Abstract
Background
Enteric fever caused by Salmonella enterica serovar Typhi (S. Typhi) is an important public health problem in developing countries like India.1 The emergence of resistance to fluoroquinolones has reduced the therapeutic options available. Currently, the uniform laboratory interpretation of ciprofloxacin and azithromycin susceptibility remains unclear.
Aims
To study the antibiogram of S. Typhi isolates with special emphasis on in-vitro activity of ciprofloxacin and azithromycin.
Method
We evaluated the antimicrobial susceptibility pattern of 16 S. Typhi isolates from January 2012 to June 2013. We also determined by Epsilometer-test (E-test) method, the minimum inhibitory concentration (MIC) of ciprofloxacin and azithromycin against these isolates and compared them with their corresponding disc diffusion sizes.
Results
Fifteen (93.75 per cent) isolates were sensitive to chloramphenicol, 14 (87.5 per cent) were sensitive to cotrimoxazole. All isolates were resistant to nalidixic acid. MICs for ciprofloxacin ranged from 6μg/ml to 15μg/ml and corresponding zone diameters ranged from 15mm to 26mm. MIC and zone diameters for ciprofloxacin had significant negative correlation. MICs for azithromycin ranged from 3μg/ml to 24μg/ml, corresponding zone diameters ranged from 13mm to 19mm. However, MIC and zone diameters for azithromycin had no significant negative correlation.
Conclusion
The widespread emergence of resistance to fluoroquinolones and reappearance of sensitivity to firstline drugs has reinforced the need for antibiotic recycling. There is a need to have uniform laboratory testing guidelines for testing susceptibility to ciprofloxacin and azithromycin for S. Typhi isolates.
Keywords: Antibiogram, ciprofloxacin, azithromycin, minimum inhibitory concentrations, S. Typhi
What this study adds:
-
What is known about this subject?
There is a changing trend in the susceptibility pattern of S. Typhi worldwide with emerging resistance to fluoroquinolones.
-
What new information is offered in this study?
There was reappearance of sensitivity to first-line drugs and emergence of nalidixic acid-resistant S. Typhi (NARST) isolates.
-
What are the implications for research, policy, or practice?
There is an urgent need for uniform laboratory testing guidelines for interpreting susceptibility of S. Typhi against ciprofloxacin and azithromycin.
Background
Enteric fever caused by Salmonella enterica serovar Typhi (S. Typhi) is an important public health problem in developing countries like India. Globally, the World Health Organization (WHO) has estimated the annual incidence of typhoid fever as 21.7 million cases, while the estimated crude incidence of typhoid fever for Southeast Asia alone is 110/100,000 persons per year.1,2
Drug resistance to Salmonella has been on the rise in India with the emergence of nalidixic acid-resistant S. Typhi (NARST) isolates. This, along with the emergence of resistance to thirdand fourth-generation cephalosporins, has diminished the therapeutic options available to newer quinolones, extended spectrum cephalosporins, azithromycin, tigecycline, and carbapenems.1,3-6
Though there are various studies proving the clinical efficacy of azithromycin as a potential therapeutic option,7,8 the lack of interpretative guidelines for testing the sensitivity of Salmonella isolates against azithromycin has hampered establishing its efficacy in laboratories. In addition, the prospect of emerging resistance to azithromycin amongst Salmonella isolates9 has made it imperative for laboratories to conduct more studies on this aspect. This prospective study was undertaken to evaluate the antibiotic susceptibility pattern of S. Typhi isolates from Pondicherry, India, with a special emphasis on comparing the MIC of azithromycin and ciprofloxacin with disc diffusion zone diameters of azithromycin and ciprofloxacin respectively.
Method
We studied a collection of 16 S. Typhi strains isolated from blood samples of febrile patients received at the Department of Microbiology, Mahatma Gandhi Medical College and Research Institute over a period of one-and-a-half years from January 2012 through June 2013.
Blood samples were collected and introduced in to brain heart infusion broth, which was then incubated aerobically at 37°C. Subcultures were then made on both blood agar and MacConkey agar 24h and 72h after collection. Identification of the isolates were done using biochemical tests and specific antisera (Central Research Institute, Kasauli, India) using standard methods.10 Isolates which were indole negative, methyl-red positive, Voges-Proskauer negative, citrate negative, urease negative, TSI–K/A with slight H2S, ornithine and lysine decarboxylase positive, arginine dihydrolase negative, glucose, mannitol, xylose and d-tartrate fermenting without production of gas, and sucrose and lactose nonfermenting were presumptively identified as S. Typhi. They were further confirmed by serotyping.
All isolates were subjected to susceptibility testing against chloramphenicol (30μg), nalidixic acid (30μg), ampicillin (10μg), co-trimoxazole (1.25/23.75μg), ceftriaxone (30μg), ciprofloxacin (5μg), and azithromycin (15μg) (Hi-Media, Mumbai, India), by Kirby‑Bauer’s disc diffusion technique.11 This uses a bacterial suspension of 0.5 McFarland turbidity inoculated onto the surface of a Mueller-Hinton agar plate (Hi-Media, Mumbai, India).
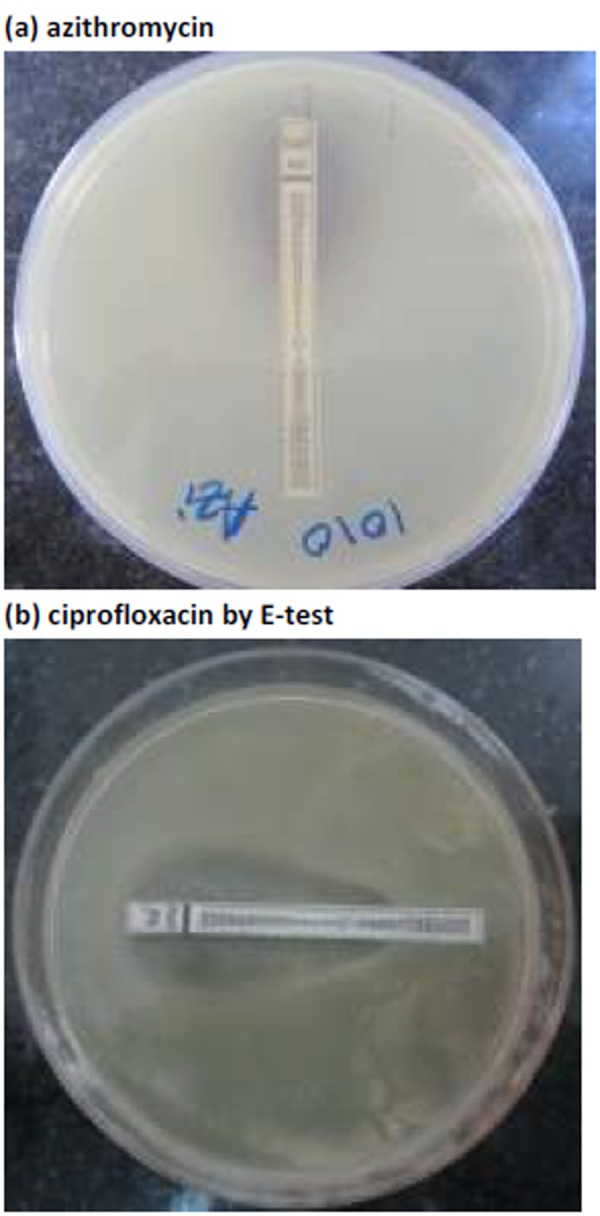
The MICs of azithromycin and ciprofloxacin were determined by E-test strips (Himedia, Mumbai, India) (Figure 1). These were set up simultaneously with the disc diffusion test, using the same 0.5 McFarland organism suspension on Mueller-Hinton agar (Hi-Media, Mumbai, India) and incubated under the same conditions. Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922 were used as controls for the disc diffusion and MIC testing, respectively.
Figure 1. MIC determination of Salmonella enterica serovar Typhi for:
Spearman’s rank correlation coefficient and regression coefficient (by linear regression) between disc diffusion and MIC was calculated for ciprofloxacin and azithromycin, respectively, taking MIC as a dependent variable and zone diameter by disc diffusion as an independent variable. Twotailed P values were calculated for the correlation and regression coefficients.
A P value of 0.05 or below was considered significant and a P value of 0.05 < P < 0.10 was considered marginally significant. A P value of > 0.10 was considered not significant.12,13 Data analysis was undertaken using the Statistical Package for Social Sciences (SPSS) version 16 for Windows (Chicago, USA).
Results
A total of 16 S. Typhi isolates were tested. Of these, 15 (93.75 per cent) isolates were sensitive to chloramphenicol, 14 (87.5 per cent) were sensitive to co-trimoxazole (Table 1). Only one of the isolates, obtained from the blood sample of a threeyear- old girl, exhibited multidrug resistance, i.e. resistance to ampicillin, co-trimoxazole, and chloramphenicol. All isolates (n=16, 100 per cent) were resistant to nalidixic acid.
Table 1. Resistance pattern of Salmonella isolates to different antimicrobials tested.
| Antimicrobial | Sensitive | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Co-trimoxazole | 14 | 87.5 | 0 | 0 | 2 | 12.5 |
| Chloramphenicol | 15 | 93.75 | 0 | 0 | 1 | 6.25 |
| Nalidixic acid | 0 | 0 | 0 | 0 | 16 | 100 |
| Ciprofloxacin By disc diffusion method | 0 | 0 | 13 | 81.25 | 3 | 18.75 |
| Ciprofloxacin By E- test | 0 | 0 | 15 | 93.75 | 1 | 6.25 |
| Ceftriaxone | 15 | 93.75 | 1 | 6.25 | 0 | 0 |
| Ampicillin | 15 | 93.75 | 0 | 0 | 1 | 6.25 |
Comparison of MIC with disk diffusion zone diameter of ciprofloxacin
MIC for ciprofloxacin ranged from 6μg/ml to 15μg/ml and the corresponding zone standardised regression coefficient (Beta) for MIC for given value of zone diameter was 0.6467 (P = 0.0068, significant). MIC values for ciprofloxacin were obtained as 0.38μg/ml for seven (43.75 per cent) isolates, 0.5 μg/ml for eight (50 per cent) isolates and one (6.25 per cent) isolate had MIC value of 32μg/ml.
The zone diameters for ciprofloxacin disc were detected to be 21–30mm in 13 (81.25 per cent) isolates and only three (18.75 per cent) isolates had zone ≤ 20 mm, while the diameters varied from 15mm to 26mm. MIC and zone diameters for ciprofloxacin had significant negative correlation (r = −0.5382; P = 0.032, considered significant).
Comparison of MIC with disk diffusion zone diameter of azithromycin
MIC for azithromycin ranged from 2μg/ml to 14μg/ml and corresponding zone standardised regression coefficient (Beta) for MIC for given value of zone diameter was – 0.4364 (P = 0.091, marginally significant). MIC values for azithromycin were obtained as 3–4μg/ml for four (25 per cent) isolates, 6–8μg/ml for 10 (62.5 per cent) isolates, and two (12.5 per cent) isolates had MIC values of 24μg/ml (Table 2).
Table 2. Azithromycin MIC values and zone diameters in S. Typhi isolates (n=16).
| MIC values (µg/ml) | Zone diameter (mm) | Number(s) |
|---|---|---|
| 3 | 15 | 1 |
| 4 | 16,17,16 | 3 |
| 6 | 16,17,16,16,13,17,16 | 7 |
| 8 | 15,17,19 | 3 |
| 24 | 14,14 | 2 |
The zone diameters for azithromycin disc were detected to be ≥ 15mm for 13/16 (81.25 per cent) and only 3/16 (18.75 per cent) isolates had zone < 15mm, while the diameters varied from 13mm to 19mm (Table 2). However, MIC and zone diameters for azithromycin had no significant negative correlation (r = −0.143; = 0.598).
Discussion
In our study, all but one S. Typhi isolate was sensitive to ampicillin, chloramphenicol, and ceftriaxone with one isolate exhibiting multidrug resistance. Most isolates were also susceptible to co-trimoxazole. A similar study from North India showed high sensitivity of Salmonella isolates to first-line agents like chloramphenicol, co-trimoxazole, and amoxycillin.13 However, they did not report any MDR Salmonella isolates. A previous study from Pondicherry showed 66 per cent of the S. Typhi isolates to be susceptible to first-line antimicrobials, while 22 per cent were multidrug resistant.1 A more recent study from South India using 322 Salmonella isolates also showed similar results, wherein all the isolates in that study were sensitive to ceftriaxone and chloramphenicol, 290 isolates (90 per cent) were sensitive to ampicillin and 306 (95 per cent) were sensitive to co-trimoxiazole.14
All isolates were resistant to nalidixic acid in our study. The high rates of nalidixic acid resistance have also been noted in other studies from various parts of India (Garg et al. reported 95.2 per cent NARST, Chowdhary et al. reported 91.9 per cent NARST).13,14 However, in sharp contrast to these findings, Afzal et al. from Pakistan reported only 23.7 per cent NARST isolates.15
The latest Clinical and Laboratory Standards Institute (CLSI) guidelines have made modified recommendations to use separate ciprofloxacin interpretative criteria for all Salmonella spp. For disc diffusion method, the modified zone sizes are: – ≥ 31mm—sensitive, 21–30mm—intermediate, and ≤ 20mm—resistant.11
The MIC interpretive criteria are: ≤ 0.06μg/ml- sensitive, 0.12– 0.5-intermediate and ≥1-resistant. According to these modified guidelines, 81.25 per cent of the isolates were categorised as intermediate susceptible. Interpretation of zone diameters as per these latest CLSI guidelines indicates that 81.25 per cent of isolates were ciprofloxacin intermediate. MIC results of these isolates, when interpreted as per the latest CLSI guidelines, showed 93.75 per cent of the isolates to be ciprofloxacin intermediate. The difference in results by these two methods was statistically significant (P < 0.0001), proving that MIC method was better than disc diffusion method for determination of intermediate susceptibility.
MIC has the power to predict efficacy in vivo. Also, an increase in MIC not detected by disc diffusion tests is documented to result in delayed response and serious complications.13,16 However, if the results of the current study are interpreted as per the latest European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines,17 more than 0.06μg/ml is to be taken as resistant, in which case, all the S. Typhi isolates in our study (100 per cent) will be resistant to ciprofloxacin by MIC method. It states that there is clinical evidence to indicate a poor response in systemic infections caused by Salmonella spp. with low-level ciprofloxacin resistance (MIC > 0.06mg/L). However, it dissuades disc diffusion testing with a ciprofloxacin 5μg disc as it will not reliably detect low-level resistance in Salmonella spp. Instead, it recommends the use of pefloxacin 5μg disc to screen for ciprofloxacin resistance in Salmonella spp. However, we could not test our isolates additionally with pefloxacin due to cost constraints.
MIC has the power to predict efficacy in vivo. Also, an increase in MIC not detected by disc diffusion tests is documented to result in delayed response and serious complications.13,16 However, if the results of the current study are interpreted as per the latest European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines,17 more than 0.06μg/ml is to be taken as resistant, in which case, all the S. Typhi isolates in our study (100 per cent) will be resistant to ciprofloxacin by MIC method. It states that there is clinical evidence to indicate a poor response in systemic infections caused by Salmonella spp. with low-level ciprofloxacin resistance (MIC > 0.06mg/L). However, it dissuades disc diffusion testing with a ciprofloxacin 5μg disc as it will not reliably detect low-level resistance in Salmonella spp. Instead, it recommends the use of pefloxacin 5μg disc to screen for ciprofloxacin resistance in Salmonella spp. However, we could not test our isolates additionally with pefloxacin due to cost constraints.
This variation in interpretation criteria for the same drug by different guideline agencies needs to be addressed, so that there is uniformity in reporting sensitivity, intermediate susceptibility or resistance to S. Typhi isolates. Previous studies on efficiency of azithromycin against S. Typhi have relied mainly on clinical criteria and less on laboratory criteria. This could be due to lack of definitive laboratory MIC breakpoints or due to the pharmacodynamics of azithromycin, whereby clinical success is reported despite peak serum levels of 0.4 mg/mL following a 500mg oral dose. This is far less than laboratory-reported MIC.19,20 The reason for therapeutic response is the high intracellular concentrations achieved by azithromycin of up to 50 to 100 times that in serum.19,21,22
Despite this, continuing treatment without taking in to consideration the laboratory MIC results is fraught with problems. Although S. Typhi is a predominately intracellular bacteria, it is estimated that one-third of bacterial cells in the blood are extracellular.23 If such isolates are exposed to sub-optimal levels of azithromycin, treatment failure and development of resistance can result.19 Even if azithromycin is used only in treatment of MDR-strains, these strains are more likely to have a higher extracellular concentration.23
Alternatively, laboratories need to contribute by addressing the following issues. Reproducibility of results is of relevance as studies have shown variation in MIC related to media pH and inoculum size,24 and there are also some differences between MIC results reported with E-test strips and agar dilution methods.25 Therefore, ensuring the uniformity of methods employed when testing is necessary. To achieve this, it is imperative for the international guideline agencies to develop universally acceptable MIC breakpoints for azithromycin susceptibility testing.
Although no MIC breakpoints are suggested, the latest EUCAST guidelines comment that for wild type S. Typhi isolates, azithromycin susceptibility can be assessed with MIC ≤16mg/L,17 while CLSI does not recommend use of azithromycin for Salmonella isolates. MIC of ≤16μg/ml and a zone diameter of ≥ 15 mm could be considered as criteria for in vitro susceptibility to azithromycin as has been concurred from various studies in India, including in this study.13,18
In accordance with these guidelines, detection of resistance for azithromycin correlates well with the MIC method [two isolates (12.5 per cent)] and disc diffusion method [three isolates (18.75 per cent)]. However, it is difficult to comment about the susceptibility of the remaining isolates to azithromycin, as 13 (81.25 per cent) of the isolates tested had a MIC between 4–8μg/ml. Furthermore, the small size of the study sample and cost constraints have limited further analysis. This further emphasises the need for more multi-centric studies on this subject and also the need to establish uniform performance standards for disk susceptibility testing for interpreting azithromycin susceptibility against S. Typhi. This would help to improve consistency between reporting laboratories.
Conclusion
To conclude, due to the emergence of nalidixic acid-resistant S. Typhi (NARST) and widespread resistance to fluoroquinolones, particularly ciprofloxacin, our results suggest the prescription of first-line drugs like ampicillin, cotrimoxazole, and chloramphenicol against S. Typhi. This reinforces the potential need for antimicrobial recycling, wherein antibiotics that have a markedly reduced effect may be withdrawn from clinical use for a period so that they may regain their efficacy.26
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
Please cite this paper as: Srirangaraj S, Kali A, Charles MVP. A study of antibiogram of Salmonella enterica serovar Typhi isolates from Pondicherry, India. AMJ 2014, 7, 4, 185-190. http//dx.doi.org/10.4066/AMJ.2014.2010
References
- 1.Harish BN, Menezes GA. Antimicrobial resistance in typhoidal salmonellae. Indian J Med Microbiol. 2011;29:223–9. doi: 10.4103/0255-0857.83904. [DOI] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Kownhar H, Shankar EM, Rajan R, Rao UA. Emergence of nalidixic acid-resistant Salmonella enterica serovar Typhi resistant to ciprofloxacin in India. J Med Microbiol. 2007;56:136–7. doi: 10.1099/jmm.0.46763-0. [DOI] [PubMed] [Google Scholar]
- 4.Capoor MR, Nair D, Hasan AS, Aggarwal P, Gupta B. Typhoid fever: narrowing therapeutic options in India. Southeast Asian J Trop Med Public Health. 2006;37:1170–4. [PubMed] [Google Scholar]
- 5.Gokul BN, Menezes GA, Harish BN. ACC-1 beta- Lactamase-producing Salmonella enterica Serovar Typhi, India. Emerg Infect Dis. 2010;16:1170–1. doi: 10.3201/eid1607.091643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capoor MR, Nair D. Quinolone and cephalosporin resistance in enteric Fever. J Glob Infect Dis. 2010;2:258–62. doi: 10.4103/0974-777X.68529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinh NT, Parry CM, Ly NT, Ha HD, Thong MX, Diep TS. et al. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob Agents Chemother. 2000;44:1855–9. doi: 10.1128/aac.44.7.1855-1859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frenck RW Jr., Mansour A, Nakhla I, Sultan Y, Putnam S, Wierzba T. et al. Short-course azithromycin for the treatment of uncomplicated typhoid fever in children and adolescents. Clin Infect Dis. 2004;38:951–7. doi: 10.1086/382359. [DOI] [PubMed] [Google Scholar]
- 9.Vlieghe ER, Phe T, De Smet B, Veng CH, Kham C, Bertrand S. et al. Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in Cambodian adults. PLoS Negl Trop Dis. 2012;6:1933. doi: 10.1371/journal.pntd.0001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmonella. Mackie & McCartney Practical Medical Microbiology. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. 14th ed. London: Churchill Livingstone: 1996. pp. 385–404. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. CLSI document M100-S23. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2013. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. [Google Scholar]
- 12.Chap TL. Hoboken, New Jersey: John Wiley and Sons, Inc; 2003; Introductory biostatistics. [Google Scholar]
- 13.Garg A, Verma S, Kanga A, Singh D, Singh B. Antimicrobial resistance pattern and in vivo activity of azithromycin in Salmonella isolates. Indian J Med Microbiol. 2013;31:287–9. doi: 10.4103/0255-0857.115641. [DOI] [PubMed] [Google Scholar]
- 14.Choudhary A, Gopalakrishnan R, Nambi PS, Ramasubramanian V, Ghafur KA, Thirunarayan MA. Antimicrobial susceptibility of Salmonella enterica serovars in a tertiary care hospital in southern India. Indian J Med Res. 2013;137:800–2. [PMC free article] [PubMed] [Google Scholar]
- 15.Afzal A, Sarwar Y, Ali A, Maqbool A, Salman M, Habeeb MA, Haque A. Molecular evaluation of drug resistance in clinical isolates of Salmonella enterica serovar Typhi from Pakistan. J Infect Dev Ctries. 2013;7:929–40. doi: 10.3855/jidc.3154. [DOI] [PubMed] [Google Scholar]
- 16.Nataro JP, Bopp CA, Fields PI, Kaper JB, Strockbire NA. In: 9th ed. Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Washington, DC: ASM Press; 2007. Escherichia, Shigella and Salmonella; Manual of Clinical Microbiology; pp. 670–87. [Google Scholar]
- 17.European Committee on anti-microbial susceptibility testing (EUCAST) Breakpoint tables for interpretation of MICs and zone diameters version 4.0, 2014. Accessed on 6-2-2014. Available from http://www.eucast.org, p–7. [Google Scholar]
- 18.Rai S, Jain S, Prasad KN, Ghoshal U, Dhole TN. Rationale of azithromycin prescribing practices for enteric fever in India. Indian J Med Microbiol. 2012;30:30–3. doi: 10.4103/0255-0857.93017. [DOI] [PubMed] [Google Scholar]
- 19.Parry CM, Ho VA, Phuong le T, Bay PV, Lanh MN, Tung le. et al. Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacinazithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever. Antimicrob Agents Chemother. 2007;51:819–25. doi: 10.1128/AAC.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;(25 Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 21.Butler T, Girard AE. Comparative efficacies of azithromycin and ciprofloxacin against experimental Salmonella Typhimurium infection in mice. J Antimicrob Chemothe. 1993;31:313–9. doi: 10.1093/jac/31.2.313. [DOI] [PubMed] [Google Scholar]
- 22.Butt F, Sultan F. In vitro activity of azithromycin in Salmonella isolates from Pakistan. J Infect Dev Ctries. 2011;5:391–5. doi: 10.3855/jidc.971. [DOI] [PubMed] [Google Scholar]
- 23.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM. et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36:1683–7. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler T, Frenck RW, Johnson RB, Khakhria R. In vitro effects of azithromycin on Salmonella Typhi: early inhibition by concentrations less than the MIC and reduction of MIC by alkaline pH and small inocula. J Antimicrob Chemother. 2001;47:455–8. doi: 10.1093/jac/47.4.455. [DOI] [PubMed] [Google Scholar]
- 25.Chayani N, Tiwari S, Sarangi G, Mallick B, Mohapatra A, Paty BP. et al. Role of azithromycin against clinical isolates of family enterobacteriaceae: A comparison of its minimum inhibitory concentration by three different methods. Indian J Med Microbiol. 2009;27:107–10. doi: 10.4103/0255-0857.45361. [DOI] [PubMed] [Google Scholar]
- 26.Saleh AA. Antimicrobial Drug Resistance: Need for an national policy. Bangabandhu Sheikh Mujib Med University J. 2009;1:1–2. [Google Scholar]


