Abstract
Gene expression of the human immunodeficiency virus type 1 (HIV-1) is a highly regulated process. Basal transcription of the integrated provirus generates early transcripts that encode for the viral products Tat and Rev. Tat promotes the elongation of RNA polymerase while Rev mediates the nuclear export of viral RNAs that contain the Rev-responsive RNA element (RRE). These RNAs are exported from the nucleus to allow expression of Gag-Pol and Env proteins and for the production of full-length genomic RNAs. A balance exists between completely processed mRNAs and RRE-containing RNAs. Rev functions as an adaptor that recruits cellular factors to re-direct singly spliced and unspliced viral RNAs to nuclear export. The aim of this review is to address the dynamic regulation of this post-transcriptional pathway in light of recent findings that implicate several novel cellular cofactors of Rev function.
Keywords: HIV-1, RNA, transcription, splicing, Rev
1. Introduction
Replication of retroviruses including the Human immunodeficiency virus type 1 (HIV-1) occurs in the nucleus of infected cells. The incoming viral RNA genomes are reverse transcribed in the cytoplasm and the resulting DNA transported to the nucleus where it is integrated into chromatin [1,2]. The chromatinized provirus becomes part of the host cell’s genome and is subjected to the complex network of regulatory pathways that control cellular gene expression [3,4]. The understanding of HIV-1 gene expression has profound pathological implications. Persistence of HIV-1 infection in patients undergoing anti-retroviral therapy is the major barrier to viral eradication [5,6,7]. Treatment intensification strategies have shown that viremia could originate both from virus replicating in sanctuaries, where drug levels are low, and from latently infected cells [8,9,10,11]. These long-lived cells harbor a provirus unable to express its genes and, as such, invisible to the immune system and to antiviral drugs. Therefore a better understanding of the specific features of HIV-1 gene expression will help in designing alternative therapies aimed at the eradication of these persistent viral reservoirs [12].
Three features distinguish the HIV-1 provirus from the host genes: (i) cis-acting DNA/RNA elements present in the provirus/viral RNA; (ii) the activity of the viral trans-activator of transcription Tat and (iii) the activity of the viral regulatory protein Rev. All other factors required for efficient viral transcription and RNA processing are of cellular origin, including the RNA polymerase (RNAPII). The generation of infectious retroviral progeny requires the synthesis and export to the cytoplasm of spliced subgenomic mRNAs for protein translation, of partially spliced RNAs that function as the mRNA for the viral proteins Gag-Pol and Env and of the viral genomic RNA [13,14]. Hence, coordinated expression of these three classes of RNAs is a hallmark of HIV-1 efficient replication. Our current understanding is that cellular transcription factors control basal transcription from the viral long terminal repeat (LTR) promoter while the viral Tat protein releases RNAPII stalled after the trans-activator response (TAR) RNA element, present at the 5’end of all transcripts and promotes RNAPII elongation [15,16]. Tat binds TAR and functions as an adaptor of the Cyclin T1/CDK9 kinase complex and for a number of cellular factors involved in the fine-tuning of the process [17,18]. Rev promotes the export of RNAs from the nucleus through the association to an RNA element called the Rev response element (RRE) that is present in the env gene [19,20,21]. Nuclear export occurs upon association of the Rev with the nuclear export factor Exportin 1 (Crm-1) and translocation of the Rev/RNA complex to the cytoplasm where it is either translated or packaged into virions [22,23,24,25]. The focus of this review will be on the journey of viral RNA from the site of viral transcription to the nuclear pore and on the role of Rev and cellular co-factors in determining the fate of HIV-1 RNA.
2. Spatial and Temporal Definition of HIV-1 Transcription
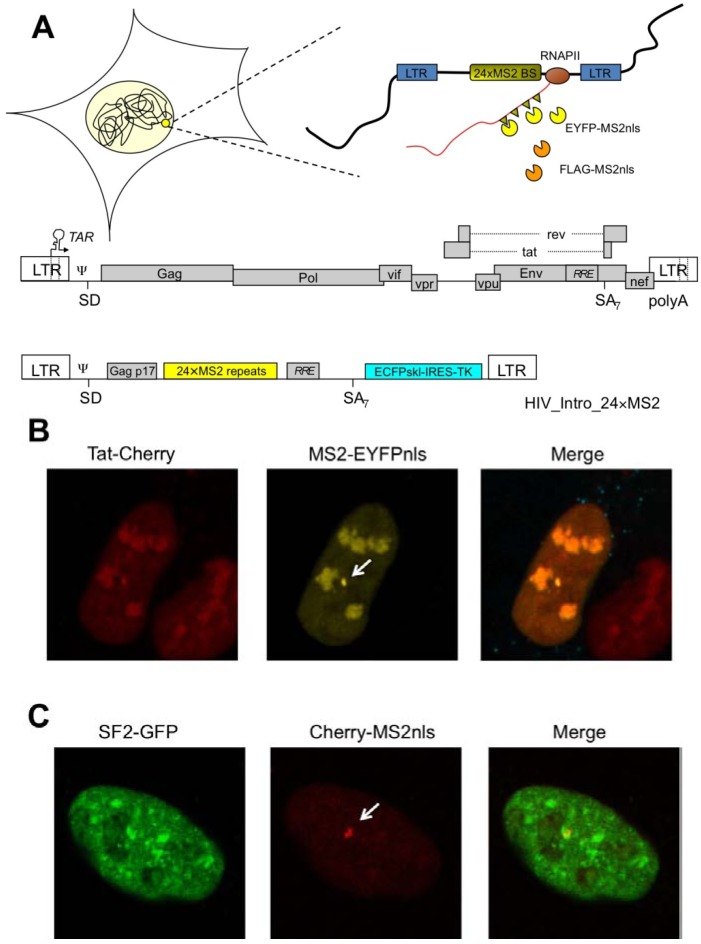
HIV-1 RNA starts its journey at the provirus integration site, where RNAPII transcribes the viral genome, and reaches the nuclear pore to exit the nucleus. HIV-1 integrates with high frequency within active genes, possibly because of a better accessibility of open chromatin [26]. Investigation of the spatial positioning of the provirus in latently infected cells demonstrated a preference towards the heterochromatic periphery of the nucleus [27]. Upon reactivation, the transcribing provirus maintains its position in the proximity of the nuclear membrane. Therefore, the pre-integration complex targets a subset of active genes at the nuclear periphery that are outside the heterochromatic regions, tightly associated to the nuclear lamina and are possibly associated to the nuclear pore [28]. These studies on the topology of HIV-1 transcription were conducted by three-dimensional in situ hybridization and by a novel technique that permits visualization of viral RNA in living cells [29]. Taking advantage of the MS2 phage core protein and its high-affinity RNA binding site it has been possible to engineer cell lines carrying an integrated provirus where arrays of MS2 binding sites were cloned in the viral genome [30,31,32]. As depicted in Figure 1A, MS2 fused to an autofluorescent protein like EYFP (enhanced yellow fluorescent protein) and to a nuclear localization signal (nls) would recognize nascent HIV-1 RNA. At steady state in a live cell the nucleus would appear yellow with a spot corresponding to the HIV-1 transcription site. This is clearly visible in Figure 1B, where HIV-1 transcription is induced by a hybrid Tat fused to the Cherry autofluorescent protein. As expected, Tat-Cherry is also present at the transcription site on nascent RNA. With this tool it has been possible to study not only the sub-nuclear localization of the transcribing provirus, but also the dynamic recruitment of factors like Tat, CDK9 or RNAPII as well as the kinetics of RNAPII transcription [30,31,33]. Recently, the generation of cell lines transduced by a HIV vector carrying the MS2 repeats allowed for the first time the analysis of RNAPII transcription rates on a single Tat-induced transcribing provirus localized at the nuclear periphery [33]. By photobleaching techniques it was shown that RNAPII could elongate at least an order-of-magnitude faster than previously thought. These data were confirmed by the quantification of single (spliced) RNAs per cell at steady state reaching the conclusion that the output rate of HIV-1 transcription was of one new RNA every 1.2–2.4 seconds. These studies demonstrate that Tat-mediated HIV-1 transcription is highly efficient and able to produce a large amount of pre-mRNA in a short time [34]. But how is the downstream processing controlled in order to produce the large variety of RNA species characteristic of HIV-1?
Figure 1.
(a) Schematic description of the HIV-1 MS2-tagging method. Top, the viral cassette carrying the MS2 repeats is integrated in the cell’s chromatin. RNAPII transcribes along the provirus and produces tagged RNAs that are bound by EYFP-MS2nls for visualization of the nascent RNA. Alternatively, FLAG-MS2nls can be used to pull-down the viral RNA for affinity purification of the associated proteome. An outline of the full-lengthviral genome is also shown below with the construct HIV_intro_24xMS2 (not drawn to scale). These constructs are described in great detail in a series of papers [30,32,35]; (b) Localization of Tat on HIV-1 RNA at the transcription site. Tat-Cherry was found associated with the transcription site marked by the accumulation of MS2 (white arrow) in U2OS cell clones expressing EYFP-MS2nls; (c) Localization of SF2 on HIV-1 RNA at the transcription site. SF2-GFP was found associated with the transcription site marked by the accumulation of MS2 (white arrow) in U2OS cell clones expressing Tat and EYFP-MS2nls.
3. A Portfolio of HIV-1 RNA Species
After RNA synthesis, the HIV-1 transcript is processed through alternative splicing. The transcripts are grouped into three size classes: the unspliced, 9 kb RNA that encodes Gag and Gag/Pol but also forms the genomic RNA, the 4 kb, singly spliced RNAs that encode Vif, Vpr, Vpu and Env, and the 2 kb, fully spliced RNAs that express Tat, Rev and Nef. Generation of the various viral RNA species is achieved through the use of five 5’ splice donors (SD1–5) and nine 3’ splice acceptors (SA1–9) [14,36,37]. HIV exploits a variety of mechanisms to make sure that all required RNAs are balanced for an efficient life cycle. Sub-optimal splice acceptor sequences and exon regulatory sequences like exon splicing enhancers (ESE) and exonic/intronic splicing silencers (ESS/ISS) and their associated factors are the players of alternative splicing control [38,39,40,41,42,43,44,45,46]. Factors that promote splicing are, for example, SF2/ASF that binds to ESEs and SC35 while association of hnRNP A1 to ESE/ISS inhibits splicing. This mechanism allows the production of both fully spliced mRNAs as well as unspliced and partially spliced RRE-containing RNAs. The latter are retained in the nucleus and require Rev action on RRE to be exported. HIV-1 also encodes for regulatory sequences called the instability (INS) or cis-acting repressor (CRS) sequences [47,48,49,50,51,52]. INS elements impair mRNA stability, nucleo-cytoplasmic transport and translation and are counteracted by the action of Rev. However Rev is unable to export an RRE-containing RNA that does not contain also a functional INS, implying their involvement in a pathway of nuclear RNA processing that is exploited by Rev [51,53]. Several cellular factors like the poly(A) binding protein 1 (PABP1), the heterogenous ribonuclear protein A1 (hnRNP), and the polypyrimidine tract-binding protein associated binding factor PSF and associated factor p54nrb complex, were shown to bind specifically to INS elements [53,54,55,56]. One possible mechanism is that these INS-binding factors divert INS/RRE containing HIV-1 RNAs from the splicing machinery and promote nuclear retention until Rev takes care of their export from the nucleus [56]. Although this hypothesis has been around since the discovery of INS sequences, there is still not a definitive answer on the intranuclear fate of unspliced HIV-1 RNAs and on the cellular factors that are involved in this pathway.
4. HIV-1 Rev & Friends
Rev is an essential HIV-1 protein encoded from two exons that are joined by splicing to produce a monocistronic transcript, early in the viral replication cycle [57,58]. In this chapter the focus will be on Rev-host factors interactions (Table 1), other aspects of Rev have been described in excellent reviews and will not be discussed in detail here [22,23,24,25]. Briefly, Rev is a small (19 kD), protein of approximately 116 amino acids in size that contains a basic arginine-rich RNA binding domain functioning also as a nuclear localization sequence (NLS), a leucine-rich nuclear export signal (NES) that binds Crm-1 (see below) and a protein self-multimerization domain. Rev is post-translationally modified by phosphorylation and possibly methylation [59,60]. Rev is a nuclear shuttling protein that specifically recognizes an RNA element located within the coding sequence of Env. This sequence, called the RRE (Rev-responsive element), forms a highly structured secondary RNA structure. Rev binds to this region through the basic arginine-rich domain. Binding of Rev to RRE is initiated first by low-affinity interaction of a single Rev monomer, followed by the cooperative binding of several Rev molecules to the RRE. Although Rev is best known to stimulate nucleocytoplasmic transport of incompletely spliced viral RNAs, other activities in translation and encapsidation, as well as integration, have been described [61,62,63].
Table 1.
Cellular cofactors that modulate Rev function.
| Cellular Protein | Proposed function in Rev Activity | References |
|---|---|---|
| Following the Order of Their Mention in the Text. | ||
| CK2 | Interacts with and phosphorylates Rev | [59] |
| PRMT6 | Methylates Rev | [60] |
| Crm-1 | Nuclear export or Rev-bound RRE containing RNAs | [64] |
| Importin-β | Import of Rev into the nucleus | [65,66] |
| DDX-3 | Helicase that promotes nuclear export of Rev-containing RNAs | [67] |
| DDX-1 | Helicase similar to DDX-3 but restricted to astrocytes | [68] |
| DDX-9 (RHA) | Helicase involved in remodeling RNA upstream of Rev | [69] |
| DDX-24 | Helicase that binds Rev and is involved in viral RNA packaging | [70] |
| B23 | Binds Rev and colocalizes to nulceoli | [71] |
| p32 | Human homologue of yeast YL2 | [72] |
| NAP-1 | Interacts with Rev nls | [73] |
| HIC | Binds Rev in the cytoplasm and modulates nuclear import through nls binding | [74] |
| eIF-5A | Binds Rev and is involved in nuclear export of viral RNA | [75,76] |
| PABP1 | Associates with HIV-1 RNAs in a Rev-dependent manner | [77] |
| hRIP | Binds Rev and promotes release of RNA from the nucleus | [78,79] |
| Sam68 | Complements Rev activity | [80,81] |
| Hax-1 | Inhibits Rev function in the cytoplasm | [82] |
| Following the Order of Their Mention in the Text. | ||
| PIMT | 5’-CAP modification of Rev-dependent transcripts | [62] |
| MATR3 | Promotes the nuclear export of Rev-dependent RNAs | [35,83] |
| SF2/ASF | Binds RRE in a Rev-dependent manner | [84] |
| Not Described in the Text. | ||
| RREBP49 | hnRNP F homologue that binds RRE | [85] |
| Pur α | Interacts with Rev and RRE | [86] |
| Prothymosin α | Interacts with Rev | [87] |
| NF90 | Inhibits Rev function | [88] |
| IkB | Negatively regulates Rev function | [89] |
| hnRNPA1 | Binds to repressor sequences in gag and stimulates Rev function | [53] |
| 16.4.1 | Interacts with Rev and Crm-1 | [90] |
| ATM | Enhances Rev function and viral replication | [91] |
| β-actin | Involved in Rev activity on nuclear export, together with eIF-5A, or translation | [92,93] |
Rev exports the RRE-containing HIV-1 mRNAs from the nucleus through interaction with Crm-1 (chromosome maintenance region 1) [94]. Crm-1 is a member of the karyopherin family of nucleocytoplasmic-transport factors. Crm-1, like other karyopherins involved in nuclear export, binds its cargo in the nucleus in the presence of the GTP-bound form of the Ran GTPase. After nuclear export, hydrolysis of the bound GTP to GDP causes a conformational shift that induces cargo release in the cytoplasm, thus providing the directionality of this export pathway. Crm-1 also interacts with components of the nuclear pore complex (NPC) and this interaction is essential for nuclear RNA export. It is known that Crm-1 is the crucial nuclear export factor for U snRNAs, rRNAs and tRNAs [64]. Their nuclear export is mediated by adaptor proteins bearing leucine-rich NESs similar to the prototypic NES first defined in Rev. A few human mRNAs might also be targeted for export through Crm-1 [95]. In the nucleus, Ran-GTP bound Crm-1 binds the NES domain of Rev, which in turn is bound to RRE-containing HIV-1 transcripts. This interaction enables Crm-1 to export the resulting RNA/protein complex into the cytoplasm, presumably through the Crm-1 connection with Nup214 and Nup98 nucleoporins. In the cytoplasm, conversion from Ran-GTP to Ran-GDP releases the Rev/RNA cargo. Rev then returns to the nucleus by binding to importin-β and Ran-GDP for subsequent rounds of export [65,66].
The Rev-RRE-Crm-1 complex also engages the activity of the cellular RNA helicase DDX3 that functions to enhance the Rev-dependent pathway and it is believed to facilitate the passage of large unspliced HIV-1 RNAs through the nuclear pore [67]. RNA helicases are a large family of cellular proteins involved at various steps of the HIV-1 life cycle [96]. Among them, DDX1 was shown to have an activity similar to DDX3 but limited to assisting Rev activity in human astrocytes [68,97]. Another helicase, RHA (DDX9), has been previously proposed to act upstream of Rev by remodeling the RRE-containing viral RNAs before completion of splicing, thus freeing them for Rev-mediated nuclear export [69]. Finally, DDX24 was shown to bind Rev and to be involved in viral RNA packaging [70].
The nucleolar protein B23 associates tightly with Rev through the basic domain [71]. Rev and B23 colocalize in the nucleoli and the permanence of Rev at that location depends on continuous preribosomal RNA transcription [98]. There have been several reports of cellular interactors of the Rev basic domain. For example, p32, a splicing factor that co-purifies with SF2/ASF, was shown to bind Rev [72] and to overcome an important post-transcriptional block to HIV replication in murine cells [99]. The nucleosome assembly protein 1 (NAP1) was co-purified with Rev and increased its nuclear import [73]. Finally, the Human I-mfa domain-Containing protein (HIC) was reported to regulate Rev nuclear import. HIC selectively blocked importin-β but not transportin-mediated Rev nuclear import via the intermolecular masking of the Rev NLS [74]. The identification of cellular factors that interact with the basic domain of Rev should be handled with great care, particularly concerning specificity. In fact, all these interactors: B23, p32, NAP1, HIC were also shown to interact with the basic domain of Tat (unpublished observations) [100,101,102,103,104]
A number of other interactions have been investigated (Table 1). Some of them were reported only once, others investigated in more detail. The Eukaryotic initiation factor-5A (eIF-5A) was shown to be a cofactor involved in Rev-mediated nuclear export [75,76]. The eIF-5A interacts with specific nucleoporins and is required for efficient interaction of Rev with Crm-1 [93]. The poly(A)-binding protein 1 (PABP1) was shown to associate with cytoplasmic HIV-1 RNAs in a Rev-dependent manner implicating Rev in translation [77]. The human Rev interacting protein (hRIP) was identified by yeast two-hybrid screen as a Rev associated factor [78,79]. The hRIP is an essential HIV cofactor required for HIV replication that promotes the release of incompletely spliced HIV-1 RNAs from the perinuclear region [105,106]. Sam68 (Src-associated protein in mitosis) was shown to be a functional homologue of Rev, able to complement its function in RRE-mediated export of RNA [80]. Sam68 synergizes with Rev in RRE-mediated gene expression and viral production [81]. It is not clear how Sam68 functions and why HIV-1 needs Rev when Sam68 alone can export RRE-containing RNAs. A partial answer has come from the observation that a partner of Sam68, the HS1-associated protein X-1 (Hax-1), interacted with Rev in the cytoplasm inhibiting its activity while Sam68 counteracted this effect [82].
HIV RRE-dependent RNAs are modified by the peroxisome proliferator-activated receptor-interacting protein with methyltransferase domain (PIMT) that induces a modification of the 7-methylguanosine (m7G) cap to create a trimethylguanosine (TMG)-capped RNA [62]. Like cellular small nuclear/nucleolar RNA (snRNA/snoRNA) and telomerase RNA, TMG-capped RNAs are exported from the nucleus through the Crm-1 pathway. Hence, TMG capping may represent another regulatory mechanism for selective expression of HIV-1 genes.
A recent novel approach to identify HIV-1 RNA binding factors exploited the MS2-tagging of RNA method to affinity purify the ribonucleprotein complex in the cell nucleus [35]. Proteins identified by mass spectrometry included the matrix-associated RNA binding protein Matrin 3 (MATR3) that was shown to interact with Rev through RRE and to be required for Rev-mediated export of RRE-containing HIV-1 RNAs. These observations were also independently confirmed by the group of Jeang [83]. Intriguingly, MATR3 was shown to form a complex with another protein identified in the screen: the polypyrimidine tract-binding protein associated binding factor PSF. MATR3 and PSF have been implicated in the nuclear retention of certain hyperedited RNAs [107]. However, PSF was already implicated in Rev-mediated export of HIV-1 RNAs by its association with the INS RNA elements that control stability of the viral RNA [56]. In addition, MATR3 has been also identified as a constituent of the nuclear pore proteome [108]. Hence, it is tempting to speculate that Rev works with MATR3 to free mRNA from INS-mediated retention through PSF, allowing export through the nuclear pore.
Rev appears to be a versatile adaptor of cellular factors on RRE-containing HIV-1 RNAs and recent literature has provided attempts to identify the interactome of Rev. Of note the work of Gerace and collaborators who carefully designed a purification scheme to dissect Rev complexes assembled in vitro with nuclear or cytosolic extracts in conditions emulating intracellular environments of Rev, where the association of functionally relevant binding factors could be predicted to be different [109]. Reassuringly, several of the already described partners of Rev were found in this analysis including Crm-1, MATR3, NAP-1, DDX24 and DDX1. On the contrary, the monumental work by Nevan Krogan and collaborators who attempted to map the global interactome of HIV-1 proteins in infected cells provided only a handful of validated hits for Rev [110]. Of note is the stringent criterium for validation that included parallel pulldowns of hits with Rev and Tat as control of specificity. None of these hits were previously identified as Rev interactors. Reasons for this low efficiency may rely on the timing of cell harvest, since Rev is an early viral protein, or on the relative abundance of Rev in the infected cell.
5. From Transcription to Nuclear Export: The Journey of HIV-1 RRE-Containing RNAs
Although it is well established that the primary effect of Rev is to promote the nuclear export of RRE- containing HIV RNAs, many aspects of this process are still poorly understood. Rev integrates several cellular posttranscriptional mechanisms, such as mRNA splicing, RNA stability and nucleocytoplasmic transport. However, Rev does not possess any enzymatic activity and functions as an adaptor between RRE-containing RNAs and cellular factors. Hence, the activity of Rev is to engage in a promiscuous menage-a-trois with RRE and cellular proteins to shift the equilibrium from spliced/RRE-containing viral RNAs towards export of the latter.
The journey of the viral RNA starts at the transcription site. As soon as RNA is synthesized by RNAPII, its post-transcriptional processing begins through binding of proteins to the nascent RNA. These include the splicing factors described previously. For example, the splicing factor SF2/ASF appears enriched at the transcription site on nascent RNA (Figure 1C). In addition, INS RNA sequences and associated factors compete with splicing and retain unspliced and partially spliced RNA that are unstable in the nucleus in the absence of Rev. When Rev is present in the nucleus, the viral RRE-containing RNAs are stabilized and exported, but where does Rev recognize its substrate?
Studies of Cochrane and co-workers found that Rev could only export newly synthesized HIV RNA indicating that Rev acts on nascent transcripts rather than on downstream pathways engaged in splicing or RNA degradation [111]. Another indirect demonstration of Rev acting co-transcriptionally comes from early studies on the transactivation potential of Tat-Rev hybrids tethered to TAR-less constructs through association with the RRE [112,113]. Therefore, rather than forming a nuclear storage compartment for viral pre-mRNA where Rev would act, as it has been previously proposed [51,53,114], HIV-1 pre-mRNA appears to be associated with Rev at the site of transcription from where it is further exported. However, direct evidence of Rev at the transcription site is still missing since most studies concerning Rev subcellular localization did not carefully investigate the nascent proviral RNA at the same time [114,115,116,117,118,119].
Rev recognizes RRE as a monomer [120] and was quickly found to form oligomers on the RNA [20,121,122,123,124,125]. The crystal structure for Rev and a structural model to describe how Rev binds to the RRE, oligomerizes, and forms the RNA-protein complex which serves as the export substrate for Crm-1 recently became available [126,127,128,129]. This has been an important achievement, particularly for the rational design of drugs that could interfere with these interactions, but how Rev/RRE binding promotes export remains obscure. Actually, as pointed out in the work from Frankel’s laboratory, multimerization per se may not be used to recruit multiple Crm-1 molecules since steric hindrance would not allow more than one or two molecules of Crm-1 to bind the complex [126]. This implies that Rev oligomerization is needed to enhance RNA-binding affinity and not necessarily to recruit additional Crm-1 molecules. This model suggests that viral RNA is bound to one face of the Rev oligomer with Crm1 at the opposite face, providing a simple architecture to facilitate interactions with the nuclear pore and promote RNA export. Very recent experiments, conducted by a gain-of-function approach in the cellular environment, further confirmed the requirement of Rev oligomerization for nuclear export of unspliced and incompletely spliced RNA [130]. However, it appears that the function of the Rev-oligomer/RRE complex goes beyond the simple recruitment of Crm-1 molecules and may be important for the assembly of additional host factors like hRIP, eIF5A, Sam68 and RNA helicases which, in addition to a putative role in translocating the RNA across the nuclear pore, could also serve in the remodeling of the RRE during Rev oligomerization [131].
The journey of viral RNAs bound to Rev in the nucleus finally ends at the nuclear pore where the Crm-1/RRE/Rev complex is translocated to the cytoplasm as described previously. Little is known about this process. Certain nucleoporins were shown to be associated to the Crm-1/RRE/Rev complex and may be involved in the initial steps of pore recognition, while helicases such as DDX3 may assist RNA translocation. The kinetics of the process is also unknown, the application of single cell live imaging could eventually address this issue [132,133].
6. Conclusions
The mechanism of Rev function in the nucleus is likely to be governed by kinetic competition. The rate of viral RNA biogenesis determines the amount of unspliced and partially spliced RNAs that are formed at the transcription site. In fact the high transcription rate observed for the HIV-1 provirus also resulted in the presence of a large fraction of unspliced RNAs at the transcription site possibly indicating an effect of RNAPII velocity on splicing efficiency as was recently proposed [33,34]. This RNA is either spliced or quickly degraded by the action of INS-binding factors and unknown nucleases. However, increasing concentrations of Rev in the nucleus compete with the splicing/degradation pathway by preserving the RRE-containing RNA for export. Levels of Rev in the nucleus are determined by the cell type and are probably an effect of the efficiency of the nuclear import/export pathway for Rev. Finally, the Rev/RRE complex must engage Crm-1, again a nuclear shuttling protein, for export.
Several questions remain unanswered. What are the cellular factors that determine the fate of unspliced HIV-1 RNA in the absence of Rev? Does Rev localize on nascent RNA? Where does Rev engage Crm-1? Future studies on HIV-1 Rev will need to address these issues with a combination of standard molecular biology techniques and the new tools that are available for high resolution live cell analysis [29,134].
Acknowledgments
We thank present and past members of the Molecular Virology Lab for useful discussions. Work in the Molecular Virology Lab of the ICGEB is supported by a HFSP Young Investigators Grant, by the Italian FIRB program of the “Ministero dell’Istruzione, Università e Ricerca” of Italy, by the AIDS Program of the “Istituto Superiore di Sanità” of Italy, by the EC STREP consortium 012182 and by the Beneficientia Stiftung.
References
- 1.Greene W.C., Peterlin B.M. Charting HIV’s remarkable voyage through the cell: Basic science as a passport to future therapy. Nat. Med. 2002;8:673–680. doi: 10.1038/nm0702-673. [DOI] [PubMed] [Google Scholar]
- 2.Marcello A., Lusic M., Pegoraro G., Pellegrini V., Beltram F., Giacca M. Nuclear organization and the control of HIV-1 transcription. Gene. 2004;326:1–11. doi: 10.1016/j.gene.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Nekhai S., Jeang K.T. Transcriptional and post-transcriptional regulation of HIV-1 gene expression: Role of cellular factors for Tat and Rev. Future Microbiol. 2006;1:417–426. doi: 10.2217/17460913.1.4.417. [DOI] [PubMed] [Google Scholar]
- 4.Karn J., Stoltzfus C.M. Transcriptional and Posttranscriptional Regulation of HIV-1 Gene Expression. Cold Spring Harb. Perspect. Med. 2012;2:1–17. doi: 10.1101/cshperspect.a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karn J. The molecular biology of HIV latency: Breaking and restoring the Tat-dependent transcriptional circuit. Curr. Opin. HIV AIDS. 2011;6:4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y., Wind-Rotolo M., Yang H.C., Siliciano J.D., Siliciano R.F. Experimental approaches to the study of HIV-1 latency. Nat. Rev. Microbiol. 2007;5:95–106. doi: 10.1038/nrmicro1580. [DOI] [PubMed] [Google Scholar]
- 7.Marcello A. Latency: The hidden HIV-1 challenge. Retrovirology. 2006;3:7. doi: 10.1186/1742-4690-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinoso J.B., Kim S.Y., Wiegand A.M., Palmer S.E., Gange S.J., Cranmer L., O’Shea A., Callender M., Spivak A., Brennan T., et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yukl S.A., Shergill A.K., McQuaid K., Gianella S., Lampiris H., Hare C.B., Pandori M., Sinclair E., Gunthard H.F., Fischer M., et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi R.T., Zheng L., Bosch R.J., Chan E.S., Margolis D.M., Read S., Kallungal B., Palmer S., Medvik K., Lederman M.M., et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: A randomized controlled trial. PLoS Med. 2010;7:8. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer S., Josefsson L., Coffin J.M. HIV reservoirs and the possibility of a cure for HIV infection. J. Intern. Med. 2011;270:550–560. doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 12.Colin L., van Lint C. Molecular control of HIV-1 postintegration latency: Implications for the development of new therapeutic strategies. Retrovirology. 2009;6:111. doi: 10.1186/1742-4690-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoltzfus C.M. Chapter 1. Regulation of HIV-1 alternative RNA splicing and its role in virus replication. Adv. Virus Res. 2009;74:1–40. doi: 10.1016/S0065-3527(09)74001-1. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane A.W., McNally M.T., Mouland A.J. The retrovirus RNA trafficking granule: From birth to maturity. Retrovirology. 2006;3:18. doi: 10.1186/1742-4690-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ott M., Geyer M., Zhou Q. The control of HIV transcription: Keeping RNA polymerase II on track. Cell Host Microbe. 2012;10:426–435. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterlin B.M., Price D.H. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Jeang K.T., Xiao H., Rich E.A. Multifaceted activities of the HIV-1 transactivator of transcription. Tat. J. Biol. Chem. 1999;274:28837–28840. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- 18.Marcello A., Zoppe M., Giacca M. Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator. IUBMB Life. 2001;51:175–181. doi: 10.1080/152165401753544241. [DOI] [PubMed] [Google Scholar]
- 19.Zapp M.L., Green M.R. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature. 1989;342:714–716. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]
- 20.Kjems J., Brown M., Chang D.D., Sharp P.A. Structural analysis of the interaction between the human immunodeficiency virus Rev protein and the Rev response element. Proc. Natl. Acad. Sci. USA. 1991;88:683–687. doi: 10.1073/pnas.88.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang D.D., Sharp P.A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 22.Cullen B.R. Nuclear RNA export pathways. Mol. Cell Biol. 2000;20:4181–4187. doi: 10.1128/MCB.20.12.4181-4187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hope T.J. The ins and outs of HIV Rev. Arch. Biochem. Biophys. 1999;365:186–191. doi: 10.1006/abbi.1999.1207. [DOI] [PubMed] [Google Scholar]
- 24.Pollard V.W., Malim M.H. The HIV-1 Rev protein. Annu. Rev. Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 25.Yedavalli V.S., Jeang K.T. Rev-ing up post-transcriptional HIV-1 RNA expression. RNA Biol. 2011;8:195–199. doi: 10.4161/rna.8.2.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroder A.R., Shinn P., Chen H., Berry C., Ecker J.R., Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/S0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 27.Dieudonne M., Maiuri P., Biancotto C., Knezevich A., Kula A., Lusic M., Marcello A. Transcriptional competence of the integrated HIV-1 provirus at the nuclear periphery. EMBO J. 2009;28:2231–2243. doi: 10.1038/emboj.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcello A., Dhir S., Dieudonne M. Nuclear positional control of HIV transcription in 4D. Nucleus. 2010;1:8–11. doi: 10.4161/nucl.1.1.10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiuri P., Knezevich A., Bertrand E., Marcello A. Real-time imaging of the HIV-1 transcription cycle in single living cells. Methods. 2011;53:62–67. doi: 10.1016/j.ymeth.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Boireau S., Maiuri P., Basyuk E., de la Mata M., Knezevich A., Pradet-Balade B., Backer V., Kornblihtt A., Marcello A., Bertrand E. The transcriptional cycle of HIV-1 in real-time and live cells. J. Cell Biol. 2007;179:291–304. doi: 10.1083/jcb.200706018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molle D., Maiuri P., Boireau S., Bertrand E., Knezevich A., Marcello A., Basyuk E. A real-time view of the TAR:Tat:P-TEFb complex at HIV-1 transcription sites. Retrovirology. 2007;4:36. doi: 10.1186/1742-4690-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Marco A., Biancotto C., Knezevich A., Maiuri P., Vardabasso C., Marcello A. Intragenic transcriptional cis-activation of the human immunodeficiency virus 1 does not result in allele-specific inhibition of the endogenous gene. Retrovirology. 2008;5:98. doi: 10.1186/1742-4690-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiuri P., Knezevich A., De Marco A., Mazza D., Kula A., McNally J.G., Marcello A. Fast transcription rates of RNA polymerase II in human cells. EMBO Rep. 2011;12:1280–1285. doi: 10.1038/embor.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcello A. RNA polymerase II transcription on the fast lane. Transcription. 2012;3:29–34. doi: 10.4161/trns.3.1.19147. [DOI] [PubMed] [Google Scholar]
- 35.Kula A., Guerra J., Knezevich A., Kleva D., Myers M.P., Marcello A. Characterization of the HIV-1 RNA associated proteome identifies Matrin 3 as a nuclear cofactor of Rev function. Retrovirology. 2011;8:60. doi: 10.1186/1742-4690-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purcell D.F., Martin M.A. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J. Virol. 1993;67:6365–6378. doi: 10.1128/jvi.67.11.6365-6378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz S., Felber B.K., Benko D.M., Fenyo E.M., Pavlakis G.N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J. Virol. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staffa A., Cochrane A. Identification of positive and negative splicing regulatory elements within the terminal tat-rev exon of human immunodeficiency virus type 1. Mol. Cell Biol. 1995;15:4597–4605. doi: 10.1128/mcb.15.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staffa A., Cochrane A. The tat/rev intron of human immunodeficiency virus type 1 is inefficiently spliced because of suboptimal signals in the 3' splice site. J. Virol. 1994;68:3071–3079. doi: 10.1128/jvi.68.5.3071-3079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dyhr-Mikkelsen H., Kjems J. Inefficient spliceosome assembly and abnormal branch site selection in splicing of an HIV-1 transcript in vitro. J. Biol. Chem. 1995;270:24060–24066. doi: 10.1074/jbc.270.41.24060. [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly M.M., McNally M.T., Beemon K.L. Two strong 5' splice sites and competing, suboptimal 3' splice sites involved in alternative splicing of human immunodeficiency virus type 1 RNA. Virology. 1995;213:373–385. doi: 10.1006/viro.1995.0010. [DOI] [PubMed] [Google Scholar]
- 42.Si Z., Amendt B.A., Stoltzfus C.M. Splicing efficiency of human immunodeficiency virus type 1 tat RNA is determined by both a suboptimal 3' splice site and a 10 nucleotide exon splicing silencer element located within tat exon 2. Nucleic Acids Res. 1997;25:861–867. doi: 10.1093/nar/25.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amendt B.A., Si Z.H., Stoltzfus C.M. Presence of exon splicing silencers within human immunodeficiency virus type 1 tat exon 2 and tat-rev exon 3: Evidence for inhibition mediated by cellular factors. Mol. Cell Biol. 1995;15:4606–4615. doi: 10.1128/mcb.15.8.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amendt B.A., Hesslein D., Chang L.J., Stoltzfus C.M. Presence of negative and positive cis-acting RNA splicing elements within and flanking the first tat coding exon of human immunodeficiency virus type 1. Mol. Cell Biol. 1994;14:3960–3970. doi: 10.1128/mcb.14.6.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caputi M., Mayeda A., Krainer A.R., Zahler A.M. hnRNP A/B proteins are required for inhibition of HIV-1 pre-mRNA splicing. Embo. J. 1999;18:4060–4067. doi: 10.1093/emboj/18.14.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tange T.O., Damgaard C.K., Guth S., Valcarcel J., Kjems J. The hnRNP A1 protein regulates HIV-1 tat splicing via a novel intron silencer element. Embo. J. 2001;20:5748–5758. doi: 10.1093/emboj/20.20.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cochrane A.W., Jones K.S., Beidas S., Dillon P.J., Skalka A.M., Rosen C.A. Identification and characterization of intragenic sequences which repress human immunodeficiency virus structural gene expression. J. Virol. 1991;65:5305–5313. doi: 10.1128/jvi.65.10.5305-5313.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maldarelli F., Martin M.A., Strebel K. Identification of posttranscriptionally active inhibitory sequences in human immunodeficiency virus type 1 RNA: Novel level of gene regulation. J. Virol. 1991;65:5732–5743. doi: 10.1128/jvi.65.11.5732-5743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasioulas G., Zolotukhin A.S., Tabernero C., Solomin L., Cunningham C.P., Pavlakis G.N., Felber B.K. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J. Virol. 1994;68:2986–2993. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider R., Campbell M., Nasioulas G., Felber B.K., Pavlakis G.N. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz S., Campbell M., Nasioulas G., Harrison J., Felber B.K., Pavlakis G.N. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J. Virol. 1992;66:7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mikaelian I., Krieg M., Gait M.J., Karn J. Interactions of INS (CRS) elements and the splicing machinery regulate the production of Rev-responsive mRNAs. J. Mol. Biol. 1996;257:246–264. doi: 10.1006/jmbi.1996.0160. [DOI] [PubMed] [Google Scholar]
- 53.Najera I., Krieg M., Karn J. Synergistic stimulation of HIV-1 rev-dependent export of unspliced mRNA to the cytoplasm by hnRNP A1. J. Mol. Biol. 1999;285:1951–1964. doi: 10.1006/jmbi.1998.2473. [DOI] [PubMed] [Google Scholar]
- 54.Afonina E., Neumann M., Pavlakis G.N. Preferential binding of poly(A)-binding protein 1 to an inhibitory RNA element in the human immunodeficiency virus type 1 gag mRNA. J. Biol. Chem. 1997;272:2307–2311. doi: 10.1074/jbc.272.4.2307. [DOI] [PubMed] [Google Scholar]
- 55.Black A.C., Luo J., Chun S., Bakker A., Fraser J.K., Rosenblatt J.D. Specific binding of polypyrimidine tract binding protein and hnRNP A1 to HIV-1 CRS elements. Virus Genes. 1996;12:275–285. doi: 10.1007/BF00284648. [DOI] [PubMed] [Google Scholar]
- 56.Zolotukhin A.S., Michalowski D., Bear J., Smulevitch S.V., Traish A.M., Peng R., Patton J., Shatsky I.N., Felber B.K. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol. Cell Biol. 2003;23:6618–6630. doi: 10.1128/MCB.23.18.6618-6630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodroski J., Goh W.C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986;321:412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- 58.Feinberg M.B., Jarrett R.F., Aldovini A., Gallo R.C., Wong-Staal F. HTLV-II expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986;46:807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- 59.Meggio F., D'Agostino D.M., Ciminale V., Chieco-Bianchi L., Pinna L.A. Phosphorylation of HIV-1 Rev protein: Implication of protein kinase CK2 and pro-directed kinases. Biochem Biophys Res Commun. 1996;226:547–554. doi: 10.1006/bbrc.1996.1392. [DOI] [PubMed] [Google Scholar]
- 60.Invernizzi C.F., Xie B., Richard S., Wainberg M.A. PRMT6 diminishes HIV-1 Rev binding to and export of viral RNA. Retrovirology. 2006;3:93. doi: 10.1186/1742-4690-3-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groom H.C., Anderson E.C., Lever A.M. Rev: Beyond nuclear export. J. Gen. Virol. 2009;90:1303–1318. doi: 10.1099/vir.0.011460-0. [DOI] [PubMed] [Google Scholar]
- 62.Yedavalli V.S., Jeang K.T. Trimethylguanosine capping selectively promotes expression of Rev-dependent HIV- RNAs. Proc. Natl. Acad. Sci. USA. 2010;107:14787–14792. doi: 10.1073/pnas.1009490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grewe B., Uberla K. The human immunodeficiency virus type 1 Rev protein: Menage a trois during the early phase of the lentiviral replication cycle. J. Gen. Virol. 2010;91:1893–1897. doi: 10.1099/vir.0.022509-0. [DOI] [PubMed] [Google Scholar]
- 64.Fornerod M., Ohno M., Yoshida M., Mattaj I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/S0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 65.Henderson B.R., Percipalle P. Interactions between HIV Rev and nuclear import and export factors: The Rev nuclear localisation signal mediates specific binding to human importin-beta. J. Mol. Biol. 1997;274:693–707. doi: 10.1006/jmbi.1997.1420. [DOI] [PubMed] [Google Scholar]
- 66.Truant R., Cullen B.R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol. Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yedavalli V.S., Neuveut C., Chi Y.H., Kleiman L., Jeang K.T. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 68.Fang J., Acheampong E., Dave R., Wang F., Mukhtar M., Pomerantz R.J. The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology. 2005;336:299–307. doi: 10.1016/j.virol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 69.Li J., Tang H., Mullen T.M., Westberg C., Reddy T.R., Rose D.W., Wong-Staal F. A role for RNA helicase A in post-transcriptional regulation of HIV type 1. Proc. Natl. Acad. Sci. USA. 1999;96:709–714. doi: 10.1073/pnas.96.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma J., Rong L., Zhou Y., Roy B.B., Lu J., Abrahamyan L., Mouland A.J., Pan Q., Liang C. The requirement of the DEAD-box protein DDX24 for the packaging of human immunodeficiency virus type 1 RNA. Virology. 2008;375:253–264. doi: 10.1016/j.virol.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 71.Fankhauser C., Izaurralde E., Adachi Y., Wingfield P., Laemmli U.K. Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: Dissociation by the Rev response element. Mol. Cell Biol. 1991;11:2567–2575. doi: 10.1128/mcb.11.5.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tange T.O., Jensen T.H., Kjems J. In vitro interaction between human immunodeficiency virus type 1 Rev protein and splicing factor ASF/SF2-associated protein, p32. J. Biol. Chem. 1996;271:10066–10072. doi: 10.1074/jbc.271.17.10066. [DOI] [PubMed] [Google Scholar]
- 73.Cochrane A., Murley L.L., Gao M., Wong R., Clayton K., Brufatto N., Canadien V., Mamelak D., Chen T., Richards D., Zeghouf M., Greenblatt J., Burks C., Frappier L. Stable complex formation between HIV Rev and the nucleosome assembly protein, NAP1, affects Rev function. Virology. 2009;388:103–111. doi: 10.1016/j.virol.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Gu L., Tsuji T., Jarboui M.A., Yeo G.P., Sheehy N., Hall W.W., Gautier V.W. Intermolecular masking of the HIV-1 Rev NLS by the cellular protein HIC: Novel insights into the regulation of Rev nuclear import. Retrovirology. 2011;8:17. doi: 10.1186/1742-4690-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruhl M., Himmelspach M., Bahr G.M., Hammerschmid F., Jaksche H., Wolff B., Aschauer H., Farrington G.K., Probst H., Bevec D., et al. Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J. Cell Biol. 1993;123:1309–1320. doi: 10.1083/jcb.123.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bevec D., Jaksche H., Oft M., Wohl T., Himmelspach M., Pacher A., Schebesta M., Koettnitz K., Dobrovnik M., Csonga R., Lottspeich F., Hauber J. Inhibition of HIV-1 replication in lymphocytes by mutants of the Rev cofactor eIF-5A. Science. 1996;271:1858–1860. doi: 10.1126/science.271.5257.1858. [DOI] [PubMed] [Google Scholar]
- 77.Campbell L.H., Borg K.T., Haines J.K., Moon R.T., Schoenberg D.R., Arrigo S.J. Human immunodeficiency virus type 1 Rev is required in vivo for binding of poly(A)-binding protein to Rev-dependent RNAs. J. Virol. 1994;68:5433–5438. doi: 10.1128/jvi.68.9.5433-5438.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bogerd H.P., Fridell R.A., Madore S., Cullen B.R. Identification of a novel cellular cofactor for the Rev/Rex class of retroviral regulatory proteins. Cell. 1995;82:485–494. doi: 10.1016/0092-8674(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 79.Fritz C.C., Zapp M.L., Green M.R. A human nucleoporin-like protein that specifically interacts with HIV Rev. Nature. 1995;376:530–533. doi: 10.1038/376530a0. [DOI] [PubMed] [Google Scholar]
- 80.Reddy T.R., Xu W., Mau J.K., Goodwin C.D., Suhasini M., Tang H., Frimpong K., Rose D.W., Wong-Staal F. Inhibition of HIV replication by dominant negative mutants of Sam68, a functional homolog of HIV-1 Rev. Nat. Med. 1999;5:635–642. doi: 10.1038/9479. [DOI] [PubMed] [Google Scholar]
- 81.Modem S., Badri K.R., Holland T.C., Reddy T.R. Sam68 is absolutely required for Rev function and HIV-1 production. Nucleic Acids Res. 2005;33:873–879. doi: 10.1093/nar/gki231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Modem S., Reddy T.R. An anti-apoptotic protein, Hax-1, inhibits the HIV-1 rev function by altering its sub-cellular localization. J. Cell Physiol. 2008;214:14–19. doi: 10.1002/jcp.21305. [DOI] [PubMed] [Google Scholar]
- 83.Yedavalli V.S., Jeang K.T. Matrin 3 is a co-factor for HIV-1 Rev in regulating post-transcriptional viral gene expression. Retrovirology. 2011;8:61. doi: 10.1186/1742-4690-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Powell D.M., Amaral M.C., Wu J.Y., Maniatis T., Greene W.C. HIV Rev-dependent binding of SF2/ASF to the Rev response element: possible role in Rev-mediated inhibition of HIV RNA splicing. Proc. Natl. Acad. Sci. USA. 1997;94:973–978. doi: 10.1073/pnas.94.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y., Reddy T.R., Fischer W.H., Wong-Staal F. A Novel hnRNP Specifically Interacts with HIV-1 RRE RNA. J. Biomed. Sci. 1996;3:82–91. doi: 10.1007/BF02255535. [DOI] [PubMed] [Google Scholar]
- 86.Kaminski R., Darbinian N., Sawaya B.E., Slonina D., Amini S., Johnson E.M., Rappaport J., Khalili K., Darbinyan A. Puralpha as a cellular co-factor of Rev/RRE-mediated expression of HIV-1 intron-containing mRNA. J. Cell Biochem. 2008;103:1231–1245. doi: 10.1002/jcb.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kubota S., Adachi Y., Copeland T.D., Oroszlan S. Binding of human prothymosin alpha to the leucine-motif/activation domains of HTLV-I Rex and HIV-1 Rev. Eur. J. Biochem. 1995;233:48–54. doi: 10.1111/j.1432-1033.1995.048_1.x. [DOI] [PubMed] [Google Scholar]
- 88.Urcuqui-Inchima S., Castano M.E., Hernandez-Verdun D., St-Laurent G., 3rd, Kumar A. Nuclear Factor 90, a cellular dsRNA binding protein inhibits the HIV Rev-export function. Retrovirology. 2006;3:83. doi: 10.1186/1742-4690-3-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu B.Y., Woffendin C., Duckett C.S., Ohno T., Nabel G.J. Regulation of human retroviral latency by the NF-kappa B/I kappa B family: Inhibition of human immunodeficiency virus replication by I kappa B through a Rev-dependent mechanism. Proc. Natl. Acad. Sci. USA. 1995;92:1480–1484. doi: 10.1073/pnas.92.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kramer-Hammerle S., Ceccherini-Silberstein F., Bickel C., Wolff H., Vincendeau M., Werner T., Erfle V., Brack-Werner R. Identification of a novel Rev-interacting cellular protein. BMC Cell Biol. 2005;6:20. doi: 10.1186/1471-2121-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ariumi Y., Trono D. Ataxia-telangiectasia-mutated (ATM) protein can enhance human immunodeficiency virus type 1 replication by stimulating Rev function. J. Virol. 2006;80:2445–2452. doi: 10.1128/JVI.80.5.2445-2452.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kimura T., Hashimoto I., Nishikawa M., Fujisawa J.I. A role for Rev in the association of HIV-1 gag mRNA with cytoskeletal beta-actin and viral protein expression. Biochimie. 1996;78:1075–1080. doi: 10.1016/S0300-9084(97)86732-6. [DOI] [PubMed] [Google Scholar]
- 93.Hofmann W., Reichart B., Ewald A., Muller E., Schmitt I., Stauber R.H., Lottspeich F., Jockusch B.M., Scheer U., Hauber J., Dabauvalle M.C. Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J. Cell Biol. 2001;152:895–910. doi: 10.1083/jcb.152.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Askjaer P., Jensen T.H., Nilsson J., Englmeier L., Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- 95.Brennan C.M., Gallouzi I.E., Steitz J.A. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J. Cell Biol. 2000;151:1–14. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeang K.T., Yedavalli V. Role of RNA helicases in HIV-1 replication. Nucleic Acids Res. 2006;34:4198–4205. doi: 10.1093/nar/gkl398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang J., Kubota S., Yang B., Zhou N., Zhang H., Godbout R., Pomerantz R.J. A DEAD box protein facilitates HIV-1 replication as a cellular co-factor of Rev. Virology. 2004;330:471–480. doi: 10.1016/j.virol.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 98.Dundr M., Leno G.H., Hammarskjold M.L., Rekosh D., Helga-Maria C., Olson M.O. The roles of nucleolar structure and function in the subcellular location of the HIV-1 Rev protein. J. Cell Sci. 1995;108:2811–2823. doi: 10.1242/jcs.108.8.2811. [DOI] [PubMed] [Google Scholar]
- 99.Zheng Y.H., Yu H.F., Peterlin B.M. Human p32 protein relieves a post-transcriptional block to HIV replication in murine cells. Nat. Cell Biol. 2003;5:611–618. doi: 10.1038/ncb1000. [DOI] [PubMed] [Google Scholar]
- 100.Li Y.P. Protein B23 is an important human factor for the nucleolar localization of the human immunodeficiency virus protein Tat. J. Virol. 1997;71:4098–4102. doi: 10.1128/jvi.71.5.4098-4102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gautier V.W., Sheehy N., Duffy M., Hashimoto K., Hall W.W. Direct interaction of the human I-mfa domain-containing protein, HIC, with HIV-1 Tat results in cytoplasmic sequestration and control of Tat activity. Proc. Natl. Acad. Sci. USA. 2005;102:16362–16367. doi: 10.1073/pnas.0503519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berro R., Kehn K., de la Fuente C., Pumfery A., Adair R., Wade J., Colberg-Poley A.M., Hiscott J., Kashanchi F. Acetylated Tat regulates human immunodeficiency virus type 1 splicing through its interaction with the splicing regulator p32. J. Virol. 2006;80:3189–3204. doi: 10.1128/JVI.80.7.3189-3204.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vardabasso C., Manganaro L., Lusic M., Marcello A., Giacca M. The histone chaperone protein Nucleosome Assembly Protein-1 (hNAP-1) binds HIV-1 Tat and promotes viral transcription. Retrovirology. 2008;5:8. doi: 10.1186/1742-4690-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Marco A., Dans P.D., Knezevich A., Maiuri P., Pantano S., Marcello A. Subcellular localization of the interaction between the human immunodeficiency virus transactivator Tat and the nucleosome assembly protein 1. Amino Acids. 2010;38:1583–1593. doi: 10.1007/s00726-009-0378-9. [DOI] [PubMed] [Google Scholar]
- 105.Sanchez-Velar N., Udofia E.B., Yu Z., Zapp M.L. hRIP, a cellular cofactor for Rev function, promotes release of HIV RNAs from the perinuclear region. Genes Dev. 2004;18:23–34. doi: 10.1101/gad.1149704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Z., Sanchez-Velar N., Catrina I.E., Kittler E.L., Udofia E.B., Zapp M.L. The cellular HIV-1 Rev cofactor hRIP is required for viral replication. Proc. Natl. Acad. Sci. USA. 2005;102:4027–4032. doi: 10.1073/pnas.0408889102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Z., Carmichael G.G. The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. doi: 10.1016/S0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 108.Cronshaw J.M., Krutchinsky A.N., Zhang W., Chait B.T., Matunis M.J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naji S., Ambrus G., Cimermancic P., Reyes J.R., Johnson J.R., Filbrandt R., Huber M.D., Vesely P., Krogan N.J., Yates J.R., Saphire A.C., Gerace L. Host cell interactome of HIV-1 Rev includes RNA helicases involved in multiple facets of virus production. Mol. Cell Prot. 2012;11:M111–015313. doi: 10.1074/mcp.M111.015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jager S., Cimermancic P., Gulbahce N., Johnson J.R., McGovern K.E., Clarke S.C., Shales M., Mercenne G., Pache L., Li K., Hernandez H., Jang G.M., Roth S.L., Akiva E., Marlett J., Stephens M., D'Orso I., Fernandes J., Fahey M., Mahon C., O'Donoghue A.J., Todorovic A., Morris J.H., Maltby D.A., Alber T., Cagney G., Bushman F.D., Young J.A., Chanda S.K., Sundquist W.I., Kortemme T., Hernandez R.D., Craik C.S., Burlingame A., Sali A., Frankel A.D., Krogan N.J. Global landscape of HIV-human protein complexes. Nature. 2012;481:365–370. doi: 10.1038/nature10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iacampo S., Cochrane A. Human immunodeficiency virus type 1 Rev function requires continued synthesis of its target mRNA. J. Virol. 1996;70:8332–8339. doi: 10.1128/jvi.70.12.8332-8339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo Y., Madore S.J., Parslow T.G., Cullen B.R., Peterlin B.M. Functional analysis of interactions between Tat and the trans-activation response element of human immunodeficiency virus type 1 in cells. J. Virol. 1993;67:5617–5622. doi: 10.1128/jvi.67.9.5617-5622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Southgate C., Zapp M.L., Green M.R. Activation of transcription by HIV-1 Tat protein tethered to nascent RNA through another protein. Nature. 1990;345:640–642. doi: 10.1038/345640a0. [DOI] [PubMed] [Google Scholar]
- 114.Berthold E., Maldarelli F. cis-acting elements in human immunodeficiency virus type 1 RNAs direct viral transcripts to distinct intranuclear locations. J. Virol. 1996;70:4667–4682. doi: 10.1128/jvi.70.7.4667-4682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalland K.H., Szilvay A.M., Langhoff E., Haukenes G. Subcellular distribution of human immunodeficiency virus type 1 Rev and colocalization of Rev with RNA splicing factors in a speckled pattern in the nucleoplasm. J. Virol. 1994;68:1475–1485. doi: 10.1128/jvi.68.3.1475-1485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolff B., Cohen G., Hauber J., Meshcheryakova D., Rabeck C. Nucleocytoplasmic transport of the Rev protein of human immunodeficiency virus type 1 is dependent on the activation domain of the protein. Exp. Cell Res. 1995;217:31–41. doi: 10.1006/excr.1995.1060. [DOI] [PubMed] [Google Scholar]
- 117.Boe S.O., Bjorndal B., Rosok B., Szilvay A.M., Kalland K.H. Subcellular localization of human immunodeficiency virus type 1 RNAs, Rev, and the splicing factor SC-35. Virology. 1998;244:473–482. doi: 10.1006/viro.1998.9110. [DOI] [PubMed] [Google Scholar]
- 118.Favaro J.P., Borg K.T., Arrigo S.J., Schmidt M.G. Effect of Rev on the intranuclear localization of HIV-1 unspliced RNA. Virology. 1998;249:286–296. doi: 10.1006/viro.1998.9312. [DOI] [PubMed] [Google Scholar]
- 119.Zhang G., Zapp M.L., Yan G., Green M.R. Localization of HIV-1 RNA in mammalian nuclei. J. Cell Biol. 1996;135:9–18. doi: 10.1083/jcb.135.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pond S.J., Ridgeway W.K., Robertson R., Wang J., Millar D.P. HIV-1 Rev protein assembles on viral RNA one molecule at a time. Proc. Natl. Acad. Sci. USA. 2009;106:1404–1408. doi: 10.1073/pnas.0807388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cook K.S., Fisk G.J., Hauber J., Usman N., Daly T.J., Rusche J.R. Characterization of HIV-1 REV protein: Binding stoichiometry and minimal RNA substrate. Nucleic Acids Res. 1991;19:1577–1583. doi: 10.1093/nar/19.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Daly T.J., Cook K.S., Gray G.S., Maione T.E., Rusche J.R. Specific binding of HIV-1 recombinant Rev protein to the Rev-responsive element in vitro. Nature. 1989;342:816–819. doi: 10.1038/342816a0. [DOI] [PubMed] [Google Scholar]
- 123.Heaphy S., Dingwall C., Ernberg I., Gait M.J., Green S.M., Karn J., Lowe A.D., Singh M., Skinner M.A. HIV-1 regulator of virion expression (Rev) protein binds to an RNA stem-loop structure located within the Rev response element region. Cell. 1990;60:685–693. doi: 10.1016/0092-8674(90)90671-Z. [DOI] [PubMed] [Google Scholar]
- 124.Malim M.H., Tiley L.S., McCarn D.F., Rusche J.R., Hauber J., Cullen B.R. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990;60:675–683. doi: 10.1016/0092-8674(90)90670-A. [DOI] [PubMed] [Google Scholar]
- 125.Madore S.J., Tiley L.S., Malim M.H., Cullen B.R. Sequence requirements for Rev multimerization in vivo. Virology. 1994;202:186–194. doi: 10.1006/viro.1994.1334. [DOI] [PubMed] [Google Scholar]
- 126.Daugherty M.D., Liu B., Frankel A.D. Structural basis for cooperative RNA binding and export complex assembly by HIV Rev. Nat. Struct. Mol. Biol. 2010;17:1337–1342. doi: 10.1038/nsmb.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Daugherty M.D., Booth D.S., Jayaraman B., Cheng Y., Frankel A.D. HIV Rev response element (RRE) directs assembly of the Rev homooligomer into discrete asymmetric complexes. Proc. Natl. Acad. Sci. USA. 2010;107:12481–12486. doi: 10.1073/pnas.1007022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Daugherty M.D., D'Orso I., Frankel A.D. A solution to limited genomic capacity: Using adaptable binding surfaces to assemble the functional HIV Rev oligomer on RNA. Mol. Cell. 2008;31:824–834. doi: 10.1016/j.molcel.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DiMattia M.A., Watts N.R., Stahl S.J., Rader C., Wingfield P.T., Stuart D.I., Steven A.C., Grimes J.M. Implications of the HIV-1 Rev dimer structure at 3.2 A resolution for multimeric binding to the Rev response element. Proc. Natl. Acad. Sci. USA. 2010;107:5810–5814. doi: 10.1073/pnas.0914946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoffmann D., Schwark D., Banning C., Brennen M., Lakshmikanth M., Krepstakies M., Schindler M., Millar D.P., Hauber J. Formation of trans-activation competent HIV-1 Rev: RRE complexes requires the recruitment of multiple protein activation domains. PLoS One. 2012 doi: 10.1371/journal.pone.0038305. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robertson-Anderson R.M., Wang J., Edgcomb S.P., Carmel A.B., Williamson J.R., Millar D.P. Single-molecule studies reveal that DEAD box protein DDX1 promotes oligomerization of HIV-1 Rev on the Rev response element. J. Mol. Biol. 2011;410:959–971. doi: 10.1016/j.jmb.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grunwald D., Singer R.H. In vivo imaging of labelled endogenous beta-actin mRNA during nucleocytoplasmic transport. Nature. 2010;467:604–607. doi: 10.1038/nature09438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mor A., Shav-Tal Y. Dynamics and kinetics of nucleo-cytoplasmic mRNA export. Wiley Interdiscip Rev. RNA. 2010;1:388–401. doi: 10.1002/wrna.41. [DOI] [PubMed] [Google Scholar]
- 134.Müller B. Novel imaging technologies in the study of HIV. Future Virol. 2011;6:929–940. doi: 10.2217/fvl.11.66. [DOI] [Google Scholar]