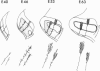
Abstract
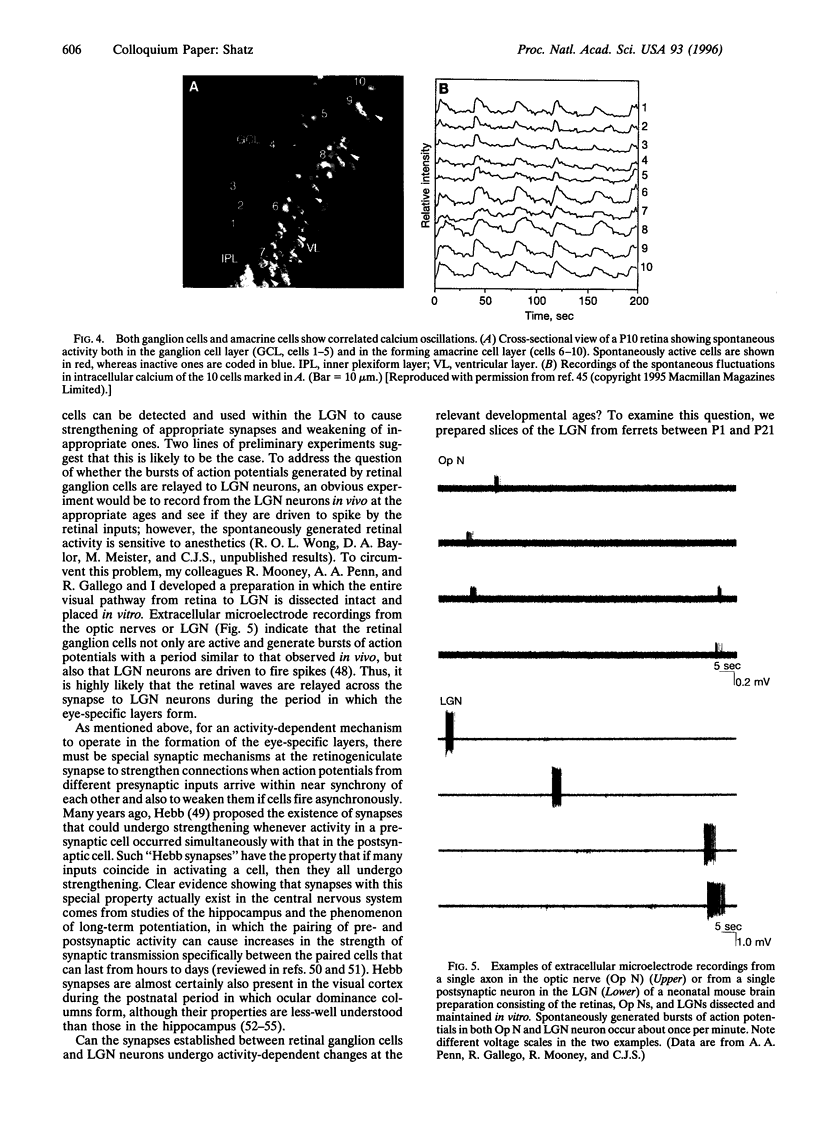
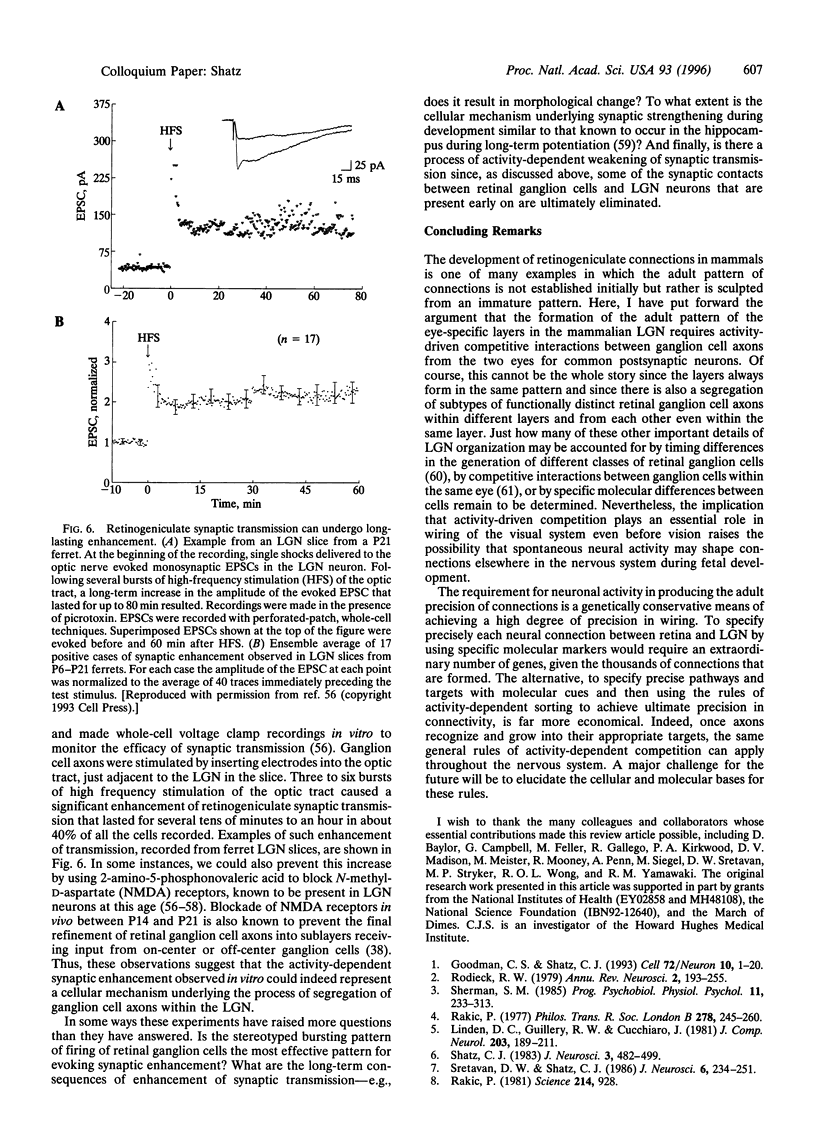
Neural connections in the adult central nervous system are highly precise. In the visual system, retinal ganglion cells send their axons to target neurons in the lateral geniculate nucleus (LGN) in such a way that axons originating from the two eyes terminate in adjacent but nonoverlapping eye-specific layers. During development, however, inputs from the two eyes are intermixed, and the adult pattern emerges gradually as axons from the two eyes sort out to form the layers. Experiments indicate that the sorting-out process, even though it occurs in utero in higher mammals and always before vision, requires retinal ganglion cell signaling; blocking retinal ganglion cell action potentials with tetrodotoxin prevents the formation of the layers. These action potentials are endogenously generated by the ganglion cells, which fire spontaneously and synchronously with each other, generating "waves" of activity that travel across the retina. Calcium imaging of the retina shows that the ganglion cells undergo correlated calcium bursting to generate the waves and that amacrine cells also participate in the correlated activity patterns. Physiological recordings from LGN neurons in vitro indicate that the quasiperiodic activity generated by the retinal ganglion cells is transmitted across the synapse between ganglion cells to drive target LGN neurons. These observations suggest that (i) a neural circuit within the immature retina is responsible for generating specific spatiotemporal patterns of neural activity; (ii) spontaneous activity generated in the retina is propagated across central synapses; and (iii) even before the photoreceptors are present, nerve cell function is essential for correct wiring of the visual system during early development. Since spontaneously generated activity is known to be present elsewhere in the developing CNS, this process of activity-dependent wiring could be used throughout the nervous system to help refine early sets of neural connections into their highly precise adult patterns.
Full text
PDF
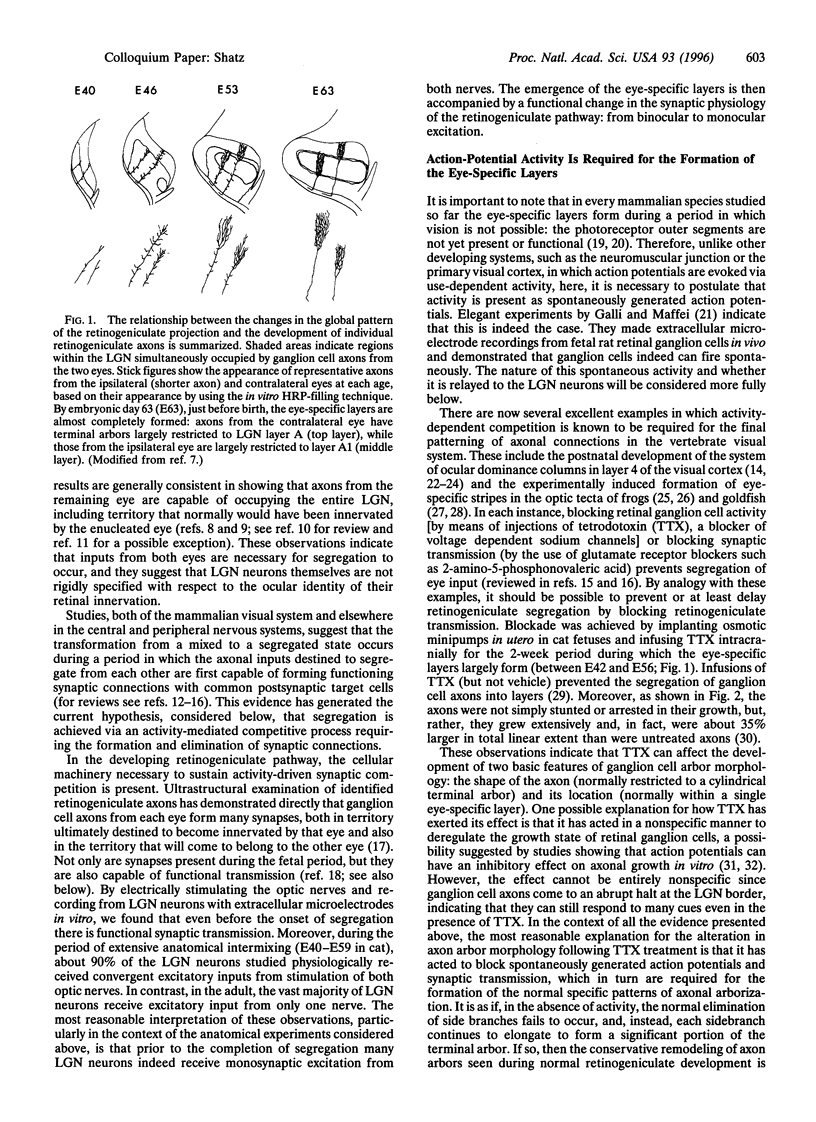
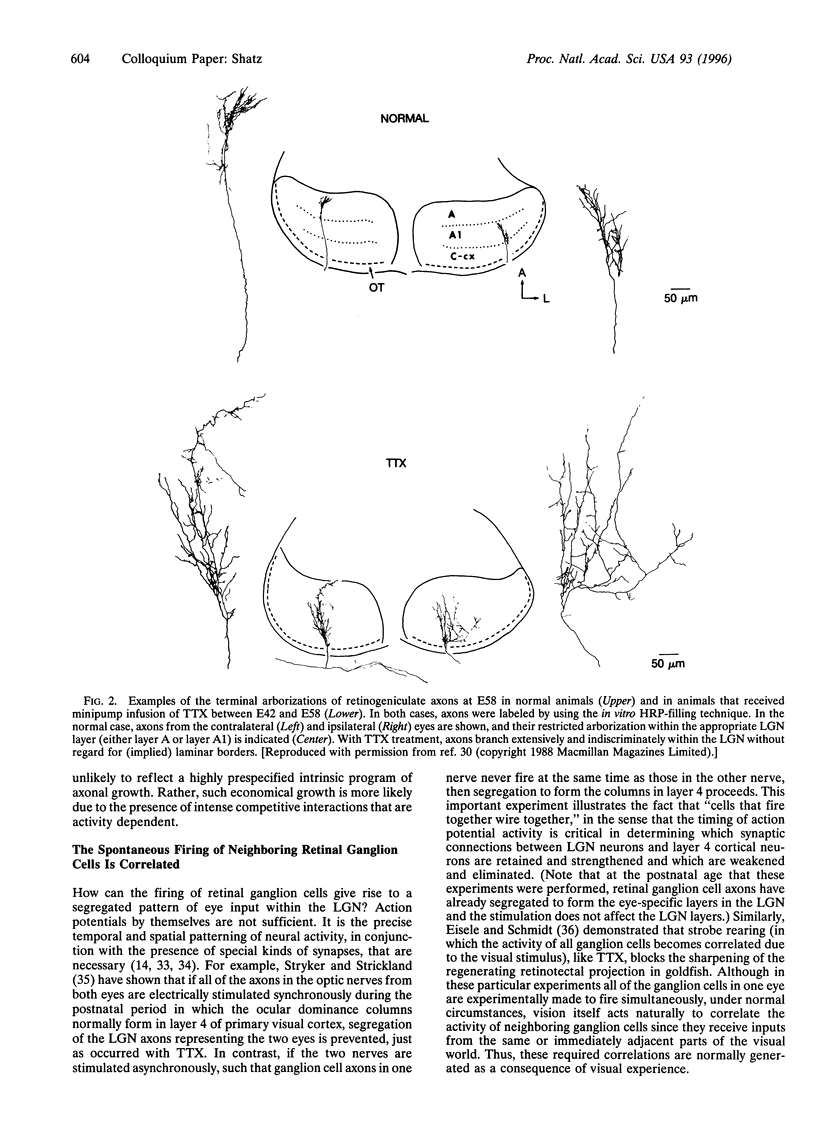
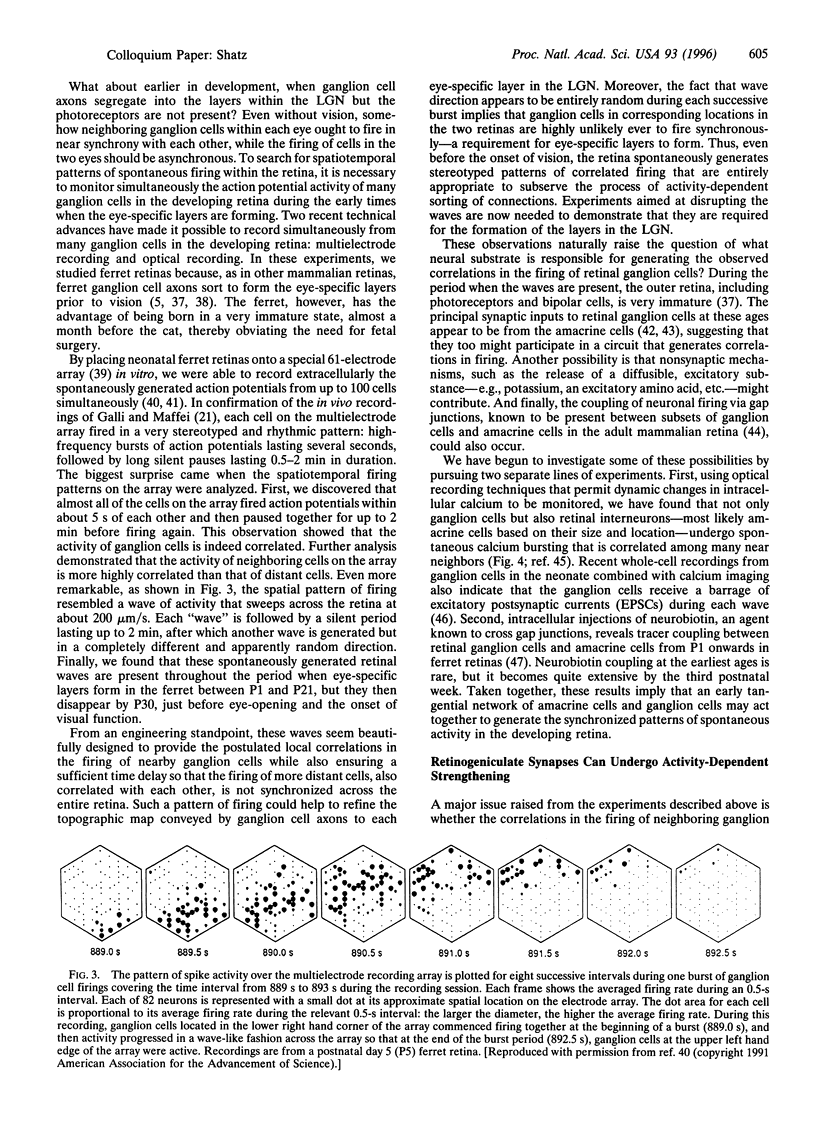

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalupa L. M., Williams R. W. Organization of the cat's lateral geniculate nucleus following interruption of prenatal binocular competition. Hum Neurobiol. 1984;3(2):103–107. [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan C. S., Kater S. B. Suppression of neurite elongation and growth cone motility by electrical activity. Science. 1986 Jun 27;232(4758):1638–1640. doi: 10.1126/science.3715470. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Donovan A. The postnatal development of the cat retina. Exp Eye Res. 1966 Oct;5(4):249–254. doi: 10.1016/s0014-4835(66)80034-9. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Stark L. A., Archer S. M. A role for action-potential activity in the development of neuronal connections in the kitten retinogeniculate pathway. J Neurosci. 1986 Apr;6(4):1021–1036. doi: 10.1523/JNEUROSCI.06-04-01021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra M., Kwon Y. H., Sur M. Retinogeniculate EPSPs recorded intracellularly in the ferret lateral geniculate nucleus in vitro: role of NMDA receptors. Vis Neurosci. 1992 Jun;8(6):545–555. doi: 10.1017/s0952523800005642. [DOI] [PubMed] [Google Scholar]
- Galli L., Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988 Oct 7;242(4875):90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Shatz C. J. Segregation of geniculocortical afferents during the critical period: a role for subplate neurons. J Neurosci. 1994 Jun;14(6):3862–3880. doi: 10.1523/JNEUROSCI.14-06-03862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W., LaMantia A. S., Robson J. A., Huang K. The influence of retinal afferents upon the development of layers in the dorsal lateral geniculate nucleus of mustelids. J Neurosci. 1985 May;5(5):1370–1379. doi: 10.1523/JNEUROSCI.05-05-01370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm J. O., Langdon R. B., Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991 Jun 13;351(6327):568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood A., Dudek S. M., Gold J. T., Aizenman C. D., Bear M. F. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993 Jun 4;260(5113):1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Fujii K., Maeda J., Sakaguchi H., Toyama K. Long-term potentiation of synaptic transmission in kitten visual cortex. J Neurophysiol. 1988 Jan;59(1):124–141. doi: 10.1152/jn.1988.59.1.124. [DOI] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Linden D. C., Guillery R. W., Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981 Dec 1;203(2):189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Maslim J., Stone J. Synaptogenesis in the retina of the cat. Brain Res. 1986 May 14;373(1-2):35–48. doi: 10.1016/0006-8993(86)90313-6. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Correlated firing of retinal ganglion cells. Trends Neurosci. 1989 Feb;12(2):75–80. doi: 10.1016/0166-2236(89)90140-9. [DOI] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Meyer R. L. Tetrodotoxin blocks the formation of ocular dominance columns in goldfish. Science. 1982 Nov 5;218(4572):589–591. doi: 10.1126/science.7123262. [DOI] [PubMed] [Google Scholar]
- Miller K. D., Keller J. B., Stryker M. P. Ocular dominance column development: analysis and simulation. Science. 1989 Aug 11;245(4918):605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- Mooney R., Madison D. V., Shatz C. J. Enhancement of transmission at the developing retinogeniculate synapse. Neuron. 1993 May;10(5):815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science. 1981 Nov 20;214(4523):928–931. doi: 10.1126/science.7302569. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Reh T. A., Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985 May;5(5):1132–1143. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Visual pathways. Annu Rev Neurosci. 1979;2:193–225. doi: 10.1146/annurev.ne.02.030179.001205. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Sretavan D. W. Interactions between retinal ganglion cells during the development of the mammalian visual system. Annu Rev Neurosci. 1986;9:171–207. doi: 10.1146/annurev.ne.09.030186.001131. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988 Oct 7;242(4875):87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulz D., Frégnac Y. Cellular analogs of visual cortical epigenesis. II. Plasticity of binocular integration. J Neurosci. 1992 Apr;12(4):1301–1318. doi: 10.1523/JNEUROSCI.12-04-01301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci. 1986 Jan;6(1):234–251. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J., Stryker M. P. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988 Dec 1;336(6198):468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C., Polley E. H., Hickey T. L., Guillery R. W. Generation of cat retinal ganglion cells in relation to central pathways. Nature. 1983 Apr 14;302(5909):611–614. doi: 10.1038/302611a0. [DOI] [PubMed] [Google Scholar]
- White C. A., Sur M. Membrane and synaptic properties of developing lateral geniculate nucleus neurons during retinogeniculate axon segregation. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9850–9854. doi: 10.1073/pnas.89.20.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willshaw D. J., von der Malsburg C. How patterned neural connections can be set up by self-organization. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):431–445. doi: 10.1098/rspb.1976.0087. [DOI] [PubMed] [Google Scholar]
- Wong R. O. L., Yamawaki R. M., Shatz C. J. Synaptic Contacts and the Transient Dendritic Spines of Developing Retinal Ganglion Cells. Eur J Neurosci. 1992;4(12):1387–1397. doi: 10.1111/j.1460-9568.1992.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Wong R. O., Chernjavsky A., Smith S. J., Shatz C. J. Early functional neural networks in the developing retina. Nature. 1995 Apr 20;374(6524):716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wong R. O., Meister M., Shatz C. J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993 Nov;11(5):923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]