitis.[25] Transient alopecia and hyperkeratosis of the palms are rare adverse effects.[26] Myalgia, arthralgia and headache are the most common systemic adverse effects.[26] In Akman study,[27] dryness of mouth were seen in 47% patients with conventional group and 9% of patients with low dose group, but in our study dryness of mouth and cheilitis was (66.4%). Epistaxis only seen in conventional group but in our study, 2.8% of patients had epistaxis, in our study alopecia and arthralgy was higher than Akman study, but pruritus and dry eyes was lower than their study.[27] In Amichai study,[10] xerosis was seen in 43% of patients and epistaxis reported in 2.5% of patients, and depression was not seen in any patients, but in our study, 3 patients (2%) developed depression.
Elevated liver function test results, and increase in serum lipid profiles was mild, and usually returned to normal within 6-8 weeks, despite continuation of treatment.
CONCLUSION
Low daily dose of isotretinoin seems to be an effective and safe treatment option for patients with moderate to severe acne, with a lower incidence of side-effects. To the best of our knowledge there is no reported use of fixed 20 mg daily isotretinoin until cumulative dose of 120 mg/kg in the literature. The present study showed that low dose 20 mg daily isotretiinoin represents a well-tolerated and efficient alternative to the standard (0.5-1 mg/kg/day) route for treatment of moderate to severe acne. The only pitfall is it is longer than 10 months duration of treatment period. The low frequency of adverse side-effects in this study, suggest that most side effects of the drug, are dose dependent; and the most serious side effects of the drug can be reduced significantly by using lower doses. Hence, we recommend this low dose therapy for treatment of moderate to severe scar prone acne. A large, prospective randomized comparative study is needed to establish the definitive response and tolerance of this protocol.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cunliffe WJ, Gould DJ. Prevalence of facial acne vulgaris in late adolescence and in adults. Br Med J. 1979;1:1109–10. doi: 10.1136/bmj.1.6171.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhir R, Gehi NP, Agarwal R, More YE. Oral isotretinoin is as effective as a combination of oral isotretinoin and topical anti-acne agents in nodulocystic acne. Indian J Dermatol Venereol Leprol. 2008;74:187. doi: 10.4103/0378-6323.39727. [DOI] [PubMed] [Google Scholar]
- 3.Cunliffe WJ, van de Kerkhof PC, Caputo R, Cavicchini S, Cooper A, Fyrand OL, et al. Roaccutane treatment guidelines: Results of an international survey. Dermatology. 1997;194:351–7. doi: 10.1159/000246134. [DOI] [PubMed] [Google Scholar]
- 4.Peck GL, Olsen TG, Butkus D, Pandya M, Arnaud-Battandier J, Gross EG, et al. Isotretinoin versus placebo in the treatment of cystic acne.A randomized double-blind study. J Am Acad Dermatol. 1982;6:735–45. doi: 10.1016/s0190-9622(82)70063-5. [DOI] [PubMed] [Google Scholar]
- 5.Layton AM, Dreno B, Gollnick HP, Zouboulis CC. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20:773–6. doi: 10.1111/j.1468-3083.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 6.Strauss JS, Rapini RP, Shalita AR, Konecky E, Pochi PE, Comite H, et al. Isotretinoin therapy for acne: Results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490–6. doi: 10.1016/s0190-9622(84)80100-0. [DOI] [PubMed] [Google Scholar]
- 7.Brelsford M, Beute TC. Preventing and managing the side effects of isotretinoin. Semin Cutan Med Surg. 2008;27:197–206. doi: 10.1016/j.sder.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Sundström A, Alfredsson L, Sjölin-Forsberg G, Gerdén B, Bergman U, Jokinen J. Association of suicide attempts with acne and treatment with isotretinoin: Retrospective Swedish cohort study. BMJ. 2010:341–c5812. doi: 10.1136/bmj.c5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marqueling AL, Zane LT. Depression and suicidal behavior in acne patients treated with isotretinoin: A systematic review. Semin Cutan Med Surg. 2007;26:210–20. doi: 10.1016/j.sder.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644–6. doi: 10.1016/j.jaad.2005.11.1061. [DOI] [PubMed] [Google Scholar]
- 11.Mandekou-Lefaki I, Delli F, Teknetzis A, Euthimiadou R, Karakatsanis G. Low-dose schema of isotretinoin in acne vulgaris. Int J Clin Pharmacol Res. 2003;23:41–6. [PubMed] [Google Scholar]
- 12.Hermes B, Praetel C, Henz BM. Medium dose isotretinoin for the treatment of acne. J Eur Acad Dermatol Venereol. 1998;11:117–21. [PubMed] [Google Scholar]
- 13.Straus JS, Thaibautat DM. Disease of the sebaceous glands. In: Freedberg I, Eisen AZ, Wolf K, editors. Dermatology in General Medicine. 5th ed. New York: McGraw-Hill; 1999. pp. 769–84. [Google Scholar]
- 14.Stainforth JM, Layton AM, Taylor JP, Cunliffe WJ. Isotretinoin for the treatment of acne vulgaris: Which factors may predict the need for more than one course? Br J Dermatol. 1993;129:297–301. doi: 10.1111/j.1365-2133.1993.tb11850.x. [DOI] [PubMed] [Google Scholar]
- 15.Layton AM, Knaggs H, Taylor J, Cunliffe WJ. Isotretinoin for acne vulgaris – 10 years later: A safe and successful treatment. Br J Dermatol. 1993;129:292–6. doi: 10.1111/j.1365-2133.1993.tb11849.x. [DOI] [PubMed] [Google Scholar]
- 16.Wessels F, Anderson AN, Kropman K. The cost-effectiveness of isotretinoin in the treatment of acne. Part 1. A meta-analysis of effectiveness literature. S Afr Med J. 1999;89:780–4. [PubMed] [Google Scholar]
- 17.Pochi PE, Shalita AR, Strauss JS, Webster SB, Cunliffe WJ, Katz HI, et al. J Am Acad Dermatol. Vol. 24. Washington, D.C: 1991. Report of the Consensus Conference on Acne Classification; pp. 495–500. March 24 and 25, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Lidén S, Göransson K, Odsell L. Clinical evaluation in acne. Acta Derm Venereol Suppl (Stockh) 1980;(Suppl 89):47–52. [PubMed] [Google Scholar]
- 19.Goulden V, Clark SM, McGeown C, Cunliffe WJ. Treatment of acne with intermittent isotretinoin. Br J Dermatol. 1997;137:106–8. [PubMed] [Google Scholar]
- 20.Seukeran DC, Cunliffe WJ. Acne vulgaris in the elderly: The response to low-dose isotretinoin. Br J Dermatol. 1998;139:99–101. doi: 10.1046/j.1365-2133.1998.02321.x. [DOI] [PubMed] [Google Scholar]
- 21.Palmer RA, Sidhu S, Goodwin PG. ‘Microdose’ isotretinoin. Br J Dermatol. 2000;143:205–6. doi: 10.1046/j.1365-2133.2000.03626.x. [DOI] [PubMed] [Google Scholar]
- 22.Chivot M, Midoun H. Isotretinoin and acne: A study of relapses. Dermatologica. 1990;180:240–3. doi: 10.1159/000248038. [DOI] [PubMed] [Google Scholar]
- 23.Lehucher-Ceyrac D, Weber-Buisset MJ. Isotretinoin and acne in practice: A prospective analysis of 188 cases over 9 years. Dermatology. 1993;186:123–8. doi: 10.1159/000247322. [DOI] [PubMed] [Google Scholar]
- 24.Sardana K, Garg VK, Sehgal VN, Mahajan S, Bhushan P. Efficacy of fixed low-dose isotretinoin (20 mg, alternate days) with topical clindamycin gel in moderately severe acne vulgaris. J Eur Acad Dermatol Venereol. 2009;23:556–60. doi: 10.1111/j.1468-3083.2008.03022.x. [DOI] [PubMed] [Google Scholar]
- 25.Katsambas A, Papakonstantinou A. Acne: Systemic treatment. Clin Dermatol. 2004;22:412–8. doi: 10.1016/j.clindermatol.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Layton AM, Cunliffe WJ. Guidelines for optimal use of isotretinoin in acne. J Am Acad Dermatol. 1992;27:S2–7. doi: 10.1016/s0190-9622(08)80252-6. [DOI] [PubMed] [Google Scholar]
- 27.Akman A, Durusoy C, Senturk M, Koc CK, Soyturk D, Alpsoy E. Treatment of acne with intermittent and conventional isotretinoin: A randomized, controlled multicenter study. Arch Dermatol Res. 2007;299:467–73. doi: 10.1007/s00403-007-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


