Abstract
We present a case of congenital malignant melanoma of the scalp in a neonate. The child was born through caesarean section with a swelling, the size of a tennis ball, on the posterior scalp. At presentation to the clinic at 25 days after birth, the swelling had significantly increased in size and ulcerated. An excision was carried out but, because of extensive haemorrhage and haemodynamic instability, the procedure was limited to subtotal resection. Later on, completion of the excision and flap coverage of the wound were performed. After an initial stable course of a few months, the child came back with local recurrence. A re-excision was planned but the child developed pneumonia resulting in sepsis leading to the demise of the child. The report adds to the literature by describing a rare entity and challenges of managing large vascular scalp lesions with complete excision and defect coverage.
Background
Congenital malignant melanomas (CMM) are a rare pathology with poor outcomes. There are three different types of CMM based on aetiology: (1) transplacental—placental metastasis from a mother with melanoma; (2) degenerative—melanoma arising from a precancerous melanocytic naevus; (3) de novo—primary CMM developing in utero.1
We are reporting this case as a primary CMM of the scalp—a condition that has been reported only twice in the literature to the best of our knowledge. The majority of melanoma cases are discovered after the age of 20. Within the prepubertal group, the cases of malignant melanoma most often develop from degeneration of a naevus. There have been few cases of CMM developing de novo and present since birth.
Our patient went through a difficult course with two surgeries, tumour regrowth and eventual mortality within a year. The case was challenging due to the large size of the lesion, low birth weight, high vascularity of the mass and limited tissue coverage options.
Case presentation
A neonate, from a remote village of our province, was brought to us on his 25th day of life with a large, ulcerated and discharging mass over the occipital region of his scalp. The lesion had been present since birth with a progressive increase in size. Born weighing 2.5 kg and full-term, this male neonate was the first of a consanguineous marriage. He was delivered through caesarean section and the abnormality was identified and documented at the time. The size of the lesion at birth was that of a tennis ball. The mother had little to no history of antenatal screening. However, there was no history of antenatal infection or radiation exposure. The patient was otherwise healthy with no history of fever or seizures. There was no major difficulty in feeding and the child's vaccination schedule was up to date. Family history did not bring forth other significant findings.
On examination, the swelling was hard, non-tender and black in colour. Dehiscence was present. Serous fluid and blood was oozing out of the ulcerations. There were no lymph nodes palpable and the liver and spleen were not palpable either. The rest of the examination was within normal limits.
Our initial clinical diagnosis was a dermoid cyst.
Investigations
MRI
Through MRI, a large and well-defined mass of heterogeneous signal intensity was seen arising from the occipital region in the midline measuring 6.7×6.3×5.3 cm in craniocaudal, anterioposterior and transverse dimensions, respectively. This mass showed hyperintense signals on T1-weighted images (figure 1) with heterogeneous enhancement on postcontrast images and heterogeneous signal on T2-weighted images (figure 2). This was suggestive of a fat containing lesion, most likely a dermoid cyst. In addition, central hyper-intense signals on T2-weighted images were also identified which appeared hypointense on T1-weighted images and showed diffusion restriction, suggestive of central necrosis. The rest of the scan was normal with no herniation, haemorrhage, infarction or mass effect.
Figure 1.
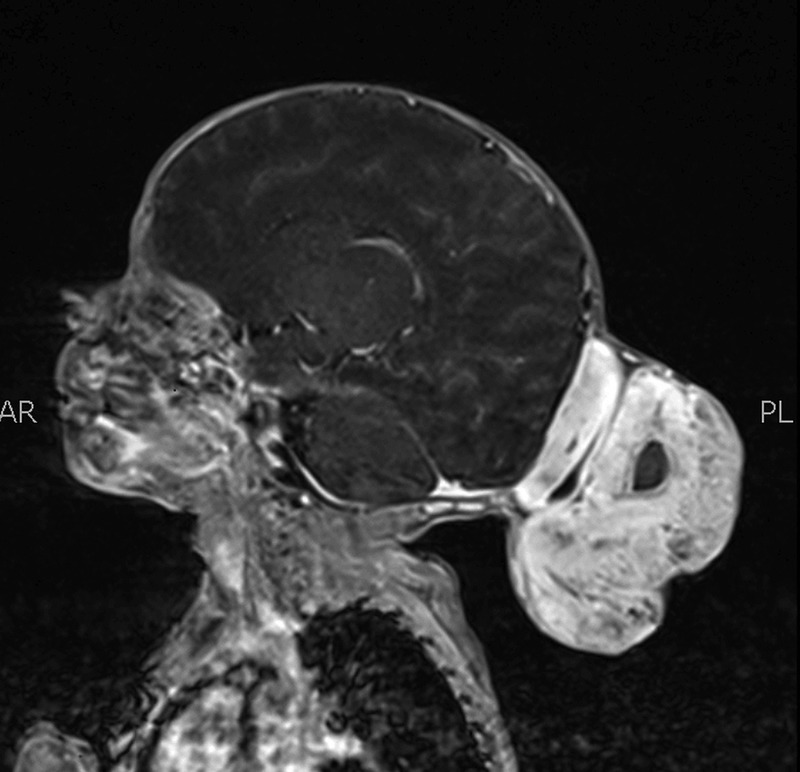
T1-weighted MRI with contrast showing hyperintensity and heterogenous enhancement of tumour with hypointense necrotic centre.
Figure 2.
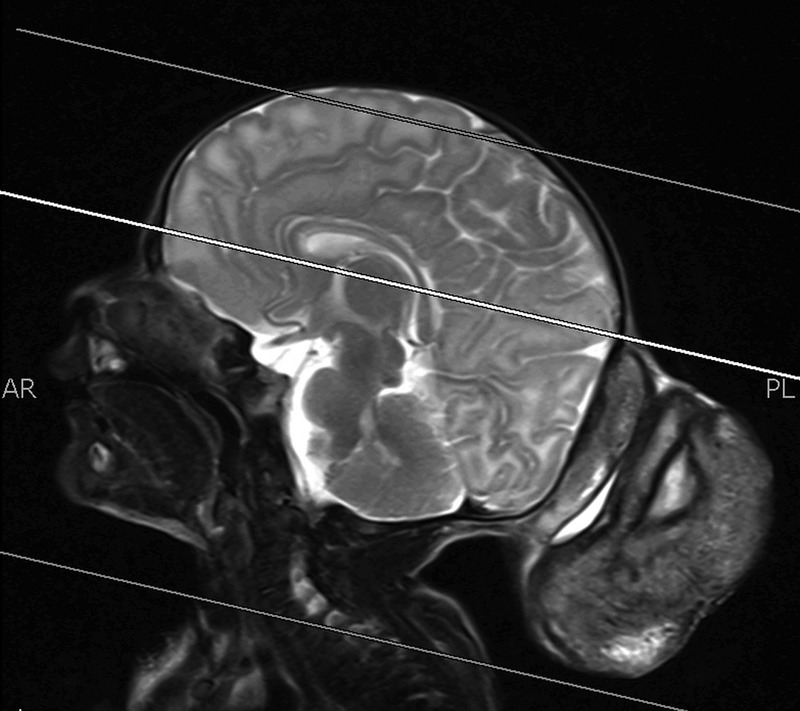
T2-weighted MRI showing heterogeneous signal and hyperintense centre.
Histopathology
Gross examination of tumour samples showed grey brown pieces of tissue covered with skin with the cut surface indicating areas of haemorrhage. There was also bone from the occipital region, grey brown and soft.
Microscopic histopathology (figures 3 and 4) of certain sections of the dermis discovered lesions composed of sheets of clusters of atypical cells containing pleomorphic nuclei with round, small nucleoli and eosinophilic cytoplasm. Dense brown pigment was also noted. These findings indicated a melanocytic lesion. Additional sections revealed a neoplastic lesion arranged in clusters and sheets. The epithelioid-shaped neoplastic cells had pleomorphic hyperchromatic nuclei with prominent eosinophilic nucleoli and abundant eosinophilic cytoplasm. Brisk mitotic activity was seen. Brown melanin pigment was also identified. A panel of immunohistochemical stains was performed (figures 5 and 6) and was positive for S-100 and HMB-45 markers. Markers such as Melan-A, epithelial membrane antigen, cytoleratin AE1/cytokeratin AE3, CD56, MIC-2 (cluster determinant 99) and glial fibrillary acidic protein were negative. These features favoured CMM.
Figure 3.
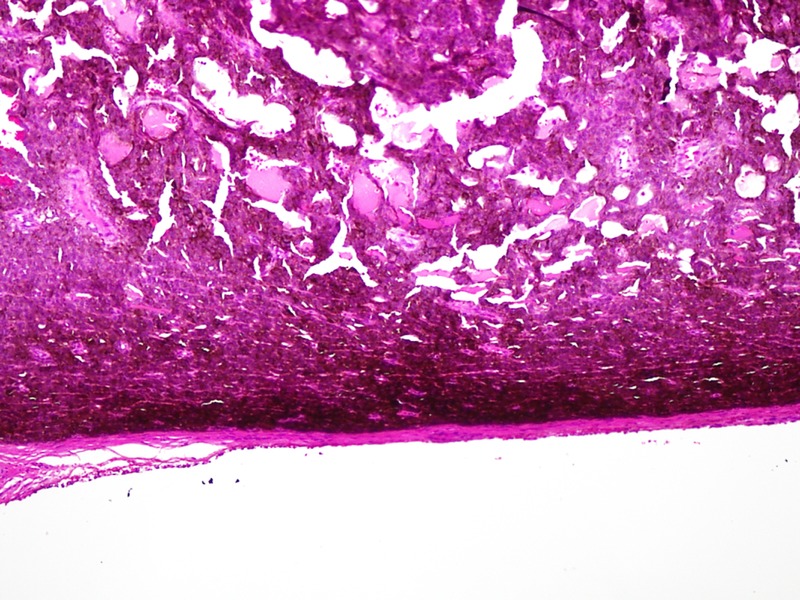
H&E staining of tumour cells (×200).
Figure 4.
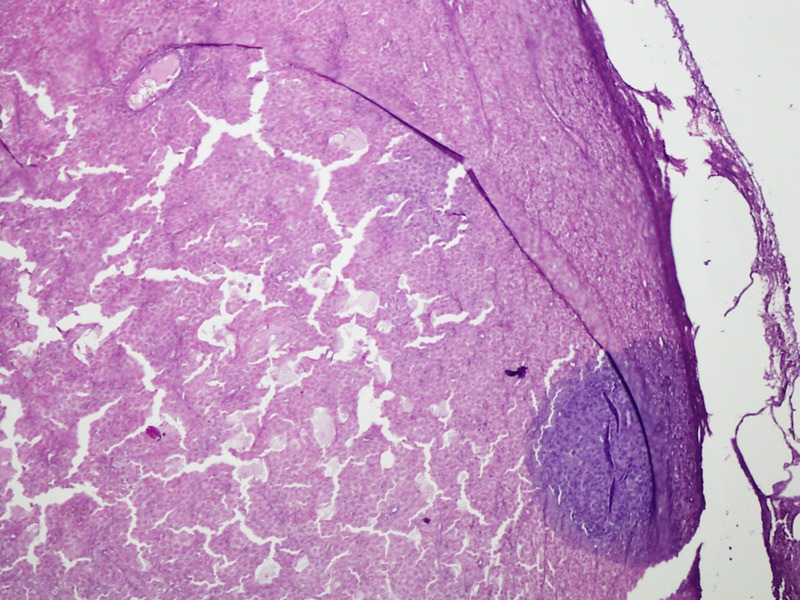
H&E staining of tumour cells bleached (×200).
Figure 5.
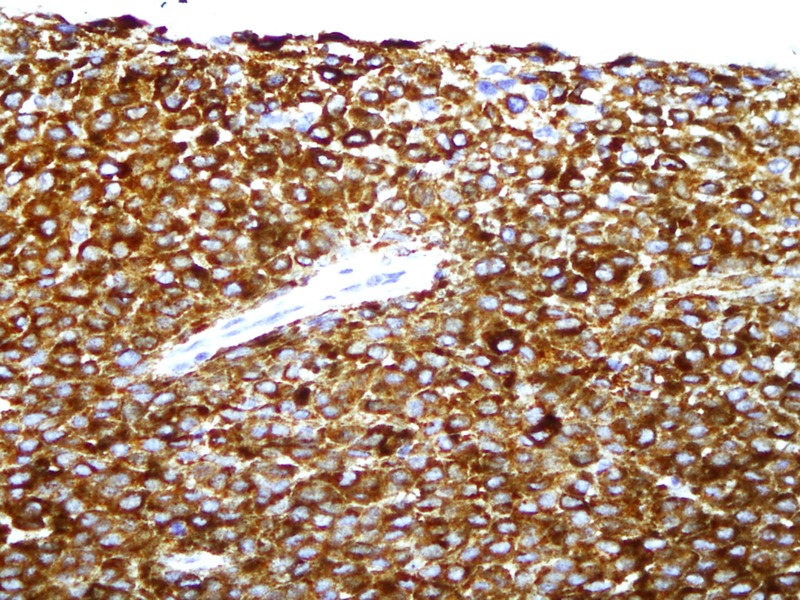
Immunohistochemistry; HMB-45 expression in tumour cells, magnified ×400.
Figure 6.
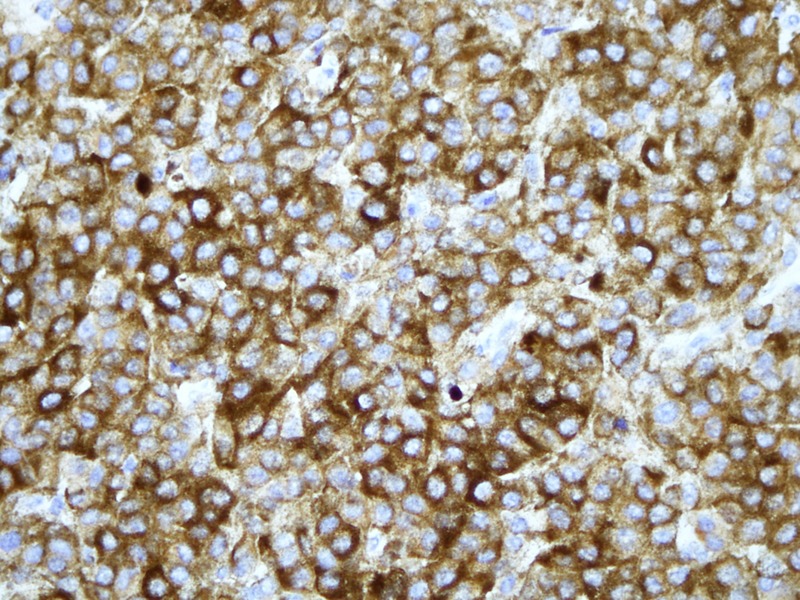
Immunohistochemistry; S-100 expression in tumour cells, magnified ×400.
There was no convincing evidence of tumour infiltration into the bone although it did reach the bone surface. Tumour margins could not be identified as the tissue samples were not large or comprehensive enough.
CT
CT of the chest, abdomen and pelvis and bone marrow biopsy were negative for any evidence of metastatic disease.
Differential diagnosis
Because of the age of the patient and location of the mass, dermoid cyst seemed to be the likely diagnosis. There were also areas of pigmentation which brought up the suspicion of a melanocytic naevus. Other possible but rare diagnoses included teratoma and rhabdomyosarcoma.
Treatment
The case was discussed in a multidisciplinary tumour board. The mother was screened for primary/metastatic disease. The patient was prepared for operation (figure 7). A complete resection was attempted but because of intraoperative haemodynamic instability the procedure was limited to a subtotal resection. The blood loss was so extensive and sudden that CPR had to be performed. Surgery was abandoned and the patient was transferred to the intensive care unit. After a few days of a stable course, the patient was discharged. Intraoperatively, the tumour was found to reach the bony surface but did not penetrate the bone.
Figure 7.
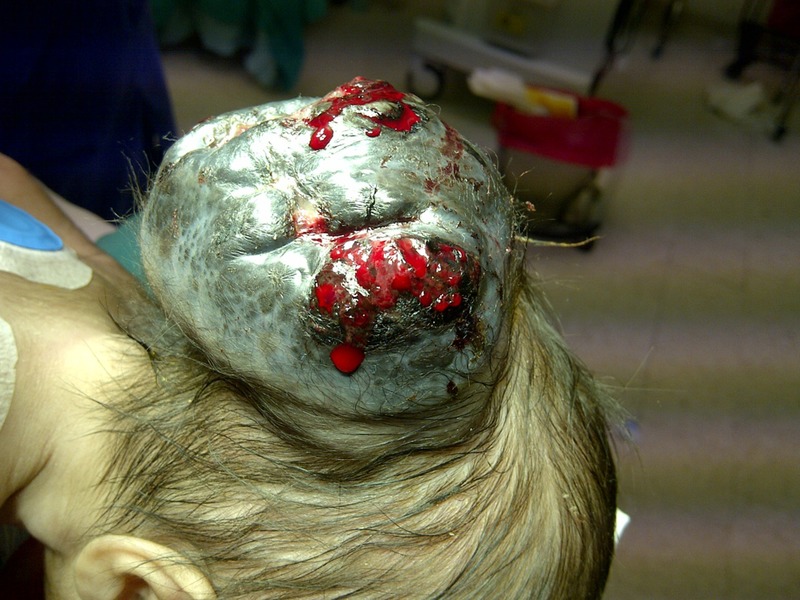
Preoperative image of the patient lying prone with visible bleeding from large posterior scalp mass.
The patient was readmitted for the second stage of the procedure after 1 month. Complete resection of the gross lesion was performed and a right superficial temporal artery transposition flap was performed to cover the tissue defect.
Six months later the patient returned with a local non-healing ulcer. The case was again discussed in a tumour board meeting. A re-excision biopsy was decided upon but the child did not return and according to the family expired of a respiratory illness.
Discussion
Little has been published on this rare condition specifically in children. The rarity is evidenced by a review in 1975,2 which found that melanomas occurred in childhood only 0.3–0.4% of the time. As described earlier, there are three different types of CMM based on aetiology: (1) transplacental; (2) degenerative and (3) de novo.
Many of the case reports of malignant melanomas that are found in children are those that develop through degeneration of a previous melanocytic naevus.1 3–5 These reports describe young patients (from 4 months to 2 years) who were initially diagnosed with a dark skin lesion which showed histopathological evidence for a naevus. These lesions then became cancerous and malignant. At times it is difficult to tell whether the melanoma is from a precancerous lesion or arose congenitally de novo and in utero. A case report described in 19906 investigated a premature baby at birth (pregnancy interrupted at 29th week of gestation) with a large mass in the occipital and neck regions. The mass was dark and histologically showed malignancy but also areas depicting melanocytic naevi. It was difficult to conclude whether the malignancy developed de novo or whether the naevi degenerated.
There have been multiple cases reported of de novo tumour origins as well. These cases and their prognosis have been covered in earlier case reports and reviews.1 7 There have been two de novo CMM cases of the scalp,8 9 one of which was observed to die within 5 months after diagnosis.8 Other case report a 6-week-old girl with a CMM in the mid-epigastric region7 from birth which was operated and the child was well and alive 1 year after. A similar report in 199110 was of a child diagnosed with CMM on the thigh since birth. This child passed away at 18 months with multiple metastases.
Treatment of CMMs is generally limited to excision with wide margins of the tumour and there has been little role of any other complementary method. For coverage of the defect, while we attempted a skin flap from the superficial temporal artery territory, another report used a skin graft.3 Their case, arising from a melanocytic naevus with no recurrence 43 months after, showed good result with the graft and the alopecic scar was also treated with cutaneous expansion.
Our case presented with a CMM from birth that progressively increased in size. Based on this history of a large mass on the scalp from birth and an absence of malignancy in the mother during gestation, it is most likely the neonate developed the malignancy de novo and in utero. The child was operated twice and then presented with recurrence 6 months later. Unfortunately, on recommendation for reoperation, the child did not return and passed away due to complications of pneumonia and sepsis.
Learning points.
Congenital malignant melanoma is rare and carries a poor outcome. It should therefore be considered as one of the differentials of congenital scalp swellings.
Antenatal screening can play a crucial role in the early detection and management since the tumour has a rapid spread.
Superficial temporal artery transposition flap is a viable option for coverage of skin defects on the scalp.
Acknowledgments
The authors would like to thank Dr Sabeeh-ud-din, Department of Pathology, Aga Khan University Hospital.
Footnotes
Contributors: Based on the International Committee of Medical Journal Editors Recommendations for the Conduct, all the authors were involved in the reporting, editing and publication of scholarly work in medical journals and meet the four criteria.
Competing interests: None.
Patient consent: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Asai J, Takenaka H, Ikada S, et al. Congenital malignant melanoma: a case report. Br J Dermatol 2004;151:693–7 [DOI] [PubMed] [Google Scholar]
- 2.Trozak DJ, Rowland WD, Hu F. Metastatic malignant melanoma in prepubertal children. Pediatrics 1975;55:191–204 [PubMed] [Google Scholar]
- 3.Galinier P, Bouali O, Lamant L, et al. Malignant melanoma on congenital naevus: a case of degeneration in a 6-month-old child with severe histological criteria. J Plast Reconstr Aesthet Surg 2009;62:96–7 [DOI] [PubMed] [Google Scholar]
- 4.Padilla RS, McConnell TS, Gribble JT, et al. Malignant melanoma arising in a giant congenital melanocytic nevus. A case report with cytogenetic and histopathologic analyses. Cancer 1988;62:2589–94 [DOI] [PubMed] [Google Scholar]
- 5.Singh K, Moore S, Sandoval M, et al. Congenital malignant melanoma: a case report with cytogenetic studies. Am J Dermatopathol 2013;35:e135–8 [DOI] [PubMed] [Google Scholar]
- 6.Song KY, Song HG, Chi JG, et al. Congenital malignant melanoma—a case report. J Korean Med Sci 1990;5:91–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prose NS, Laude TA, Heilman ER, et al. Congenital malignant melanoma. Pediatrics 1987;79:967–70 [PubMed] [Google Scholar]
- 8.Coe H. Malignant pigmented mole in an infant. Northwest Med 1925;24(April):181–2 [Google Scholar]
- 9.Pratt CB, Palmer MK, Thatcher N, et al. Malignant melanoma in children and adolescents. Cancer 1981;47:392–7 [DOI] [PubMed] [Google Scholar]
- 10.Ishii N, Ichiyama S, Saito S, et al. Congenital malignant melanoma. Br J Dermatol 1991;124:492–4 [DOI] [PubMed] [Google Scholar]


