Abstract
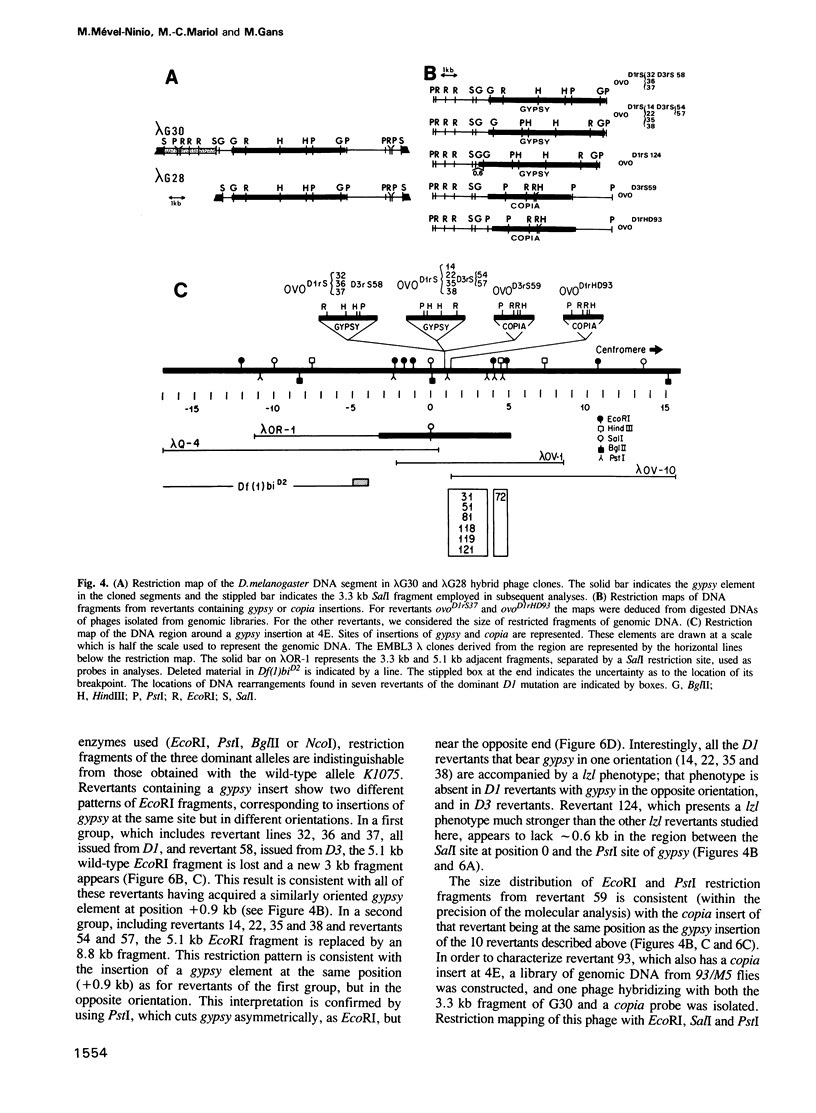
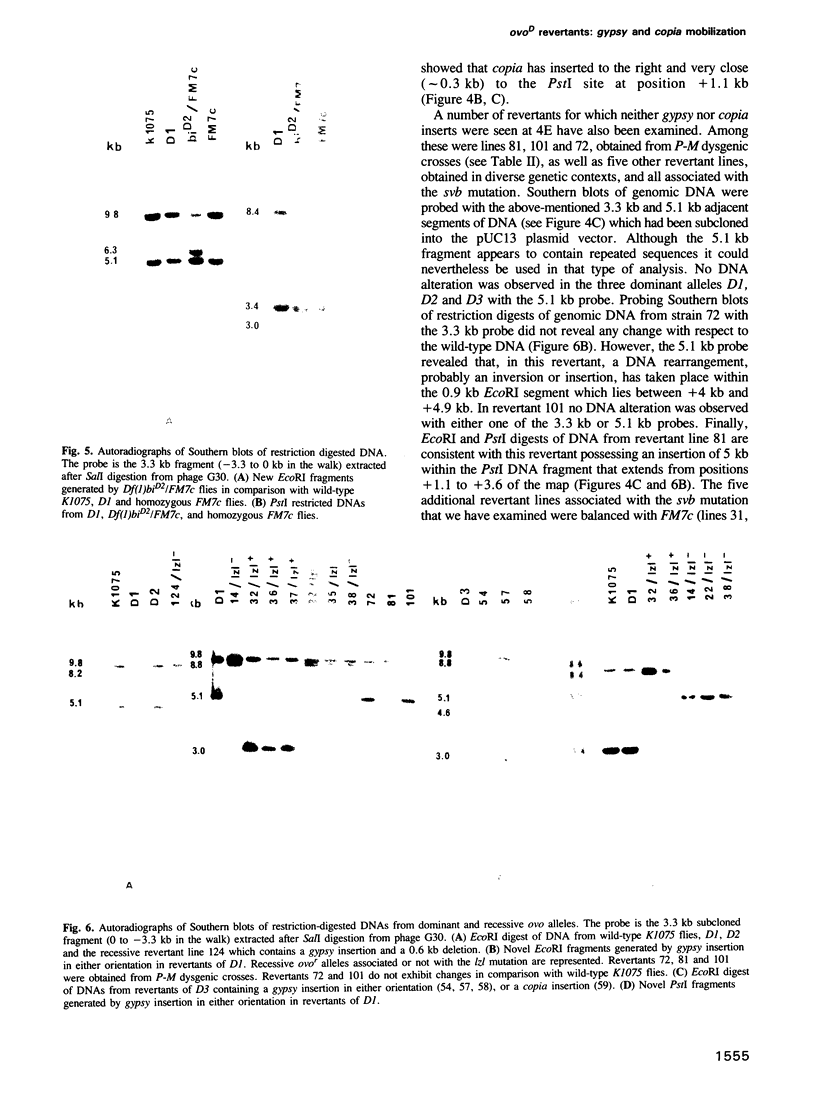
The ovo locus is required for the maintenance of the female germ line in Drosophila melanogaster. In the absence of an ovo+ gene, males are completely normal but females have no germ-line stem cells. Three dominant mutations at the ovo locus, called ovoD, were observed to revert towards recessive alleles at high frequency when ovoD males were crossed to females of the strain y v f mal. We have found that this strain contains an inordinately high number of gypsy transposable elements, and crossing it with the ovoD strains results in the mobilization of both gypsy and copia, with high-frequency insertions into the ovo locus: of 16 revertants examined 12 have gypsy and four have copia inserted at 4E, the ovo cytological site. Using gypsy DNA as a tag we have cloned 32 kb of wild-type DNA sequences surrounding a gypsy insertion and characterized molecular rearrangements in several independent revertants: in 10 of them gypsy appears to be inserted into the same site. The orientation of gypsy is strictly correlated with whether the neighbouring lozenge-like mutation appears in the revertants. A distal limit of the ovo locus was molecularly determined from the breakpoint of a deletion affecting closely flanking regions.
Keywords: Drosophila, ovoD reversion, gypsy mobilization, shavenbaby
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga S. S., Bloomquist B. T., Brodberg R. K., Pye Q. N., Larrivee D. C., Mason J. M., Boyd J. B., Pak W. L. Cytogenetic characterization of the 4BC region on the X chromosome of Drosophila melanogaster: localization of the mei-9, norpA and omb genes. Chromosoma. 1986;93(4):341–346. doi: 10.1007/BF00327593. [DOI] [PubMed] [Google Scholar]
- Bayev A. A., Jr, Lyubomirskaya N. V., Dzhumagaliev E. B., Ananiev E. V., Amiantova I. G., Ilyin Y. V. Structural organization of transposable element mdg4 from Drosophila melanogaster and a nucleotide sequence of its long terminal repeats. Nucleic Acids Res. 1984 Apr 25;12(8):3707–3723. doi: 10.1093/nar/12.8.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Grimaila R., Koehler M. M., Gelbart W. M. Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell. 1987 May 22;49(4):497–505. doi: 10.1016/0092-8674(87)90452-1. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. Ecdysone-stimulated RNA synthesis in imaginal discs of Drosophila melanogaster. Assay by in situ hybridization. Chromosoma. 1976 Oct 12;58(1):87–99. doi: 10.1007/BF00293443. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
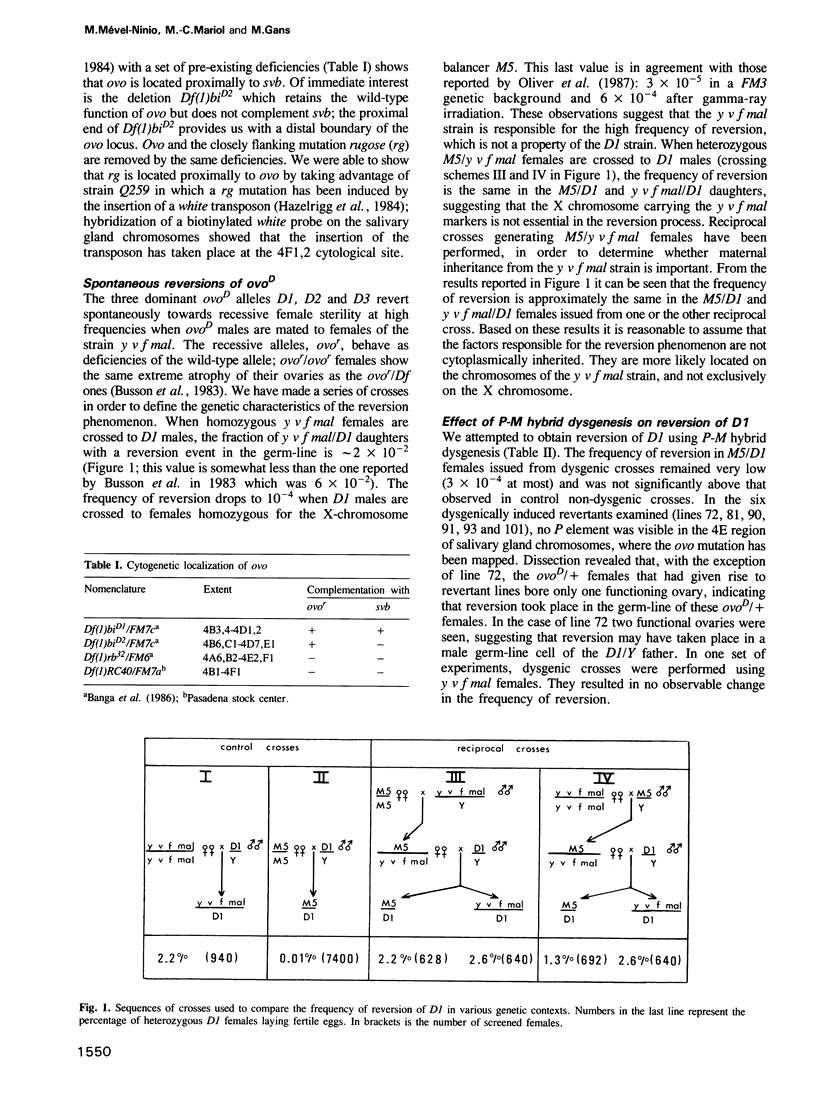
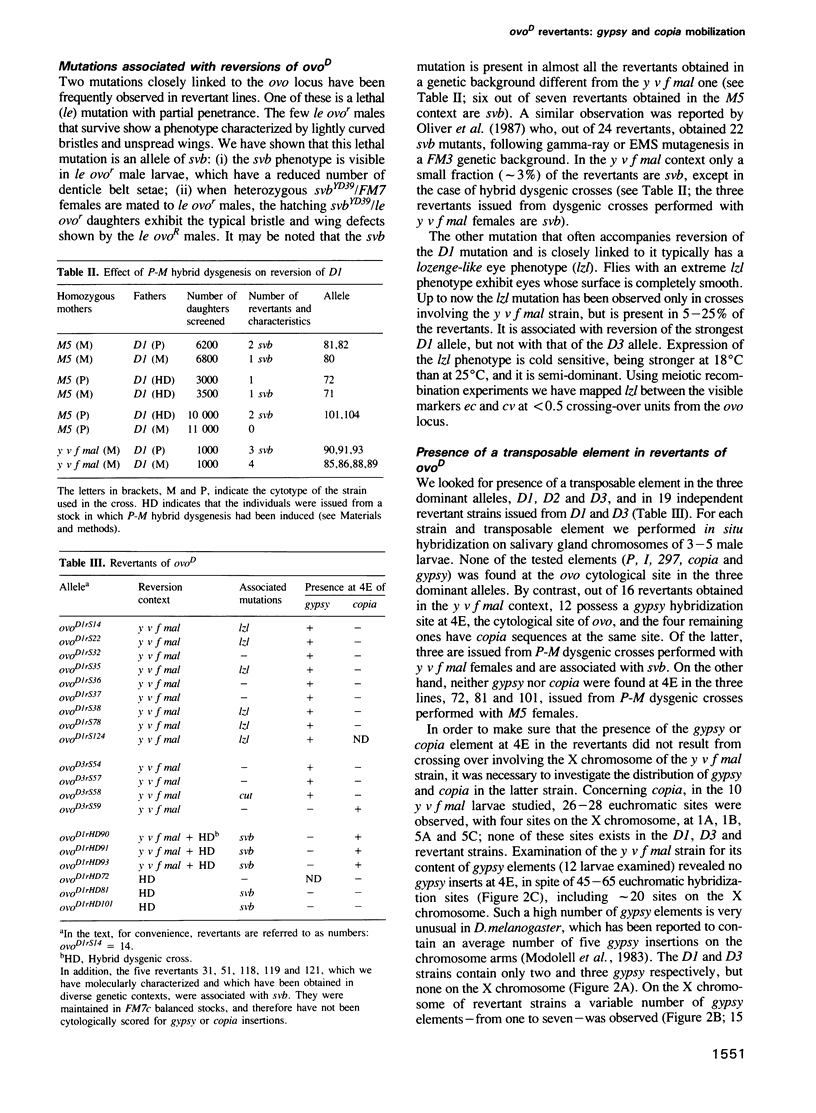
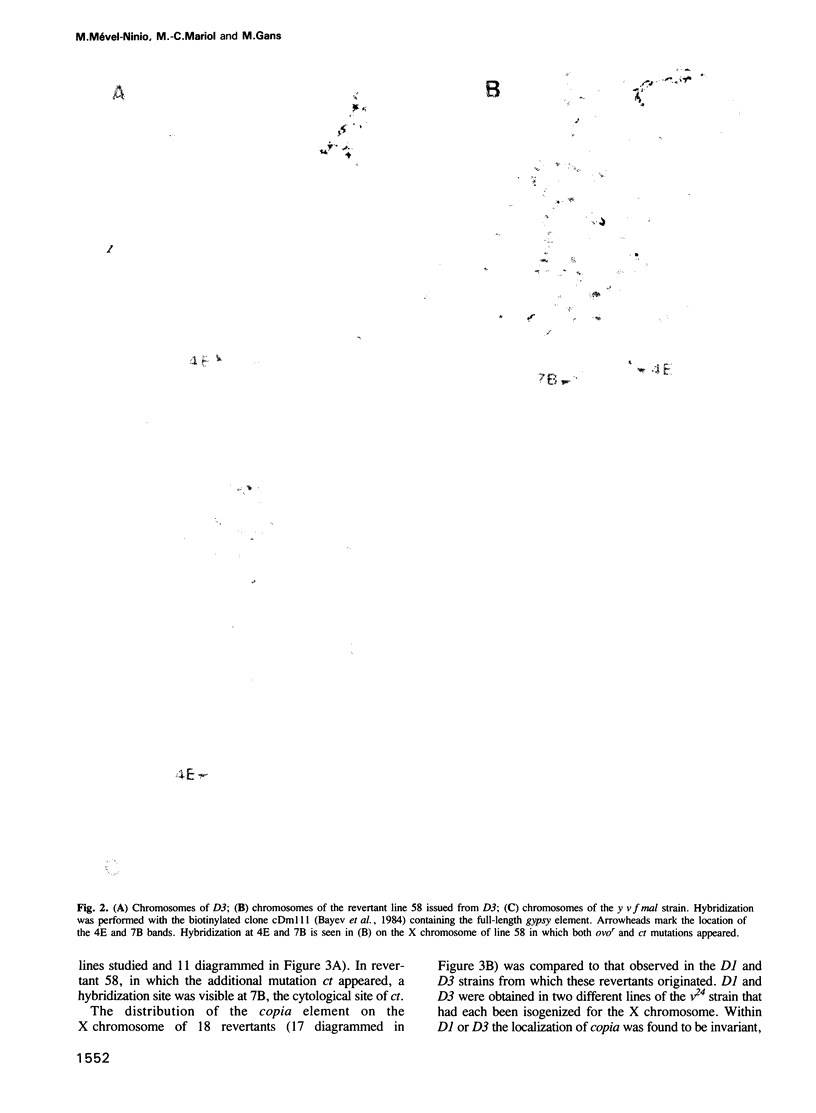
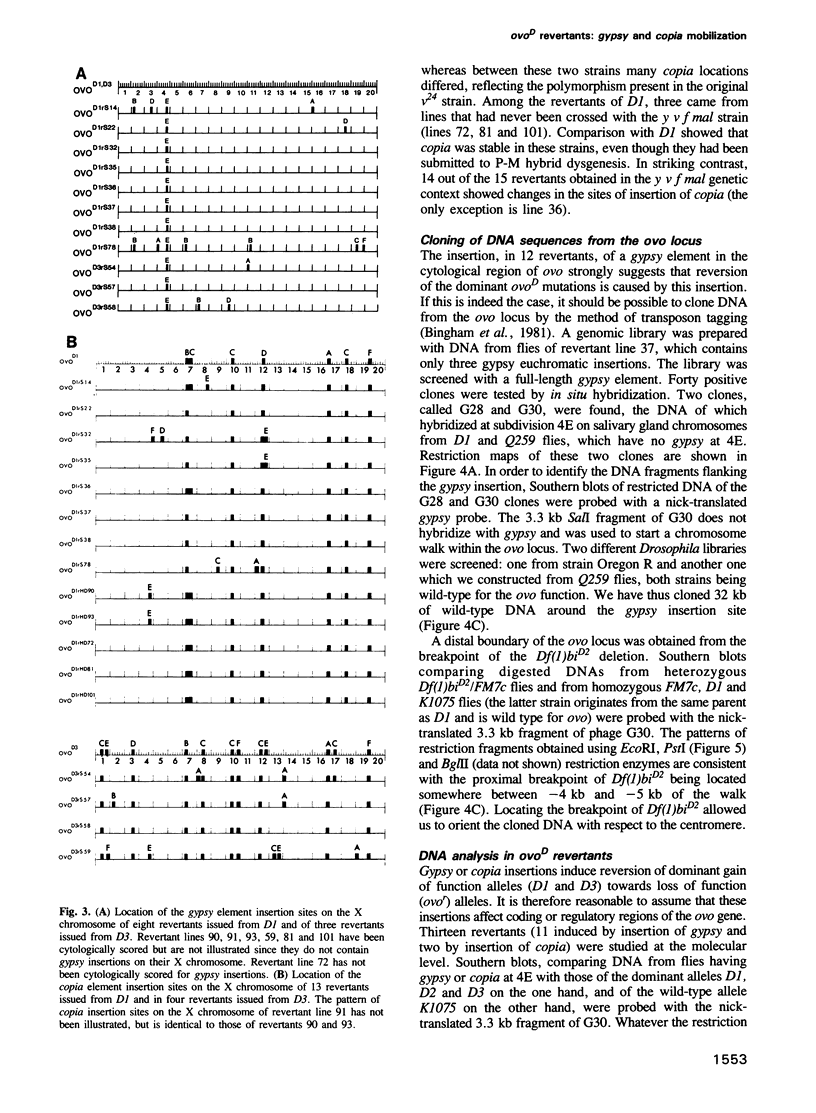
- Busson D., Gans M., Komitopoulou K., Masson M. Genetic Analysis of Three Dominant Female-Sterile Mutations Located on the X Chromosome of DROSOPHILA MELANOGASTER. Genetics. 1983 Oct;105(2):309–325. doi: 10.1093/genetics/105.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano S., Balcells L., Villares R., Carramolino L., García-Alonso L., Modolell J. Excess function hairy-wing mutations caused by gypsy and copia insertions within structural genes of the achaete-scute locus of Drosophila. Cell. 1986 Jan 31;44(2):303–312. doi: 10.1016/0092-8674(86)90764-6. [DOI] [PubMed] [Google Scholar]
- Dunsmuir P., Brorein W. J., Jr, Simon M. A., Rubin G. M. Insertion of the Drosophila transposable element copia generates a 5 base pair duplication. Cell. 1980 Sep;21(2):575–579. doi: 10.1016/0092-8674(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Freund R., Meselson M. Long terminal repeat nucleotide sequence and specific insertion of the gypsy transposon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4462–4464. doi: 10.1073/pnas.81.14.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans M., Audit C., Masson M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 1975 Dec;81(4):683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimova T. I., Matjunina L. V., Mizrokhi L. J., Georgiev G. P. Successive transposition explosions in Drosophila melanogaster and reverse transpositions of mobile dispersed genetic elements. EMBO J. 1985 Dec 30;4(13B):3773–3779. doi: 10.1002/j.1460-2075.1985.tb04147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Spana C., Corces V. G. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 1986 Oct;5(10):2657–2662. doi: 10.1002/j.1460-2075.1986.tb04548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrigg T., Levis R., Rubin G. M. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984 Feb;36(2):469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Komitopoulou K., Gans M., Margaritis L. H., Kafatos F. C., Masson M. Isolation and Characterization of Sex-Linked Female-Sterile Mutants in DROSOPHILA MELANOGASTER with Special Attention to Eggshell Mutants. Genetics. 1983 Dec;105(4):897–920. doi: 10.1093/genetics/105.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Bingham P. M., Rubin G. M. Physical map of the white locus of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Jan;79(2):564–568. doi: 10.1073/pnas.79.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Bender W., Meselson M. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobase mobile element. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1678–1682. doi: 10.1073/pnas.80.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B., Perrimon N., Mahowald A. P. The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes Dev. 1987 Nov;1(9):913–923. doi: 10.1101/gad.1.9.913. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Forked, gypsys, and suppressors in Drosophila. Cell. 1985 Jun;41(2):429–437. doi: 10.1016/s0092-8674(85)80016-7. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Woodruff R. C., Blount J. L., Thompson J. N., Jr Hybrid dysgenesis in D. melanogaster is not a general release mechanism for DNA transpositions. Science. 1987 Sep 4;237(4819):1206–1218. doi: 10.1126/science.2820057. [DOI] [PubMed] [Google Scholar]
- Yannopoulos G., Stamatis N., Monastirioti M., Hatzopoulos P., Louis C. hobo is responsible for the induction of hybrid dysgenesis by strains of Drosophila melanogaster bearing the male recombination factor 23.5MRF. Cell. 1987 May 22;49(4):487–495. doi: 10.1016/0092-8674(87)90451-x. [DOI] [PubMed] [Google Scholar]






