Abstract
Polar Regions are unique and highly prolific ecosystems characterized by extreme environmental gradients. Photosynthetic autotrophs, the base of the food web, have had to adapt physiological mechanisms to maintain growth, reproduction and metabolic activity despite environmental conditions that would shut-down cellular processes in most organisms. High latitudes are characterized by temperatures below the freezing point, complete darkness in winter and continuous light and high UV in the summer. Additionally, sea-ice, an ecological niche exploited by microbes during the long winter seasons when the ocean and land freezes over, is characterized by large salinity fluctuations, limited gas exchange, and highly oxic conditions. The last decade has been an exciting period of insights into the molecular mechanisms behind adaptation of microalgae to the cryosphere facilitated by the advancement of new scientific tools, particularly “omics” techniques. We review recent insights derived from genomics, transcriptomics, and proteomics studies. Genes, proteins and pathways identified from these highly adaptable polar microbes have far-reaching biotechnological applications. Furthermore, they may provide insights into life outside this planet, as well as glimpses into the past. High latitude regions also have disproportionately large inputs into global biogeochemical cycles and are the region most sensitive to climate change.
Keywords: polar microalgae, physiology, genomics, proteomics, biogeochemistry, sea ice, oceanography, adaptation, evolution, environment
1. Introduction
Low-temperature environments represent probably the largest untouched biological resource on our planet because the largest proportion of the Earth’s biomass exists in low temperate environments, largely marine. Polar microalgae, which form the base of a largely bottom-up controlled polar food web [1] have successfully adapted to the extreme and oscillating polar environmental gradients. In addition to freezing temperatures, these cold environments coincide with a host of other environmental challenges including solar, osmotic, oxidative and nutrient stress which have been well described in previous reviews [2,3,4]. The ephemeral nature of one of polar microalgae’s major niches, sea-ice, makes it one of the most dynamic of the extreme environments on earth. The semi-enclosed sea-ice habitat harbours a very diverse community of organisms interacting on a very small scale, continually acclimating and adapting to strong and oscillating environmental conditions [5]. This promotes fast evolution through horizontal exchange and recombination of genetic material. Thus, these organisms represent a resource for identification of new species, new physiological mechanisms of adaptation and new genes. However, global warming due to increased atmospheric carbon dioxide concentrations has begun to seriously threaten the coldest environments on our planet, polar ecosystems. This could mean a loss of a vast pool of genetic diversity yet to be uncovered. And only through advances in our understanding of molecular mechanisms driving metabolism and community structure of polar microalgae will scientists be able to better estimate the current and future biogeochemical inputs and niche adaptability of polar microalgae.
The focus of this review is to describe the physiological mechanisms involved in microalgae adaptations to cryospheric conditions, emphasizing insights “omics” techniques have recently provided. The later section, “Using systems biology to understand a changing world,” highlights gaps in knowledge and suggested priorities for future research with particular emphasis on “omics” applications. We also refer readers to previous reviews that have given insights into microalgae adaptations to polar environments, including several which have gone into great detail on sea-ice structure, biodiversity, primary production and niche adaptation [5,6,7], and those that have focused on polar lake microalgae [8,9] and cyanobacteria [10,11], as well as reviews of polar macroalgae [12] and bacteria [13,14,15,16,17]. There have also been very informative reviews of the metabolic and biogeochemical insights derived from the first two temperate microalgae genomes sequenced [18,19]. More general reviews of metagenomic [20], proteomic [21], and metabolomic [22] environmental applications are also available.
2. Polar Significance
Polar regions are unique, prolific ecosystems despite their inhospitable appearance [23]. The Antarctic continent covers an area of 14 million km2 and amidst the world’s largest desert are numerous perennial frozen lakes. It is surrounded by the Southern Ocean, a high nutrient low chlorophyll (HNLC) region, which is covered by seasonal sea-ice that can extend up to 20 million km2, covering ~40% of the Southern Ocean during austral winters [24]. Southern polar glaciation arose ~40 million years ago coinciding with the separation of the Antarctic and South American continents and formation of the Antarctic Circumpolar Current [25]. In the northern hemisphere, regions of North America, Europe and Asia continents all lie within the Arctic Circle (66°33'N) and surround a shallow Arctic basin covered by up to 16 million km2 of perennial and seasonal sea-ice [26]. However, the most recent northern glaciation event did not occur until ~3.5 million years ago, making this a much younger cryospheric region [25].
Together, these polar regions account for a large proportion of the Earth’s surface area and have great impacts on global biogeochemical cycles. Due to increased CO2 solubility at low water temperatures, deep water formation in the Southern Ocean sequesters large amounts of carbon (i.e., 30% of global uptake despite accounting for only 10% of the surface area; (i.e., 30% of global uptake despite accounting for only 10% of the surface area; [27]). High river inputs and the largely refractory nature of the terrestrial dissolved organic matter entering the Arctic basin also leads to large CO2 sequestration [28]. However, warming Arctic temperatures are mobilizing frozen methane deposits, a greenhouse gas that can further exacerbate climate warming [29]. Polar regions have also been shown to produce large biogenic sulfur fluxes to the atmosphere through the breakdown of the phytoplankton metabolite dimethylsulfoniopropionate (DMSP) to dimethylsulfide (DMS), a volatile gas [30]. Oxidized sulfate particles from DMS, in turn, help seed clouds which mitigate climate warming [31]. The qualitative and quantitative inputs polar ecosystems have on the various biogeochemical cycles and food webs are largely dependent on the microbial populations that control primary production and remineralization processes. While perennial permafrost severely limits terrestrial photoautotrophs, algae and cyanobacteria have successfully adapted to polar marine and/or fresh water niches. They form the base of these productive food webs, converting light energy and nutrients into chemical energy despite a physiologically challenging environment.
3. Microalgal Mechanisms to Thrive
3.1. Membrane Fluidity
Cell membranes control transport of nutrients and metabolic waste products in and out of the cells and are integral to the electron transport chains of cellular metabolism. Therefore, maintaining their fluidity under freezing temperatures is of utmost importance. Increases in unsaturated bonds promote a looser packing of lipids and decreased temperature of solidification. Increased concentrations of polyunsaturated fatty acids (PUFAs) is one of the most well-documented cold tolerance mechanisms and has been shown in polar diatoms [32], dinoflagellates [33], and chlorophytes [34,35,36]. Polar microalgae not only increase PUFA concentrations in cell membrane phospholipids, but perhaps more importantly, in the galactolipids integral to the chloroplast membrane [37,38,39,40]. The recent publication of the genome of a psychrotolerant green algae, Coccomyxa subellipsoidea, found amongst the most enriched gene families FA synthases, elongases, lipases, and desaturases [41], highlighting the importance of lipid metabolism under polar conditions. Desaturase enzymes are responsible for inserting double bonds into FAs at specific carbon locations and differential regulation of desaturases indicates locations of double bonds are tightly controlled [42,43]. Upregulation in response to salt stress indicates they are also likely involved in more than just temperature acclimation [44,45]. Unlike bacterial desaturases, de novo PUFA synthesis by eukaryotic desaturases can function independent of growth, which is important for rapid acclimation [9]. However, the sensory and signal pathways involved in PUFA synthesis remain to be elucidated in eukaryotic phytoplankton.
3.2. Enzyme Kinetics
Microbes are poikilothermic (i.e., they are in thermal equilibrium with their surrounding environment). Thus, bioenergetic demands of the cell must overcome the inhibiting effects of a low kinetic environment, most notably the freezing of molecules and decreased rates of catalysis. Recently, thanks to the sequencing of >30 prokaryotic genomes, specific protein structural changes promoting cryospheric enzyme flexibility were identified and statistically validated [14]. They include amino acid substitutions which decrease hydrophobic interactions, H-bonds and salt-bridges, particularly around the active site, which in turn can increase reaction rates by requiring less energy than induced fit mechanisms. A detailed review of enzymes kinetics in polar prokaryotes is given by Gerday and colleagues in this issue [15]. While the availability of only two polar microalgae genomes does not enable statistical investigations into amino acid substitutions, physiological and molecular techniques have shown the temperature hardiness of polar microalgae metabolic enzymes utilizes various mechanisms depending on the enzyme. For example, studies of ice diatoms found enzymes involved in nitrate, ammonium and carbon uptake have optimal temperature ranges at near freezing temperatures, while other metabolic enzymes such as nitrate reductase (NR) have more moderate optimal temperatures but less sensitivity to temperature changes [46]. Comparisons between psychrophilic and mesophilic chlorophytes also showed shifts to higher and more stable activity of psychrophilic metabolic enzymes at low temperatures [47,48]. Interestingly, NR from a psychrophilic chlorophyte was capable of utilizing both NADPH and NADH as energetic reductants, whereas the mesophilic chlorophyte could only use NADH [47]. Thus, in response to polar conditions which can reduce metabolic production of energy units, enzymes from polar microalgae seem to have evolved to be more flexible in their energy source. This is supported by the fact that measurements of NR:NADH activity showed maximal activities at higher temperatures than in vivo assays; Ferrara and colleagues [49] suggest this can be explained by the in vivo presence of enzymes such as glucose 6-phosphate dehydrogenase whose synthesize of NADPH improves other enzymes (i.e., NR) cold activity. Another example of polar microalgae kinetic adaptations was the elevation of pyrophosphate-dependent phospho-fructo-kinase detected in the polar diatom Fragilariopsis cylindrus during salinity acclimation [50]. This ATP-independent form of an important glycolysis enzyme saves chemical energy units for other processes, such as osmolyte synthesis. A gene encoding a rhodopsin has also been found in F. cylindrus which has been suggested to serve as a trace-metal independent method for additional ATP synthesis [51,52]. Surprisingly, ribulose-1,6-bisphosphate carboxylase (RUBISCO), which is fundamental to phototrophic carbon fixation, shows an opposite trend of greater decreases in activity under cold temperatures when psychrophilic chlorophytes where compared to their temperate counterparts [53]. However, the cellular concentrations of RUBISCO enzymes in the polar species were twice that found in temperate algae [53]. A similar mechanism was observed in molecular studies in Chlamydomonas subcaudata which showed increased concentrations of ATP synthase proteins within the chloroplast [54]. Elevations in this enzyme’s abundance may partly explain the ability for psychrophiles, but not mesophiles, to increase ATP production following cold-shock [55]. Most recently ribosomal proteins have also been found to be significantly increased at colder temperatures, presumable to maintain adequate translation within a low kinetics environment [56].
3.3. Compatible Solutes and Cryoprotectants
Cellular compatible solutes, typically associated with their role in salinity acclimation, also serve as freeze protection molecules and contribute to the high in vivo enzyme activities within psychrophilic organisms. These compounds reduce the intracellular freezing point and help maintain enzyme hydration spheres to stabilize catalytic activity [57]. There is a wide array of compatible solutes including: sugars, polyols, amino acids and their derivatives such as betaine and DMSP. The amino acid proline is an abundant compatible solute in many cryophilic microalgae, including the model ice diatom F. cylindrus. Genes for proline synthesis were strongly represented in F. cylindrus cold and salt stress EST libraries [58]. Proteomics studies further validated the importance of proline synthesis within this ice diatom with seven of the 36 proteins that increased in relative abundance in response to high salinity involved in the amino acid synthesis pathway of proline ([50]; Figure 1). Two isoforms of a homologue to the bacterial/archaeal glycine betaine methyltransferase protein were also elevated. This is an alternate enzyme for synthesis of the compatible solute betaine that, in contrast to the more typical eukaryotic choline oxidase pathway, produces large betaine concentrations without damaging H2O2 as a by-product [59]. High concentrations of DMSP, a compatible solute with important feedbacks in climate and biogeochemical cycles, are found in ice-diatom communities [60] in contrast to typically low levels in temperate diatoms [61]. This compound has been shown to stabilize enzymes against cold-induced denaturation [62]. Proteomics following the salinity shift of F. cylindrus identified candidate genes for all four steps of the proposed synthesis pathway (from previous radiolabeling metabolite studies by [63]) demonstrating the utility of this “omics” technique to identify candidate enzymes for poorly characterized metabolic pathways [50].
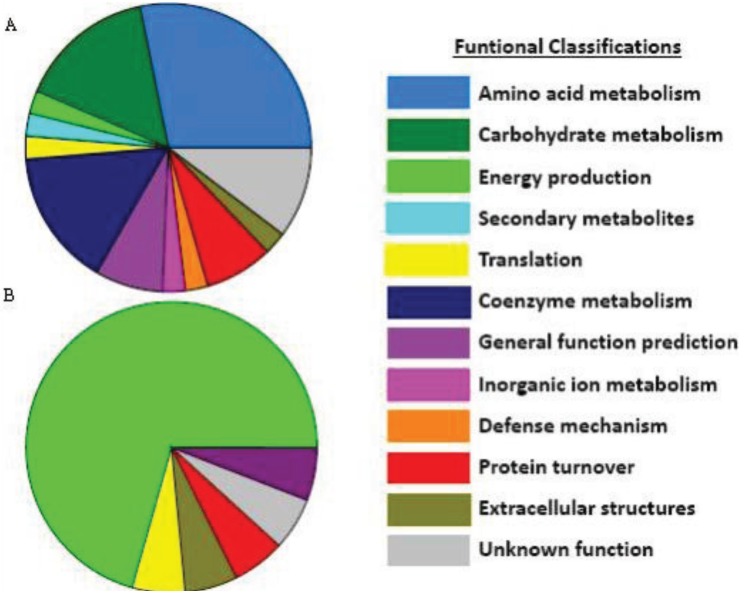
Figure 1.
Functional groups for elevated (A) and reduced (B) proteins in F. cylindrus following a shift to high salinity. This is an example of the major functional groups associated with polar algae acclimation processes. Note that most proteins resolved on 2DE gels had predicted functions in contrast to the much larger percentage of unknown proteins in genomic and transcriptomic datasets, most likely due to gel bias towards highly abundant metabolic proteins.
Late embryogenesis abundant (LEA) proteins are also considered multi-faceted cryoprotectants believed to work through hydrogen-bond stabilizing effects on enzymes [64]. LEA genes were found to be highly expressed in clone libraries from the polar chlorophyte Chlorella vulgaris (which was recently reclassified as C. subellipsoidea) and associated with cold-hardiness [65]. Additional novel LEA proteins were later isolated from this species by suppression subtractive hybridization and shown to protect lactate dehydrogenase activity from freeze inactivation [66].
A category of “cold-shock response” proteins has also been identified and is present across all taxa, from microbes to mammals [14]. These encompass a family of highly conserved small molecular weight proteins which bind to cold shock domains of single-stranded nucleic acids, as well as a number of RNA helicases. Cold-shock proteins are highly abundant in F. cylindrus two-dimensional protein gels (equivalent intensity to the highly abundant light harvesting complex proteins) and were significantly elevated following shifts from 4 °C to 0 °C [67]. Furthermore, six DNA/RNA helicases were identified within the F. cylindrus cold stress EST library [68]. It is believed that they work together to promote replication, transcription and translation under low temperature conditions by minimizing cold denaturation which otherwise forms kinks, coils and secondary structures that impede such processes.
3.4. Extracellular Compounds
Under freezing conditions it is also important for phytoplankton to maintain an aqueous external environment. To this end various extracellular modifiers are produced by microalgae within the ice such as ice-binding proteins (IBPs) which are excreted from the cells and inhibit ice growth and recrystallization and enhance brine retention through changes in ice channel structure [69]. IBPs were first identified in ice diatoms simultaneously in the salt shock EST library of F. cylindrus and through protein isolation and mass spectrometry from the ice-diatom Navicula glaciei [70]. The presence of these genes in all ice-algae tested to date (diatoms, prymnesiophytes, prasinophytes and chlorophytes), and complete absence from temperate species, suggests that IBPs play an important role in sea-ice adaptation [71]. Furthermore, phylogenetic analysis strongly suggests the genes were acquired through horizontal gene transfer (HGT) of bacterial IBPs [71,72]. Exopolymeric substances (EPS), composed of polysaccharides, amino acids, and proteins, are also highly abundant within the sea-ice [73]. Diatom EPS also helps retain salts, increase liquid brine fraction and thus create microhabitats within sea-ice [74]. Polysaccharide and cell wall metabolism gene families, which likely includes enzymes for synthesis of EPS and antifreeze glycoproteins, were enriched in the polar green algae C. subellipsoidea compared to temperate chlorophytes [41]. The role of EPS in polar adaptation is nicely reviewed by Ewert and Deming [7] within this special issue.
3.5. Light Acclimation
Light availability is highly variable in the polar environment and polar microalgae must both avoid photodamage under periods of high light and adapt to low light levels. Ice-diatoms are well adapted to low light levels. High photosynthetic efficiencies enable them to reach saturated growth at 20 µE m−2 s−1 and active photosynthesis has been observed at irradiances < 0.5 µE m−2 s−1, 0.01% of incident irradiance [75,76]. Physiological studies revealed steady-state F. cylindrus cultures growing at 2 µE m−2 s−1 versus those grown at 15 µE m−2 s−1 exhibited increases in specific chloroplast PUFAs that enhance the fluidity of the thylakoid membrane and thus the flow of electrons, and this was associated with a near doubling of pigment concentrations and 50% reduction in carbohydrate concentration [38]. Fucoxanthin-chlorophyll binding proteins (FCPs), complex families of proteins that appear to have different specializations (e.g., light harvesting versus dissipation of excess energy), were the most redundant genes identified in F. cylindrus and C. neogracile EST libraries [68,77] but it has yet to be tested whether elevated FCP concentrations are also part of the high shade adaptability of polar diatoms. However, it appears that in ice diatoms a dense packaging of pigments and their binding proteins in conjunction with enhanced thylakoid fluidity enable high photosynthetic efficiencies at very low light, while carbohydrate utilization and alternate energy sources help offset any energetic deficiencies. The chlorophyte Chlamydomonas raudensis, which dominates the highly saline bottom layers of permanently ice-covered Antarctic lakes, is also highly shade adapted, but utilizes quite unique mechanisms (reviewed in [9]). It has lost many conserved short-term and long-term photoacclimation mechanisms such as non-photochemical quenching (NPQ), light harvesting complex state transitions between PSI and PSII, and alterations in pigment concentrations. Instead, C. raudensis has an extremely high PSII to PSI stoichiometry to maximize harvesting of low levels of blue light. Furthermore, in response to increased irradiance (up to 10-fold higher than natural conditions) C. raudensis increases growth rates to dissipate increased energy rather than exhibiting photoinhibition. In contrast, the chlorophyte species (Chlorella BI) isolated from an Antarctic pond, with higher and more fluctuating light levels, has maintained its ability for state transitions to balance PSI and PSII light absorption and thus maintain optimal photosynthetic activity under changing light conditions [78]
Despite their adaption to low light levels, ice diatoms are still capable of acclimating to high light (>350 µE m−2 s−1) at temperatures down to −5 °C [79]. Like diatoms from other habitats, they utilize NPQ mechanisms, such as the diatoxanthin - diadinoxanthin xanthophyll cycle, to dissipate excess energy and prevent photoinhibition and cellular damage [80]. Xanthophyll cycle pigments can bind to the LHCx family of FCPs associated with the dissipation of excess energy [81]. The genome of F. cylindrus revealed a large expansion in the LHCx gene family compared to the two sequenced temperate diatom species [82]. Microarray studies with the polar diatom Chaetoceros neogracile found shifts from 20 to 600 µE m−2 s−1 resulted in significant elevations in LHCx proteins and antioxidant proteins, while those associated with light harvesting were significantly reduced [83]. The fact that growth rate was only 20-35% reduced over a 10 day period at this very high light level illustrates the photoacclimation capabilities of this species. Interestingly, work from various labs has shown low temperatures to elicit photoacclimation responses similar to high light, such as increased NPQ, PSII proteins, and photoprotective pigments [35,79,84]. Presumably these changes were a result of increased excitation pressures caused by low temperatures (i.e., decreased enzyme kinetics inhibits efficiency of metabolic electron sinks leading to a build-up of reduced plastoquinone). The redox status of the plastoquinone pool in turn triggers phosphorylation cascades which initiate photoacclimation mechanisms [81,85]. Salinity shifts can also elicit photoacclimation mechanisms in polar chlorophytes and diatoms [67,86], again likely through the common mechanism of changes in excitation pressures. Thus, multiple environmental pressures require robust photoacclimation mechanisms for microalgae to thrive within the cryosphere.
3.6. Antioxidants
Photosynthesis creates an oxic environment intracellularly that can be exacerbated by reactive oxygen species (ROS) formation caused by low temperature and other stress induced metabolic imbalances. This is further magnified by the increased solubility of oxygen at low temperatures and restricted diffusion within sea-ice and permanently ice-covered lakes leading to hyperoxic extracellular environments. Thus, robust antioxidant systems are important for polar microalgae to cope with ROS. Polar diatoms [87], chlorophytes [88] and dinoflagellates [89] are resistant to UV damage, indicating highly effective antioxidant systems. High catalase activity in the sea-ice diatom Entomoneis kufferathii following exposure to high light and low temperatures [90] is one of many antioxidant systems that can help protect cells from oxidative damage. Studies with the polar diatom Chaetoceros brevis showed elevated levels in superoxide dismutase activity, in addition to xanthophyll cycling, also to be important for dissipating ROS brought on by irradiance shifts [91]. A survey of antioxidant systems showed polar algae also utilize ascorbate peroxidase and glutathione reductase as antioxidant systems; however, the activity levels following light stress did not show a clear geographic trend between polar and temperate species but rather indicated species specific responses that could be due to differences in photoacclimation capabilities and utilization of alternate antioxidant systems [92]. In fact a microarray study with the polar diatom Chaetoceros neogracile found amongst thermal stress response genes (following shift from 4 °C to 10 °C) a spectrum of antioxidant enzymes, including monoascorbate reductase, glutaredoxin, glutathione peroxidase, glutathione S-transferase (GST), and alternative oxidase, illustrating the diverse suite of ROS defense enzymes this psychrophile can utilize to mitigate oxidative damage [93]. Various compatible solutes such as proline and DMSP also have secondary benefits as antioxidants [94,95] and their high concentrations in polar microalgae likely contribute to protection from oxidative damage. Furthermore, symbiotic relationships between ice-diatom and epiphytic bacteria who scavenge ROS have been described [96].
Given the importance of PUFAs to cryoprotection and their sensitivity to oxidation, protection against ROS damage to these lipids is of utmost importance. GST conjugates reduced glutathione to electrophilic centers, particularly abundant within PUFAs, and protects them from more damaging oxidation such as lipid peroxidation and other reactions with H2O2. In addition to the GST transcript elevations in response to elevated temperatures mentioned above, proteomics studies have found significantly elevated GST protein levels to be involved in low temperature acclimation of the sea-ice chlorophyte, Chlamydomonas sp. [97] and high salinity acclimation of the polar diatom, F. cylindrus [50], further emphasizing its importance in environmental stress tolerance across diverse taxa of polar microalgae. Another important enzyme for protecting cellular proteins from oxidative damage is methionine sulfoxide reductase (Msr) which reduces methionine residues that become oxidized by ROS. MsrB has been shown to be a cold response protein in the Arabidopsis plant and knock-down mutants show decreased cold tolerance in the form of increased methionine oxidation, H2O2 formation, and electrolyte leakage [98]. Fourteen Msr homologues are present in the F. cylindrus genome (versus seven and ten in the temperate diatom genomes of Thalassiosira pseudonana and Phaeodactylum tricornutum, respectively) supporting an important role for Msr enzymes in cold and oxidative stress acclimation of polar microalgae. Comparisons between the genome of the psychrotolerant chlorophyte C. subellipsoidea and its temperate counterpart found enrichment in the polar species of a family of short-chain dehydrogenase/reductase enzymes whose substrates vary from alcohols, sugars, and steroids to xenobiotics [41]. Most proteins in this family have oxidoreductase activity and enrichment could indicate an important role in maintaining a balanced cellular redox state under polar conditions, perhaps complementary to aldehyde dehydrogenases mitigation of chemically reactive oxidized lipid aldehydes that can accumulate when environmental stress perturbs metabolic balance as shown in plants [99]. Interestingly, an aldehyde dehydrogenase protein was found to be significantly elevated in F. cylindrus during high salinity acclimation at 0 °C [50]. While there is still much work to be done to understand more thoroughly the molecular mechanisms enabling oxidative stress tolerance in polar microalgae, studies thus far indicate a diverse suite of mechanisms to prevent and mitigate ROS damage.
3.7. Dark Adaptation
Survival through winter’s extended periods of darkness is key to photoautotrophic success in polar regions. Dark adaptation is also important in controlling microalgae seasonal and spatial distributions [100]. Incubation experiments found temperate diatoms to survive 21–35 days of darkness [100], while Antarctic species survived 4-9 month periods in the dark [101]. However, little is known of physiological mechanisms behind polar overwintering as logistics severely limits austral winter field studies. It is known that carbohydrate storage molecules such as glucan in diatoms and starch in chlorophytes are accumulated within polar algae and utilized during periods of darkness [9,102]. Furthermore, polar microalgae can also uptake dissolved organic material such as sugars and starches for energetic breakdown [103]. A recent study of polar pelagic algae also described a high plasticity in regards to inorganic carbon uptake in Southern Ocean phytoplankton [104]. Comparison of the recent polar chlorophyte genome C. subellipsoidea to its temperate counterparts showed gene enrichment in amino acid transporters and permeases which would promote enhanced uptake of organic nutrient sources [41]. Additionally, a number of carbohydrate metabolism gene families were present in C. subellipsoidea that did not have homologs in temperate chlorophyte species but instead appeared to have HGT origins. In diatoms, the presence of the urea cycle has been proposed as a means for recovering carbon and nitrogen depleted during photorespiration [18], while the FA β-oxidation pathway means lipids can be used as metabolic intermediates and for ATP synthesis [105]. This metabolic flexibility being revealed through diatom genomes is likely fundamental to their ability to thrive within the extreme and highly-variable environmental conditions which characterize polar regions.
Ice-algae cells can go into a winter resting stage during which time minimal changes in carbon and chlorophyll concentrations occur [106]. Low temperature shift in F. cylindrus (5 °C to −1.8 °C) were associated with reductions in photosynthesis and carbon fixation genes, and this was hypothesized to indicate a preparation towards a winter resting stage cued by decreasing temperatures [107]. Xanthonema, a class of heterokont snow algae, has been shown to disassemble PSII but keep LHC proteins intact in response to extended dark adaptation [108]. Thus, it appears they maintain thylakoid structural proteins primed to quickly reassembly PS in order to utilize the first short periods of irradiance in austral spring. Extended dark adaption studies in the chlorophyte Koliella antarctica showed similar PS changes, but also detected hallmarks of programmed cell death (PCD) in a subpopulation of cells [109]. However, return of cultures to low light after 60 days of darkness showed rapid recovery of growth and photosynthesis, clearly demonstrating viability of cells that did not undergo PCD and raising the question of PCD as an adaptive benefit to unicellular communities. There is still much debate to whether shifts to heterotrophic metabolism also play a role in adaption to extended periods of darkness. Transformation of P. tricornutum with a single gene, a glucose transporter, enabled this diatom to switch from photoautotrophic to heterotrophic growth [110], illustrating the ease for such a shift to occur given the high degree of HGT believed to occur within the sea-ice environment. Indeed, the chlorophyte Chlorella BI is capable of switching between autotrophic growth and heterotrophic growth, with highest growth rates achieved during mixotrophic growth in light with a glucose carbon source [78]. Many dinoflagellate species are also capable of heterotrophic growth, and shifts to mixotrophic sea-ice communities have been detected from late winter ice cores [111]. However, more research is needed to understand metabolic shifts occurring within species and through changes in community composition during seasonal periods of extended darkness.
4. Using Systems Biology to Understand a Changing World
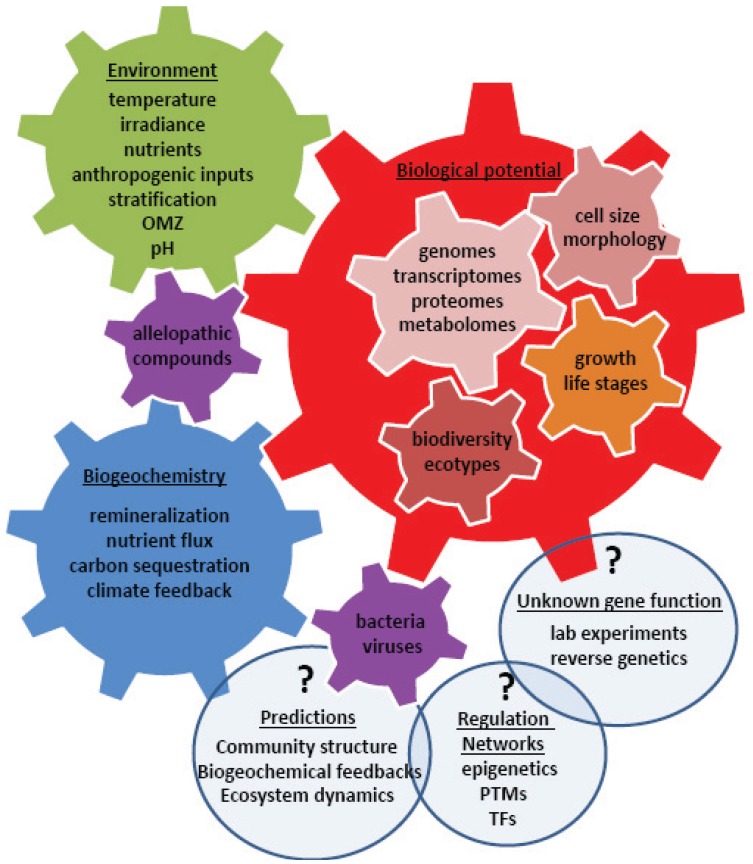
Improving our understanding of the molecular mechanisms behind environmental acclimation and adaptation processes is key to predicting ecological and biogeochemical inputs of polar primary producers (Figure 2). Our molecular tool box has greatly advanced over the past couple decades, and the next hurdle is linking this knowledge to processes on the global scale with ever increasing resolution. While some mechanisms may be unique to polar environments, many are likely utilized in other environments to overcome similar bioenergetic pressures that may arise from common or quite distinct stressors. Temperature, light, nutrients, allelopathic and anthropogenic compounds, and chemical-physical processes (e.g., stratification, oxygen minimum zones, carbonate saturation depth) collectively control temporal and spatial taxonomic distributions depending on the biological potential of organisms (i.e., genetic adaptability). Different evolutionary histories (e.g., endosymbiotic events, HGT, gene loss/expansion during niche specialization) provide different suites of genes which result in metabolic diversity. Differences in metabolism between species, in turn, ultimately result in different impacts on biogeochemical cycles. A systems biology approach to understand the complex genomic, transcriptomic, proteomic, and metabolomic interactions is required if we are to achieve a holistic understanding of feedbacks between organisms and their environment and eventually develop mathematical models capable of representing past, current, and predicting future biological inputs on various ecosystem parameters, such as climate, biodiversity, and nutrient availability (Figure 2). Measuring the effects of various environmental variables, individually and in combinations, on the vast array of phytoplankton is a laborious and logistically difficult matrix of experiments. Alternatively, environmental “omic” approaches reveals the importance of genes in the natural environment and can be correlated with environmental metadata to tease out significant relationships and important drivers of biodiversity, gene expression, and biogeochemical inputs. But in order to move beyond the current low resolution, broad taxonomic group models there are some important gaps (highlighted in the following paragraphs) that must be addressed, particularly with regard to polar primary producers due to their significance in global biogeochemical processes and the sensitivity of this region to climate change.
Figure 2.
An array of advanced, high-throughput molecular techniques is enabling a global effort to link environmental parameters to biological distributions, physiological capabilities, protein expression, metabolic functions, and biogeochemical cycles. The ultimate goal is the ability to make high resolution predictions of biogeochemical and ecosystem inputs under current and future climates. The light blue bubbles represent areas to focus future research to improve our understanding and modeling efforts. OMZ–oxygen minimum zone, PTM – post-translational modification, TF–transcription factor.
Polar microalgae genomes provide valuable information on their genetic repertoire, as well as promoter and intergenic regions whose diverse roles in gene regulation we are still just beginning to understand. Polar microalgae genomes also can provide novel proteins and pathways for biotechnological applications and insights into cryosphere bioenergetics that may even help us understand possibilities for life outside of this planet [112]. The F. cylindrus genome was the first psychrophilic microalgae genome to become publicly available [113] and insights from its annotation should be published in the near future. Recently the genome of a psychrotolerant green algae, C. subellipsoidea, was published [41]. Sequencing of a dominant polar haptophyte, P. antarctica, has also been started by the U.S. Department of Energy Joint Genome Institute in 2010 and likely will become public in 2014 (Arrigo, personal communication). Thanks to the rapid advancement of sequencing and annotation techniques, genome sequencing costs and time has been greatly reduced and the number of sequencing facilities has increased substantially. Thus science is now capable of an important next step to understanding polar microalgae physiology and global biogeochemical inputs: expand reference genomes and/or transcriptomes to include multiple species from various taxa representing different niche specialists, and importantly environmental isolates rather than clones that have been cultured for decades in the laboratory. Comparisons between ecotypes of the same species (i.e., cosmopolitan species like C. neogracile and C. raudensis with polar and warm water strains) will also be incredibly valuable to understanding genes fundamental to cryospheric life. To help fill this gap The Gordon and Betty Moore Foundation has undertaken the Marine Microbial Eukaryote Transcriptome Sequencing Project [114] to sequence the transcriptomes of 750 samples from a diverse range of habitats, including many more polar species. This will enable us to better understand competitive advantages between species and ecotypes and differences in their metabolic potentials. Importantly, it will also substantially increase our database of reference genomes for identifying the taxonomic source of environmental metatranscriptome and metaproteome sequences and prevent misassignment due to limited reference libraries. Furthermore, a new technique of single-cell sequencing can now be used to provide genetic information on non-cultured environmental samples and detect differences and interactions at the individual cell level [115].
Determining which species are present and contributing to biogeochemical fluxes is not a simple task. Since traditional microscopy methods are laborious, molecular techniques have been gaining prominence. Hypervariable regions of the eukaryotic 18s small subunit ribosomal RNA (rRNA) sequence have been used to define eukaryotic phytoplankton taxonomic units at the level of species supergroups (i.e., division or family level) and this has greatly expanded our appreciation of polar microalgae diversity, particularly with regards to the more fragile single-celled organisms [116,117]. Using 18s rRNA Charvet and colleagues [118] found the diversity of Antarctic lake flagellates was significantly underestimated by traditional microscopy and HPLC pigment methods. Similarly, 18s rRNA studies of Antarctic sea-ice communities under different irradiance regimes identified a very diverse eukaryotic community and identified three seasonal stages in community structure: mixed, dinoflagellate-dominated, and diatom-dominated [119]. However, it is important to note that 18s rRNA methods have their own biases towards particular species depending on the primer pairs used [120]. Furthermore, 18s rRNA copy number varies substantially between species [121]. And many 18s rRNA environmental sequences cannot be assigned to known taxa, as evidenced by preliminary analysis of the recent TARA expedition which could not assign 31% of 18s rRNA v9 sequences to known supergroups [122]. So while molecular taxonomic techniques increase sensitivity to species richness and provide a high-throughput means for gathering phenotypic information to link with metatranscriptome and metaproteome data, to apply 18s rRNA metagenomic techniques beyond a presence/absence assessment will require a greatly expanded reference database and increased efforts to quantify and normalize inherent sequencing biases. Furthermore, to truly improve our understanding of who is where and doing what will require the development of molecular markers able to define microalgae taxa with ever increasing specificity, eventually at the species and ecotype level.
Genes of unknown function made up six of the 10 most abundant genes in the F. cylindrus EST libraries [68] and 45% of the 1700 unique ESTs in C. neogracile [77]. While genes of unknown function are a common feature of all genomes, there are great possibilities for metabolic ingenuity and novel functions amongst these genes in polar microalgae. Environmental expression and functional characterization studies will be key to understanding their effect on an organisms biological potential and biogeochemical feedbacks (Figure 2). Even the assignment of genes to putative functions (i.e., aldehyde dehydrogenase mentioned earlier) are usually only based on sequence similarity and conserved domains that place them in broad enzyme classes with a diverse array of cellular functions, which in a eukaryotic cell are also dependent on subcellular localization. This is further compounded by redundant enzymes and paralogs within a genome. Thus, while comparative “omics” using natural and laboratory controlled conditions provides candidate genes for important and novel regulatory and metabolic processes, a true understanding of function and activity will require protein characterization studies using over-expression and silencing transformations within appropriate host organisms. Such work is easy and well-established in bacterial systems, but these hosts lack eukaryotic organelles, chaperones, post-translational systems and the upstream and downstream signaling networks native to that gene. Gene overexpression and silencing techniques have been developed in temperate diatom species P. tricornutum and T. pseudonana [110,123,124]. However, the establishment of methods to transform polar diatoms and other phytoplankton taxa would greatly enhance our ability to functionally characterize novel polar genes. New techniques such as viral promoters and transcription activator-like effector nucleases are promising approaches to expand this technology into polar microalgae host models.
Various cell organelles and metabolic systems are involved in polar acclimation processes and interact through a complex network of sensory, signaling, and regulatory mechanisms. Understanding and predicting biogeochemical feedbacks requires untangling and ultimately quantifying this collection of synergistic and antagonistic intracellular interactions. In contrast to the large proportion of putative genes with unknown functions found in F. cylindrus transcript studies, only three of the 56 proteins identified in an F. cylindrus proteomics study were of unknown function (Figure 1; [50]). Protein gels are biased towards highly abundant and soluble proteins. Perhaps these strongly differentially-regulated gene transcripts of unknown function code for unique signaling and transcription factor proteins which do not need to be expressed at the same concentrations as metabolic enzymes to have immense physiological effects, hence their limited presence in differentially expressed 2D gel proteins. A high priority for understanding gene expression on a systems biology level must be deciphering gene networks, transcription factors and promoter binding regions, sensory/signaling pathways and post-translation regulation. Such studies have been applied to plants and cyanobacteria but are still in their infancy within temperate microalgae and have yet to be applied to polar counterparts.
The ability of polar microalgae to acclimate to a wide range of environmental gradients and their broader range in photosynthetic and metabolic responses compared to temperate counterparts [125] has led to the conclusion that they possess a high degree of phenotypic plasticity. Phenotypic plasticity is like a “cellular memory” that responds to environment and may be selected for in environments with multiple stressors and steep or rapidly changing gradients [19,126]. Epigenetic modifications, such as cytosine methylations and histone modifications can serve as “soft” heritable changes which effect transcription. Recently, a high level of methylation of transposable elements (TEs) and a subset of genes which tended to be involved in important metabolic activities of nutrient resource management were identified in the “methylome” of the diatom P. tricornutum [127]. Environmental cues, such as nitrate limitation, decreased methylation of specific genes and triggered an increase in transcript levels. On the other hand, TEs are mobile genetic units and activation by environmental stressors (likely through changes in methylation) can lead to genetic rearrangements, another mechanism facilitating evolution and environmental adaptation at a rapid rate [128]. Small non-coding (silencing) RNAs are another method for controlling phenotypic plasticity in plants and animals [129] and cDNA libraries generated from T. pseudonana small RNAs indicate that these are also likely important transcription and translation regulators in microalgae [130]. Clearly, there are still many gaps in our understanding of the molecular mechanisms behind the high level of phenotypic plasticity within polar microalgae which must be addressed if we are to begin quantifying environmental regulation of cellular feedbacks into biogeochemical processes.
Microalgae community structure, genetic mobility, and nutrient availability are all regulated by bacteria and viruses [131] which are abundant in polar environments [132]. Recently a study from an Antarctic hypersaline lake described a virophage-virus-prasinophyte interaction whereby the virophage limited virus-induced mortality and increased phytoplankton blooms [133]. Viruses have also been shown to stimulate PCD pathways in phytoplankton [134]. On the other hand, bacteria-derived infochemicals may stimulate diatom EPS capsule formation [135] and species-specific interaction between a bacterium and diatom may enhance diatom growth, likely through a bacteria produced phytohormone [136]. Yet only a few of the potential bioactive secondary metabolites (aka allelopathic compounds or infochemicals) responsible for microbial intra/interspecies communication have been described so far. These include diatom derived aldehydes shown to trigger nitric oxide signalling and PCD in phytoplankton [137] and diatom derived oxylipins, formed from oxygenated PUFAs and shown to disrupt zooplankton reproduction and development [138]. Studies specific to polar microbial communities are still lacking. The role of intra/interspecies communication and predator-prey interactions in polar ecosystems, particularly in regard to viral and bacterial regulation of primary production, bioremineralization processes, community structure, and biogeochemical cycling, should be a high priority of future research.
For some time now polar regions have shown an amplified sensitivity to climate change [139]. Increased temperatures and winds have increased sea-ice retreat, upper ocean freshening and nutrient upwelling in the relatively shallow Arctic basin; this, in turn, has increased pelagic primary production but with a shift from nano to picoplankton populations [140]. Thinning sea-ice, allowing for increased light penetration, has also led to large under ice phytoplankton blooms in the Arctic [141]. In the Southern Ocean increased stratification, resulting in a shallower mixed layer with increased light, has been postulated to favor diatom growth, but at the same time decreases in upwelling also due to stratification have been predicted to more negatively impact large diatoms compared to small phytoplankton [142]. Meanwhile a region around the Antarctic Peninsula has seen overall summer phytoplankton abundance decrease by 12% over the past 30 years [143]. Warming temperatures may also alter adaptation to other polar conditions, such as darkness [144].
Microalgae play a key role in biogeochemical cycling. Species composition, abundance, cell size and life history all determine the drawdown of organic matter and C, N, and P are sequestered in different ratios depending on such factors [145]. Furthermore, changes in lipid composition and other metabolic shifts associated with adaptations of polar microalgae to altered niches [32,146] will have complex effects on food quality, community structure and biogeochemical processes [147]. Importantly, polar microalgae are key players in two major climate feedback loops that mitigate global warming trends: deep ocean carbon sequestration [27] and cloud condensation sulfate particles [148]. Changes in species composition and/or environmental variables significantly affect the fluxes within these feedback loops but to what extant is still largely uncertain. Clearly, the need to better understand feedbacks between climate, primary production, and biogeochemical loops in polar regions is paramount, yet models are ripe with uncertainties inherent in attempts to define function based on broad taxonomic classifications, such as nano versus picoplankton or diatom versus haptophyte [149]. “Omics” techniques continue to generate a wealth of data towards understanding acclimation potentials and metabolic fluxes, as well as elucidating niche separation and climate change adaptability. The next major hurdle will be advancing our ability to quantify and model different molecular/metabolic strategies to give a finer resolution on functional groups and biogeochemical fluxes, particularly in the face of new ecological pressures. This requires close collaborations between molecular biologists and modelers in order to develop a holistic approach based on genomic and biochemical data. Such an integrative systems ecology approach will provide mechanistic insights into how climate change will impact polar phytoplankton communities.
5. Conclusions
Only over the past decade have modern molecular genomic, transcriptomic, proteomic, and metabolomic tools been applied to polar microalgae. Although still in its infancy, great insights have already been made in regards to adaptation and acclimation mechanisms of polar microalgae using these new techniques. As we improve our understanding of polar bioenergetics, resource management, metabolic fluxes, and community composition, our ability to understand feedbacks of polar microalgae on global biogeochemical processes will become clearer. Furthermore, discovery of novel genes and pathways could have profound impacts on biotechnological applications.
Acknowledgments
The authors thank Jan Strauss for thoughtful comments on the manuscript. Funding for BR Lyon was provided by NSF OISE (Award Number 1159163).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Smith W.O., Lancelot C. Bottom-up versus top-down control in phytoplankton of the Southern Ocean. Antarct. Sci. 2004;16:531–539. doi: 10.1017/S0954102004002305. [DOI] [Google Scholar]
- 2.Kirst G.O., Wiencke C. Ecophysiology of polar algae. J. Phycol. 1995;31:181–199. [Google Scholar]
- 3.Thomas D.N., Dieckmann G.S. Antarctic Sea ice—A habitat for extremophiles. Science. 2002;295:641–644. doi: 10.1126/science.1063391. [DOI] [PubMed] [Google Scholar]
- 4.Mock T., Thomas D.N. Microalgae in Polar Regions: Linking Functional Genomics and Physiology with Environmental Conditions. In: Margesin R., Schinner F., Marx J.-C., Gerday C., editors. Psychrophiles: From Biodiversity to Biotechnology. Springer; Berlin/Heidelberg, Germany: 2008. pp. 285–312. [Google Scholar]
- 5.Arrigo K.R., Mock T., Lizotte M.P. Primary production in sea ice. In: Thomas D., Dieckmann G., editors. Sea Ice: An Introduction to Its Physics, Chemistry, Biology and Geology. Wiley-Blackwell; Malden, MA, USA: 2010. pp. 143–183. [Google Scholar]
- 6.Mock T., Thomas D.N. Recent advances in sea-ice microbiology. Environ. Microbiol. 2005;7:605–619. doi: 10.1111/j.1462-2920.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 7.Ewert M., Deming J. Sea ice microorganisms: Environmental constraints and extracellular responses. Biology. 2013;2:603–628. doi: 10.3390/biology2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolhi J., Maxwell D., Morgan-Kiss R. Review: The Antarctic Chlamydomonas raudensis: An emerging model for cold adaptation of photosynthesis. Extremophiles. 2013;17:711–722. doi: 10.1007/s00792-013-0571-3. [DOI] [PubMed] [Google Scholar]
- 9.Morgan-Kiss R.M., Priscu J.C., Pocock T., Gudynaite-Savitch L., Huner N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006;70:222–252. doi: 10.1128/MMBR.70.1.222-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S.M., Elster J. Cyanobacteria in Antarctic Lake Environments. In: Seckbach J., editor. Algae and Cyanobacteria in Extreme Environments. Volume 11. Springer; Dordrecht, The Netherlands: 2007. pp. 303–320. [Google Scholar]
- 11.Vincent W. Cold Tolerance in Cyanobacteria and Life in the Cryosphere. In: Seckbach J., editor. Algae and Cyanobacteria in Extreme Environments. Volume 11. Springer; Dordrecht, The Netherlands: 2007. pp. 287–301. [Google Scholar]
- 12.Wiencke C., Amsler C.D. Seaweeds and Their Communities in Polar Regions. In: Wiencke C., Bischof K., editors. Seaweed Biology. Volume 219. Springer; Berlin/Heidelberg, Germany: 2012. pp. 265–291. [Google Scholar]
- 13.D’Amico S. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006;7:385–389. doi: 10.1038/sj.embor.7400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanueva A., Tuffin M., Cary C., Cowan D.A. Molecular adaptations to psychrophily: The impact of ‘omic’ technologies. Trends Microbiol. 2010;18:374–381. doi: 10.1016/j.tim.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Gerday C. Psychrophily and catalysis. Biology. 2013;2:719–741. doi: 10.3390/biology2020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh E.Y., Martin A.R., McMinn A., Ryan K.G. Recent Advances and Future Perspectives in Microbial Phototrophy in Antarctic Sea Ice. Biology. 2012;1:542–556. doi: 10.3390/biology1030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkins D., Yau S., Williams T.J., Allen M.A., Brown M.V., DeMaere M.Z., Lauro F.M., Cavicchioli R. Key microbial drivers in Antarctic aquatic environments. FEMS Microbiol. Rev. 2013;37:303–335. doi: 10.1111/1574-6976.12007. [DOI] [PubMed] [Google Scholar]
- 18.Parker M.S., Mock T., Armbrust E.V. Genomic insights into marine microalgae. Annu. Rev. Genet. 2008;42:619–645. doi: 10.1146/annurev.genet.42.110807.091417. [DOI] [PubMed] [Google Scholar]
- 19.Bowler C., Vardi A., Allen A.E. Oceanographic and biogeochemical insights from diatom genomes. J. Rev. Mar. Sci. 2010;2:333–365. doi: 10.1146/annurev-marine-120308-081051. [DOI] [PubMed] [Google Scholar]
- 20.Allen A.E., LaRoche J., Maheswari U., Lommer M., Schauer N., Lopez P.J., Finazzi G., Fernie A.R., Bowler C. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. PNAS. 2008;105:10438–10443. doi: 10.1073/pnas.0711370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomanek L. Environmental proteomics: Changes in the proteome of marine organisms in response to environmental stress, pollutants, infection, symbiosis, and development. J. Rev. Mar. Sci. 2011;3:373–399. doi: 10.1146/annurev-marine-120709-142729. [DOI] [PubMed] [Google Scholar]
- 22.Weber A.P.M., Horst R.J., Barbier G.G., Oesterhelt C. Metabolism and metabolomics of eukaryotes living under extreme conditions. In: Kwang W.J., editor. International Review of Cytology. Volume 256. Academic Press; Salt Lake City, UT, USA: 2007. pp. 1–34. [DOI] [PubMed] [Google Scholar]
- 23.Bluhm B.A., Gebruk A.V., Gradinger R., Hopcroft R.R., Huettmann F., Kosobokova K.N., Sirenko B.I., Weslawski J.M. Arctic marine biodiversity: An update of species richness and examples of biodiversity change. Oceanography. 2011;24:232. doi: 10.5670/oceanog.2011.75. [DOI] [Google Scholar]
- 24.Lizotte M.P. The contributions of sea ice algae to Antarctic marine primary production. Am. Zool. 2001;41:57–73. doi: 10.1668/0003-1569(2001)041[0057:TCOSIA]2.0.CO;2. [DOI] [Google Scholar]
- 25.Zachos J., Pagani M., Sloan L., Thomas E., Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 26.Comiso J.C. Large-scale characteristics and variability of the global sea ice cover. In: Thomas D.N., Dieckmann G.S., editors. Sea Ice: An Introduction to Its Physics, Chemistry, Biology, and Geology. Blackwell Science Ltd.; Oxford, UK: 2003. pp. 112–142. [Google Scholar]
- 27.Sabine C.L., Feely R.A., Gruber N., Key R.M., Lee K., Bullister J.L., Wanninkhof R., Wong C.S., Wallace D.W.R., Tilbrook B., Millero F.J., Peng T.-H., Kozyr A., Ono T., Rios A.F. The Oceanic Sink for Anthropogenic CO2. Science. 2004;305:367–371. doi: 10.1126/science.1097403. [DOI] [PubMed] [Google Scholar]
- 28.Dittmar T., Kattner G. The biogeochemistry of the river and shelf ecosystem of the Arctic Ocean: a review. Mar. Chem. 2003;83:103–120. doi: 10.1016/S0304-4203(03)00105-1. [DOI] [Google Scholar]
- 29.Shakhova N., Semiletov I., Salyuk A., Yusupov V., Kosmach D., Gustafsson Ö. Extensive methane venting to the atmosphere from sediments of the East Siberian Arctic Shelf. Science. 2010;327:1246–1250. doi: 10.1126/science.1182221. [DOI] [PubMed] [Google Scholar]
- 30.Kiene R., Kieber D., Slezak D., Toole D., del Valle D., Bisgrove J., Brinkley J., Rellinger A. Distribution and cycling of dimethylsulfide, dimethylsulfoniopropionate, and dimethylsulfoxide during spring and early summer in the Southern Ocean south of New Zealand. Aquat. Sci. 2007;69:305–319. doi: 10.1007/s00027-007-0892-3. [DOI] [Google Scholar]
- 31.Gunson J.R., Spall S.A., Anderson T.R., Jones A., Totterdell I.J., Woodage M.J. Climate sensitivity to ocean dimethylsulphide emissions. Geophys. Res. Lett. 2006;33 doi: 10.1029/2005GL024982. [DOI] [Google Scholar]
- 32.Teoh M.-L., Phang S.-M., Chu W.-L. Response of Antarctic, temperate, and tropical microalgae to temperature stress. J. Appl. Phycol. 2012;1:1–13. [Google Scholar]
- 33.Thomson P.G., Wright S.W., Bolch C.J.S., Nichols P.D., Skerratt J.H., McMinn A. Antarctic distribution, pigment and lipid composition, and molecular identification of the brine dinoflagellate Polarella glacialis (Dinophyceae) J. Phycol. 2004;40:867–873. doi: 10.1111/j.1529-8817.2004.03169.x. [DOI] [Google Scholar]
- 34.Osipova S., Dudareva L., Bondarenko N., Nasarova A., Sokolova N., Obolkina L., Glyzina O., Timoshkin O. Temporal variation in fatty acid composition of Ulothrix Zonata (Chlorophyta) from ice and benthic communities of Lake Baikal. Phycologia. 2009;48:130–135. doi: 10.2216/08-49.1. [DOI] [Google Scholar]
- 35.Fogliano V., Andreoli C., Martello A., Caiazzo M., Lobosco O., Formisano F., Carlino P.A., Meca G., Graziani G., Rigano V.D.M., Vona V., Carfagna S., Rigano C. Functional ingredients produced by culture of Koliella antarctica. Aquaculture. 2010;299:115–120. doi: 10.1016/j.aquaculture.2009.11.008. [DOI] [Google Scholar]
- 36.Chen Z., He C., Hu H. Temperature responses of growth, photosynthesis, fatty acid and nitrate reductase in Antarctic and temperate Stichococcus. Extremophiles. 2012;16:127–133. doi: 10.1007/s00792-011-0412-1. [DOI] [PubMed] [Google Scholar]
- 37.Mock T., Kroon B.M.A. Photosynthetic energy conversion under extreme conditions—I: Important role of lipids as structural modulators and energy sink under N-limited growth in Antarctic sea ice diatoms. Phytochemistry. 2002;61:41–51. doi: 10.1016/S0031-9422(02)00216-9. [DOI] [PubMed] [Google Scholar]
- 38.Mock T., Kroon B.M.A. Photosynthetic energy conversion under extreme conditions—II: The significance of lipids under light limited growth in Antarctic sea ice diatoms. Phytochemistry. 2002;61:53–60. doi: 10.1016/S0031-9422(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 39.Gray C.G., Lasiter A.D., Leblond J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. III. Four cold-adapted, peridinin-containing taxa and the presence of trigalactosyldiacylglycerol as an additional glycolipid. Eur. J. Phycol. 2009;44:439–445. doi: 10.1080/09670260902787977. [DOI] [Google Scholar]
- 40.Morgan-Kiss R., Ivanov A.G., Williams J., Mobashsher K., Huner N.P.A. Differential thermal effects on the energy distribution between photosystem II and photosystem I in thylakoid membranes of a psychrophilic and a mesophilic alga. Biochim. Biophys. Acta. 2002;1561:251–265. doi: 10.1016/S0005-2736(02)00352-8. [DOI] [PubMed] [Google Scholar]
- 41.Blanc G., Agarkova I., Grimwood J., Kuo A., Brueggeman A., Dunigan D.D., Gurnon J., Ladunga I., Lindquist E., Lucas S., Pangilinan J., Proschold T., Salamov A., Schmutz J., Weeks D., Yamada T., Lomsadze A., Borodovsky M., Claverie J.M., Grigoriev I.V., Van Etten J.L. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 2012;13 doi: 10.1186/gb-2012-13-5-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suga K., Honjoh K.-I., Furuya N., Shimizu H., Nishi K., Shinohara F., Hirabaru Y., Maruyama I., Miyamoto T., Hatano S., Iio M. Two low-temperature-inducible Chlorella genes for Δ-12 and omega-3 fatty acid desaturase (FAD): Isolation of Δ-12 and omega-3 fad cDNA clones. Biosci. Biotechnol. Biochem. 2002;66:1314–1327. doi: 10.1271/bbb.66.1314. [DOI] [PubMed] [Google Scholar]
- 43.An M., Mou S., Zhang X., Ye N., Zheng Z., Cao S., Xu D., Fan X., Wang Y., Miao J. Temperature regulates fatty acid desaturases at a transcriptional level and modulates the fatty acid profile in the Antarctic microalga Chlamydomonas sp. ICE-L. Bioresour. Technol. 2013;134:151–157. doi: 10.1016/j.biortech.2013.01.142. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P., Liu S., Cong B., Wu G., Liu C., Lin X., Shen J., Huang X. A novel omega-3 fatty acid desaturase involved in acclimation processes of polar condition from Antarctic ice algae Chlamydomonas sp. ICE-L. Mar. Biotechnol. 2011;13:393–401. doi: 10.1007/s10126-010-9309-8. [DOI] [PubMed] [Google Scholar]
- 45.An M., Mou S., Zhang X., Zheng Z., Ye N., Wang D., Zhang W., Miao J. Expression of fatty acid desaturase genes and fatty acid accumulation in Chlamydomonas sp. ICE-L under salt stress. Bioresour. Technol. 2013;149:77–83. doi: 10.1016/j.biortech.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 46.Priscu J., Palmisano A., Priscu L., Sullivan C. Temperature dependence of inorganic nitrogen uptake and assimilation in Antarctic sea-ice microalgae. Polar Biol. 1989;9:443–446. doi: 10.1007/BF00443231. [DOI] [Google Scholar]
- 47.Di Martino Rigano V., Vona V., Lobosco O., Carillo P., Lunn J.E., Carfagna S., Esposito S., Caiazzo M., Rigano C. Temperature dependence of nitrate reductase in the psychrophilic unicellular alga Koliella antarctica and the mesophilic alga Chlorella sorokiniana. Plant Cell Environ. 2006;29:1400–1409. doi: 10.1111/j.1365-3040.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- 48.Vona V., Di Martino Rigano V., Lobosco O., Carfagna S., Esposito S., Rigano C. Temperature responses of growth, photosynthesis, respiration and NADH: Nitrate reductase in cryophilic and mesophilic algae. New Phytol. 2004;163:325–331. doi: 10.1111/j.1469-8137.2004.01098.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara M., Guerriero G., Cardi M., Esposito S. Purification and biochemical characterisation of a glucose-6-phosphate dehydrogenase from the psychrophilic green alga Koliella antarctica. Extremophiles. 2012;17:53–62. doi: 10.1007/s00792-012-0492-6. [DOI] [PubMed] [Google Scholar]
- 50.Lyon B.R., Lee P.A., Bennett J.M., DiTullio G.R., Janech M.G. Proteomic analysis of a sea-ice diatom: Salinity acclimation provides new insight into the dimethylsulfoniopropionate production pathway. Plant Physiol. 2011;157:1926–1941. doi: 10.1104/pp.111.185025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strauss J., Gao S., Morrissey J., Bowler C., Nagel G., Mock T. A light-driven rhodopsin proton pump from the psychrophilic diatom Fragilariopsis cylindrus; Proceeding of EMBO Workshop: The Molecular Life of Diatoms; Paris, France. 25–28 June 2013. [Google Scholar]
- 52.Marchetti A., Schruth D.M., Durkin C.A., Parker M.S., Kodner R.B., Berthiaume C.T., Morales R., Allen A.E., Armbrust E.V. Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. PNAS. 2012;109:E317–E325. doi: 10.1073/pnas.1118408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devos N., Ingouff M., Loppes R., Matagne R.F. RUBISCO adaptation to low temperatures: A comparative study in psychrophilic and mesophilic unicellular algae. J. Phycol. 1998;34:655–660. [Google Scholar]
- 54.Morgan R.M., Ivanov A.G., Priscu J.C., Maxwell D.P., Huner N.P.A. Structure and composition of the photochemical apparatus of the antarctic green alga, Chlamydomonas subcaudata. Photosynth. Res. 1998;56:303–314. doi: 10.1023/A:1006048519302. [DOI] [Google Scholar]
- 55.Napolitano M.J., Shain D.H. Distinctions in adenylate metabolism among organisms inhabiting temperature extremes. Extremophiles. 2005;9:93–98. doi: 10.1007/s00792-004-0424-1. [DOI] [PubMed] [Google Scholar]
- 56.Toseland A.D.S.J., Clark J.R., Kirkham A., Strauss J., Uhlig C., Lenton T.M., Valentin K., Pearson G.A., Moulton V., Mock T. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Change. 2013;3:979–984. doi: 10.1038/nclimate1989. [DOI] [Google Scholar]
- 57.Welsh D.T. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiol. Rev. 2000;24:263–290. doi: 10.1111/j.1574-6976.2000.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 58.Krell A. Ph.D. Thesis. University of Bremen; Bremen, Germany: 2006. Salt stress tolerance in the psychrophilic diatom Fragilariopsis cylindrus. [Google Scholar]
- 59.Waditee R., Bhuiyan M.N.H., Rai V., Aoki K., Tanaka Y., Hibino T., Suzuki S., Takano J., Jagendorf A.T., Takabe T., Takabe T. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc. Natl. Acad. Sci. USA. 2005;102:1318–1323. doi: 10.1073/pnas.0409017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiTullio G.R., Garrison D.L., Mathot S. Dimethylsulfonioproprionate in sea ice algae from the Ross Sea polynya. In: Arrigo K.R., Lizotte M.P., editors. Antarctic Sea Ice: Biological Processes, Interactions and Variability. American Geophysical Union; Washington, DC, USA: 1998. pp. 139–146. [Google Scholar]
- 61.Keller M.D., Bellows W.K., Guillard R.R.L. Dimethyl sulfide production in marine phytoplankton. In: Saltzman E.S., Cooper W.J., editors. Biogenic Sulfur in the Environment. American Chemical Society; Washington, DC, USA: 1989. pp. 131–142. [Google Scholar]
- 62.Nishiguchi M.K., Somero G.N. Temperature- and concentration-dependence of compatibility of the organic osmolyte [beta]-dimethylsulfoniopropionate. Cryobiology. 1992;29:118–124. doi: 10.1016/0011-2240(92)90011-P. [DOI] [PubMed] [Google Scholar]
- 63.Gage D.A., Rhodes D., Nolte K.D., Hicks W.A., Leustek T., Cooper A.J.L., Hanson A.D. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature. 1997;387:891–894. doi: 10.1038/43160. [DOI] [PubMed] [Google Scholar]
- 64.Tunnacliffe A., Wise M.J. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- 65.Honjoh K.-I., Yoshimoto M., Joh T., Kajiwara T., Miyamoto T., Hatano S. Isolation and characterization of hardening-induced proteins in Chlorella vulgaris C-27: Identification of late embryogenesis abundant proteins. Plant Cell Physiol. 1995;36:1421–1430. [PubMed] [Google Scholar]
- 66.Liu X., Wang Y., Gao H., Xu X. Identification and characterization of genes encoding two novel LEA proteins in Antarctic and temperate strains of Chlorella vulgaris. Gene. 2011;482:51–58. doi: 10.1016/j.gene.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Lyon B.R. (Medical University of South Carolina-Hollings Marine Lab, Charleston, SC, USA). 2011. Unpublished work.
- 68.Mock T., Krell A., Glockner G., Kolukisaoglu U., Valentin K. Analysis of expressed sequence tags (ESTs) from the polar diatom Fragilariopsis cylindrus. J. Phycol. 2005;42:78–85. [Google Scholar]
- 69.Raymond J.A. Algal ice-binding proteins change the structure of sea ice. PNAS. 2011;108:E198. doi: 10.1073/pnas.1106288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janech M.G., Krell A., Mock T., Kang J.S., Raymond J.A. Ice-binding proteins from sea ice diatoms (Bacillariophyceae) J. Phycol. 2006;42:410–416. doi: 10.1111/j.1529-8817.2006.00208.x. [DOI] [Google Scholar]
- 71.Raymond J.A., Kim H.J. Possible role of horizontal gene transfer in the colonization of sea ice by algae. PLoS ONE. 2012;7:e35968. doi: 10.1371/journal.pone.0035968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raymond J.A., Morgan-Kiss R. Separate origins of ice-binding proteins in Antarctic Chlamydomonas species. PLoS ONE. 2013;8:e59186. doi: 10.1371/journal.pone.0059186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krembs C., Eicken H., Junge K., Deming J.W. High concentrations of exopolymeric substances in Arctic winter sea ice: Implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res. Part I. 2002;49:2163–2181. [Google Scholar]
- 74.Krembs C., Eicken H., Deming J.W. Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic. PNAS. 2011;108:3653–3658. doi: 10.1073/pnas.1100701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mock T., Gradinger R. Determination of Arctic ice algal production with a new in situ incubation technique. Mar. Ecol. Prog. Ser. 1999;177:15–26. doi: 10.3354/meps177015. [DOI] [Google Scholar]
- 76.Cota G.F. Photoadaptation of high Arctic ice algae. Nature. 1985;315:219–222. doi: 10.1038/315219a0. [DOI] [Google Scholar]
- 77.Jung G., Lee C.G., Kang S.H., Jin E. Annotation and expression profile analysis of cDNAs from the Antarctic diatom Chaetoceros neogracile. J. Microbiol. Biotechnol. 2007;17:1330–1337. [PubMed] [Google Scholar]
- 78.Morgan-Kiss R., Ivanov A., Modla S., Czymmek K., Hüner N., Priscu J., Lisle J., Hanson T. Identity and physiology of a new psychrophilic eukaryotic green alga, Chlorella sp., strain BI, isolated from a transitory pond near Bratina Island, Antarctica. Extremophiles. 2008;12:701–711. doi: 10.1007/s00792-008-0176-4. [DOI] [PubMed] [Google Scholar]
- 79.Ralph P.J., McMinn A., Ryan K.G., Ashworth C. Short-term effect of temperature on the photokinetics of microalgae from the surface layers of Antarctic pack ice. J. Phycol. 2005;41:763–769. doi: 10.1111/j.1529-8817.2005.00106.x. [DOI] [Google Scholar]
- 80.Robinson D., Kolber Z., Sullivan C. Photophysiology and photoacclimation in surface sea ice algae from McMurdo Sound, Antarctica. Mar. Ecol. Prog. Ser. 1997;147:243–256. doi: 10.3354/meps147243. [DOI] [Google Scholar]
- 81.Lepetit B., Sturm S., Rogato A., Gruber A., Sachse M., Falciatore A., Kroth P.G., Lavaud J. High light acclimation in the secondary plastids containing diatom Phaeodactylum tricornutum is triggered by the redox state of the plastoquinone pool. Plant. Physiol. 2013;161:853–865. doi: 10.1104/pp.112.207811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Green B., Alami M., Zhu S., Guo J., Maldonado M. The LHC superfamily and the complex roles of its members in photoacclimation; Proceedings of EMBO Workshop: The Molecular Life of Diatoms; Paris, France. 25–28 June 2013. [Google Scholar]
- 83.Park S., Jung G., Hwang Y.-s., Jin E. Dynamic response of the transcriptome of a psychrophilic diatom, Chaetoceros neogracile, to high irradiance. Planta. 2010;231:349–360. doi: 10.1007/s00425-009-1044-x. [DOI] [PubMed] [Google Scholar]
- 84.Mock T., Hoch N. Long-term temperature acclimation of photosynthesis in steady-state cultures of the polar diatom Fragilariopsis cylindrus. Photosynth. Res. 2005;85:307–317. doi: 10.1007/s11120-005-5668-9. [DOI] [PubMed] [Google Scholar]
- 85.Szyszka B., Ivanov A.G., Huner N.P. Psychrophily is associated with differential energy partitioning, photosystem stoichiometry and polypeptide phosphorylation in Chlamydomonas raudensis. Biochimica et Biophysica Acta. 2007;1767:789–800. doi: 10.1016/j.bbabio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Takizawa K., Takahashi S., Huner N.P., Minagawa J. Salinity affects the photoacclimation of Chlamydomonas raudensis Ettl UWO241. Photosynth. Res. 2009;99:195–203. doi: 10.1007/s11120-008-9397-8. [DOI] [PubMed] [Google Scholar]
- 87.Ryan K.G., McMinn A., Hegseth E.N., Davy S.K. The effects of ultraviolet-b radiation on Antarctic sea-ice algae. J. Phycol. 2012;48:74–84. doi: 10.1111/j.1529-8817.2011.01104.x. [DOI] [PubMed] [Google Scholar]
- 88.Miao J., Li G., Hou X., Zhang Y., Jiang Y., Wang B., Zhang B. Study on induced synthesis of anti-UV substances in the Antarctic algae. High. Tech. Lett. 2002;6:179–183. [Google Scholar]
- 89.Obertegger U., Camin F., Guella G., Flaim G. Adaptation of a psychrophilic freshwater dinoflagellate to ultraviolet radiation. J. Phycol. 2011;47:811–820. doi: 10.1111/j.1529-8817.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 90.Schriek R. Effects of light and temperature on the enzymatic antioxidative defense systems in the Antarctic ice diatom Entomoneis kufferathii Manguin. Rep. Polar Res. 2000;349:1–130. [Google Scholar]
- 91.Janknegt P.J., Van De Poll W.H., Visser R.J.W., Rijstenbil J.W., Buma A.G.J. Oxidative stress responses in the marine antarctic diatom Chaetoceros brevis (bacillariophyceae) during photoacclimation. J. Phycol. 2008;44:957–966. doi: 10.1111/j.1529-8817.2008.00553.x. [DOI] [PubMed] [Google Scholar]
- 92.Janknegt P.J., De Graaff C.M., Van De Poll W.H., Visser R.J.W., Rijstenbil J.W., Buma A.G.J. Short-term antioxidative responses of 15 microalgae exposed to excessive irradiance including ultraviolet radiation. Eur. J. Phycol. 2009;44:525–539. doi: 10.1080/09670260902943273. [DOI] [Google Scholar]
- 93.Hwang Y.-S., Jung G., Jin E. Transcriptome analysis of acclimatory responses to thermal stress in Antarctic algae. Biochem. Biophys. Res. Commun. 2008;367:635–641. doi: 10.1016/j.bbrc.2007.12.176. [DOI] [PubMed] [Google Scholar]
- 94.Sunda W., Kieber D.J., Kiene R.P., Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–320. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- 95.Chen C., Dickman M.B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA. 2005;102:3459–3464. doi: 10.1073/pnas.0407960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hünken M., Harder J., Kirst G.O. Epiphytic bacteria on the Antarctic ice diatom Amphiprora kufferathii Manguin cleave hydrogen peroxide produced during algal photosynthesis. Plant. Biol. 2008;10:519–526. doi: 10.1111/j.1438-8677.2008.00040.x. [DOI] [PubMed] [Google Scholar]
- 97.Kan G.-F., Miao J.-L., Shi C.-J., Li G.-Y. Proteomic alterations of Antarctic ice microalga Chlamydomonas sp. under low-temperature stress. J. Integr. Plant Biol. 2006;48:965–970. doi: 10.1111/j.1744-7909.2006.00255.x. [DOI] [Google Scholar]
- 98.Kwon S.J., Kwon S.I., Bae M.S., Cho E.J., Park O.K. Role of the methionine sulfoxide reductase MsrB3 in cold acclimation in Arabidopsis. Plant Cell. Physiol. 2007;48:1713–1723. doi: 10.1093/pcp/pcm143. [DOI] [PubMed] [Google Scholar]
- 99.Kirch H.-H., Bartels D., Wei Y., Schnable P.S., Wood A.J. The ALDH gene superfamily of Arabidopsis. Trends Plant Sci. 2004;9:371–377. doi: 10.1016/j.tplants.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 100.Peters E. Prolonged darkness and diatom mortality: II. Marine temperate species. J. Exp. Mar. Biol. Ecol. 1996;207:43–58. doi: 10.1016/0022-0981(95)02519-7. [DOI] [Google Scholar]
- 101.Peters E., Thomas D.N. Prolonged darkness and diatom mortality I: Marine Antarctic species. J. Exp. Mar. Biol. Ecol. 1996;207:25–41. doi: 10.1016/S0022-0981(96)02520-8. [DOI] [Google Scholar]
- 102.van Oijen T., Leeuwe M., Gieskes W.C. Variation of particulate carbohydrate pools over time and depth in a diatom-dominated plankton community at the Antarctic Polar Front. Polar Biol. 2003;26:195–201. [Google Scholar]
- 103.Palmisano A., Garrison D. Microorganisms in Antarctic sea ice. In: Friedmann E., editor. Antarctic Microbiology. Wiley-Liss; New York, NY, USA: 1993. pp. 167–218. [Google Scholar]
- 104.Neven I.A., Stefels J., van Heuven S.M.A.C., de Baar H.J.W., Elzenga J.T.M. High plasticity in inorganic carbon uptake by Southern Ocean phytoplankton in response to ambient CO2. Deep Sea Res. Part II. 2011;58:2636–2646. doi: 10.1016/j.dsr2.2011.03.006. [DOI] [Google Scholar]
- 105.Armbrust E.V., Berges J.A., Bowler C., Green B.R., Martinez D., Putnam N.H., Zhou S., Allen A.E., Apt K.E., Bechner M., Brzezinski M.A., Chaal B.K., Chiovitti A., Davis A.K., Demarest M.S., Detter J.C., Glavina T., Goodstein D., Hadi M.Z., Hellsten U., Armbrust E.V. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolis. Science. 2004;306:79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- 106.Doucette G.J., Fryxell G.A. Thalassiosira antarctica: vegetative and resting stage chemical composition of an ice-related marine diatom. Mar. Biol. 1983;78:1–6. doi: 10.1007/BF00392964. [DOI] [Google Scholar]
- 107.Mock T., Valentin K. Photosynthesis and cold acclimation: Molecular evidence from a polar diatom. J. Phycol. 2004;40:732–741. doi: 10.1111/j.1529-8817.2004.03224.x. [DOI] [Google Scholar]
- 108.Baldisserotto C., Ferroni L., Moro I., Fasulo M.P., Pancaldi S. Modulations of the thylakoid system in snow xanthophycean alga cultured in the dark for two months: comparison between microspectrofluorimetric responses and morphological aspects. Protoplasma. 2005;226:125–135. doi: 10.1007/s00709-005-0127-1. [DOI] [PubMed] [Google Scholar]
- 109.Ferroni L., Baldisserotto C., Zennaro V., Soldani C., Fasulo M.P., Pancaldi S. Acclimation to darkness in the marine chlorophyte Koliella antarctica cultured under low salinity: hypotheses on its origin in the polar environment. Eur. J. Phycol. 2007;42:91–104. doi: 10.1080/09670260600960850. [DOI] [Google Scholar]
- 110.Zaslavskaia L.A., Lippmeier J.C., Kroth P.G., Grossman A.R., Apt K.E. Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J. Phycol. 2000;36:379–386. [Google Scholar]
- 111.Bachy C., Lopez-Garcia P., Vereshchaka A., Moreira D. Diversity and vertical distribution of microbial eukaryotes in the snow, sea ice and seawater near the North Pole at the end of the polar night. Front. Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duarte R.T.D., Nóbrega F., Nakayama C.R., Pellizari V.H. Brazilian research on extremophiles in the context of astrobiology. Int. J. Astrobiol. 2012;11:325–333. doi: 10.1017/S1473550412000249. [DOI] [Google Scholar]
- 113.Homepage of Fragilariopsis cylindrus Genome. [(accessed on 7 September 2013)]. Available online: http://genome.jgi-psf.org/Fracy1/Fracy1.home.html.
- 114.Marine MIcrobial Eukaryote Transcriptome Sequencing Project. [(accessed on 7 September 2013)]. Available online: www.marinemicroeukaryotes.org.
- 115.Yoon H.S., Price D.C., Stepanauskas R., Rajah V.D., Sieracki M.E., Wilson W.H., Yang E.C., Duffy S., Bhattacharya D. Single-cell genomics reveals organismal interactions in uncultivated marine protists. Science. 2011;332:714–717. doi: 10.1126/science.1203163. [DOI] [PubMed] [Google Scholar]
- 116.Lovejoy C., Massana R., Pedros-Alio C. Diversity and distribution of marine microbial eukaryotes in the Arctic Ocean and adjacent seas. Appl. Environ. Microbiol. 2006;72:3085–3095. doi: 10.1128/AEM.72.5.3085-3095.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amaral-Zettler L.A., McCliment E.A., Ducklow H.W., Huse S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE. 2009;4:e6372. doi: 10.1371/journal.pone.0006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Charvet S., Vincent W., Lovejoy C. Chrysophytes and other protists in High Arctic lakes: molecular gene surveys, pigment signatures and microscopy. Polar Biol. 2012;35:733–748. doi: 10.1007/s00300-011-1118-7. [DOI] [Google Scholar]
- 119.Piquet A.M.T., Bolhuis H., Davidson A.T., Thomson P.G., Buma A.G.J. Diversity and dynamics of Antarctic marine microbial eukaryotes under manipulated environmental UV radiation. FEMS Microbiol. Ecol. 2008;66:352–366. doi: 10.1111/j.1574-6941.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 120.Potvin M., Lovejoy C. PCR-based diversity estimates of artificial and environmental 18S rRNA gene libraries. J. Eukaryotic Microbiol. 2009;56:174–181. doi: 10.1111/j.1550-7408.2008.00386.x. [DOI] [PubMed] [Google Scholar]
- 121.Zhu F., Massana R., Not F., Marie D., Vaulot D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol. Ecol. 2005;52:79–92. doi: 10.1016/j.femsec.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Malviya S., Veluchamy A., Bittner L., Tanaka A., Bowler C. Comprehensive biogeographical insights into the complexity of marine diatom communities; EMBO Workshop: The Molecular Life of Diatoms; Paris, France. 25–28 June 2013. [Google Scholar]
- 123.Poulsen N., Chesley P.M., Kröger N. Molecular genetic manipulation of the diatom Thalassiosira pseudonana (bacillariophyceae) J. Phycol. 2006;42:1059–1065. doi: 10.1111/j.1529-8817.2006.00269.x. [DOI] [Google Scholar]
- 124.De Riso V., Raniello R., Maumus F., Rogato A., Bowler C., Falciatore A. Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res. 2009;37:e96. doi: 10.1093/nar/gkp448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pocock T., Vetterli A., Falk S. Evidence for phenotypic plasticity in the Antarctic extremophile Chlamydomonas raudensis Ettl. UWO 241. J. Exp. Bot. 2011;62:1169–1177. doi: 10.1093/jxb/erq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Konstantinidis K.T., Braff J., Karl D.M., DeLong E.F. Comparative metagenomic analysis of a microbial community residing at a depth of 4,000 meters at station ALOHA in the North Pacific Subtropical Gyre. Appl. Environ. Microbiol. 2009;75:5345–5355. doi: 10.1128/AEM.00473-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Veluchamy A., Lin X., Maumus F., Rivarola M., Bhavsar J., Creasy T., O’Brien K., Sengamalay N.A., Tallon L.J., Smith A.D., Rayko E., Ahmed I., Crom S.L., Farrant G.K., Sgro J.-Y., Olson S.A., Bondurant S.S., Allen A., Rabinowicz P.D., Sussman M.R., Bowler C., Tirichine L. Insights into the role of DNA methylation in diatoms by genome-wide profiling in Phaeodactylum tricornutum. Nat. Commun. 2013;4 doi: 10.1038/ncomms3091. [DOI] [PubMed] [Google Scholar]
- 128.Maumus F., Allen A.E., Mhiri C., Hu H., Jabbari K., Vardi A., Grandbastien M.A., Bowler C. Potential impact of stress activated retrotransposons on genome evolution in a marine diatom. BMC Genomics. 2009;10:624. doi: 10.1186/1471-2164-10-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richards E.J. Inherited epigenetic variation—Revisiting soft inheritance. Nat. Rev. Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 130.Norden-Krichmar T.M., Allen A.E., Gaasterland T., Hildebrand M. Characterization of the small RNA transcriptome of the diatom, Thalassiosira pseudonana. PLoS ONE. 2011;6:e22870. doi: 10.1371/journal.pone.0022870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anesio A.M., Bellas C.M. Are low temperature habitats hot spots of microbial evolution driven by viruses? Trends Microbiol. 2011;19:52–57. doi: 10.1016/j.tim.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Pearce I., Davidson A.T., Bell E.M., Wright S. Seasonal changes in the concentration and metabolic activity of bacteria and viruses at an Antarctic coastal site. Aquat. Microb. Ecol. 2007;47:11–23. doi: 10.3354/ame047011. [DOI] [Google Scholar]
- 133.Yau S., Lauro F.M., DeMaere M.Z., Brown M.V., Thomas T., Raftery M.J., Andrews-Pfannkoch C., Lewis M., Hoffman J.M., Gibson J.A., Cavicchioli R. Virophage control of antarctic algal host–virus dynamics. PNAS. 2011;108:6163–6168. doi: 10.1073/pnas.1018221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vardi A., Haramaty L., Van Mooy B.A., Fredricks H.F., Kimmance S.A., Larsen A., Bidle K.D. Host-virus dynamics and subcellular controls of cell fate in a natural coccolithophore population. Proc. Natl. Acad. Sci. USA. 2012;109:19327–19332. doi: 10.1073/pnas.1208895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kroth P., Windler M., Leinweber K., Schulze B., Spiteller D., Buhmann M. Interactions of diatoms and bacteria in biofilms; Proceedings of EMBO Workshop: The Molecular Life of Diatoms; Paris, France. 25–28 June 2013. [Google Scholar]
- 136.Amin S., Hmelo L., Parsek M., Armbrust E.V. Multiple complex interactions between a toxigenic diatom and an associated bacterium revealed using whole cell transcriptomics; Proceedings of EMBO: The Molecular Life of Diatoms; Paris, France. 25–28 June 2013. [Google Scholar]
- 137.Vardi A., Bidle K.D., Kwityn C., Hirsh D.J., Thompson S.M., Callow J.A., Falkowski P., Bowler C. A diatom gene regulating nitric-oxide signaling and susceptibility to diatom-derived aldehydes. Curr. Biol. 2008;18:895–899. doi: 10.1016/j.cub.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 138.Caldwell G.S. The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs. 2009;7:367–400. doi: 10.3390/md7030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smetacek V., Nicol S. Polar ocean ecosystems in a changing world. Nature. 2005;437:362–368. doi: 10.1038/nature04161. [DOI] [PubMed] [Google Scholar]
- 140.Tremblay J.-É., Robert D., Varela D., Lovejoy C., Darnis G., Nelson R., Sastri A. Current state and trends in Canadian Arctic marine ecosystems: I. Primary production. Clim. Change. 2012;115:161–178. doi: 10.1007/s10584-012-0496-3. [DOI] [Google Scholar]
- 141.Arrigo K.R., Perovich D.K., Pickart R.S., Brown Z.W., van Dijken G.L., Lowry K.E., Mills M.M., Palmer M.A., Balch W.M., Bahr F., Bates N.R., Benitez-Nelson C., Bowler B., Brownlee E., Ehn J.K., Frey K.E., Garley R., Laney S.R., Lubelczyk L., Mathis J., Matsuoka A., Mitchell B.G., Moore G.W.K., Ortega-Retuerta E., Pal S., Polashenski C.M., Reynolds R.A., Schieber B., Sosik H.M., Stephens M., Swift J.H. Massive phytoplankton blooms under Arctic sea ice. Science. 2012;336:1408. doi: 10.1126/science.1215065. [DOI] [PubMed] [Google Scholar]
- 142.Marinov I., Doney S.C., Lima I.D. Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature and light. Biogeosciences. 2010;7:3941–3959. doi: 10.5194/bg-7-3941-2010. [DOI] [Google Scholar]
- 143.Montes-Hugo M., Doney S.C., Ducklow H.W., Fraser W., Martinson D., Stammerjohn S.E., Schofield O. Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science. 2009;323:1470–1473. doi: 10.1126/science.1164533. [DOI] [PubMed] [Google Scholar]
- 144.Karsten U., Schlie C., Woelfel J., Becker B. Benthic diatoms in Arctic waters -ecological functions and adaptations. Polarforschung. 2012;81:77–84. [Google Scholar]
- 145.Dunbar R.B., Arrigo K.R., Lutz M., DiTullio G.R., Leventer A.R., Lizotte M.P., Van Woert M.L., Robinson D.H. Non-Redfield production and export of marine organic matter: A recurrent part of the annual cycle in the Ross Sea, Antarctica. Antarct. Sci. Ser. 2003;78:179–195. doi: 10.1029/078ARS11. [DOI] [Google Scholar]
- 146.Lee S.H., Whitledge T.E., Kang S.-H. Spring time production of bottom ice algae in the landfast sea ice zone at Barrow, Alaska. J. Exp. Mar. Biol. Ecol. 2008;367:204–212. doi: 10.1016/j.jembe.2008.09.018. [DOI] [Google Scholar]
- 147.Comeau A.M., Li W.K.W., Tremblay J.-É., Carmack E.C., Lovejoy C. Arctic Ocean microbial community structure before and after the 2007 record sea ice minimum. PLoS ONE. 2011;6:e27492. doi: 10.1371/journal.pone.0027492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gabric A.J., Shephard J.M., Knight J.M., Jones G., Trevena A.J. Correlations between the satellite-derived seasonal cycles of phytoplankton biomass and aerosol optical depth in the Southern Ocean: Evidence for the influence of sea ice. Glob. Biogeochem. Cycle. 2005;19:1–10. [Google Scholar]
- 149.Boyd P.W., Strzepek R., Fu F., Hutchinsc D.A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 2010;55:1353–1376. doi: 10.4319/lo.2010.55.3.1353. [DOI] [Google Scholar]