Abstract
A rational and appropriate evaluation of liver biochemical tests is essential, given the increased number of abnormal laboratory results in asymptomatic patients. Critical judgement allows early diagnosis in the absence of typical clinical signs. Autoimmune hepatitis is a rare disease with high clinical variability. We present a child investigated for unexplained increase in aminotransferases, discovered accidentally 2 months earlier in a standard laboratory panel approach. She was asymptomatic and no physical signs of chronic or acute liver disease were found. Laboratory investigation showed hypergammaglobulinaemia with selective elevation of IgG and a positive anti-liver cytosol type 1. Severe interface hepatitis was found on liver biopsy and treatment was initiated with steroids and azathioprine with good response. This case highlights the importance of trusting in any serum aminotransferase abnormality, even in asymptomatic children and emphasises the value of clinical suspicion and specific immunosuppressive therapy in prognosis.
Background
Liver biochemical tests often are part of standard blood chemistries for screening symptomatic and asymptomatic adult outpatients, for example, in routine check-ups, before surgical procedures or in screening tests for blood donors.1 2 This has resulted in increased incidental findings of abnormal liver chemistry tests that must be interpreted by physicians. The prevalence and aetiology of hypertransaminasemia in childhood are not well defined, but it is known to be present in several other situations than viral-induced liver disease. Critical judgement of these findings allows early diagnosis in the absence of typical clinical signs.2
Autoimmune hepatitis (AIH) is a possible cause of elevated aminotransferases, characterised by a progressive hepatic necroinflammatory disorder of unknown cause with increased levels of serum IgG, circulating autoantibodies and histological features of interphase hepatitis. In paediatric population, it is uncommon, but its real prevalence is still unknown.3 4 Clinical spectrum is very wide and occasionally AIH is diagnosed in asymptomatic individuals (‘presymptomatic’ stage).4 There is also a large heterogeneity in genetic, laboratory, histological and serological features of the disease.5 We present a type 2 AIH case, in which investigation was triggered by unexpected results in routine laboratory test.
Case presentation
An 11-year-old Caucasian girl was referred due to a casual finding of persistent elevated serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), detected 2 months earlier in a routine laboratory assessment delivered by her primary care physician although she was completely asymptomatic.
Her medical history was innocent, except for an isolated episode of epistaxis 2 years earlier, with medical follow-up. At that time, laboratory results already revealed abnormal biochemistry with moderate elevated aminotransferases but no investigation was conducted. She had no history of drug allergies or blood transfusion and was not taking any medications (including herbal supplements or vitamins) or drinking alcohol. On physical examination, no signs of chronic or acute liver disease were found. A body mass index ≥85th centile and <95th centile and normal blood pressure was confirmed.
Investigations
Laboratory investigation confirmed persistent increased transaminases (AST 127 UI/L and ALT 198 UI/L), with normal albumin (4.7 g/dL), total bilirubin (0.5 mg/dL), γ glutamyltransferase (21 UI/L) and alkaline phosphatase (215 U/L). It also showed a normal blood count (haemoglobin 12 g/dL, white cell count 5.9×109/L and platelet count 259×109/L), activated partial thromboplastin time 33.8 s (normal range (NR) 26–37 s) and prothrombin time 12.8 s (NR 10–13 s). Hypergammaglobulinaemia was noted and serum IgG was elevated (3114 mg/dL; NR 552–1631 mg/dL). Serological testing for viral hepatitis was negative (hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), anti-hepatitis C virus and hepatitis A virus, Epstein-Barr virus, cytomegalovirus). Values of fasting glucose and lipid panel were normal. Ceruloplasmin, copper and α-1-antitrypsin levels were normal. Tests for antinuclear (ANA), antimitochondrial, anti-smooth muscle (ASMA) and anti-liver/kidney microsomal type 1 (anti-LKM1) antibodies were negative. Anti-liver cytosol type 1 (anti-LC1) was positive. Coeliac antibodies were negative. Abdominal ultrasound showed a normal homogeneous echo texture of liver and normal spleen size. Liver biopsy performed 1 month later revealed an inflammatory lymphocytic infiltrate in portal areas and severe interface hepatitis; bile ducts were normal (figures 1 and 2). No cholangiographic study was performed.
Figure 1.
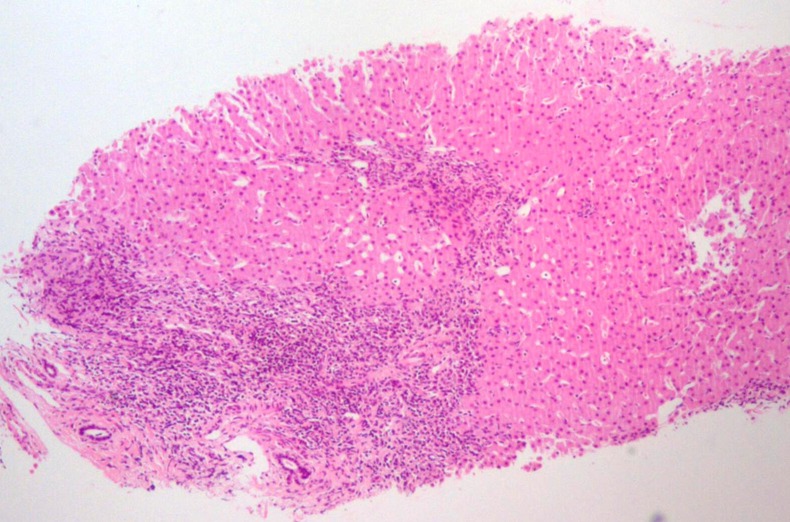
Inflammatory lymphoplasmacytic infiltrate in portal areas and severe interface hepatitis. No lesions or ductal proliferation were observed in bile ducts.
Figure 2.
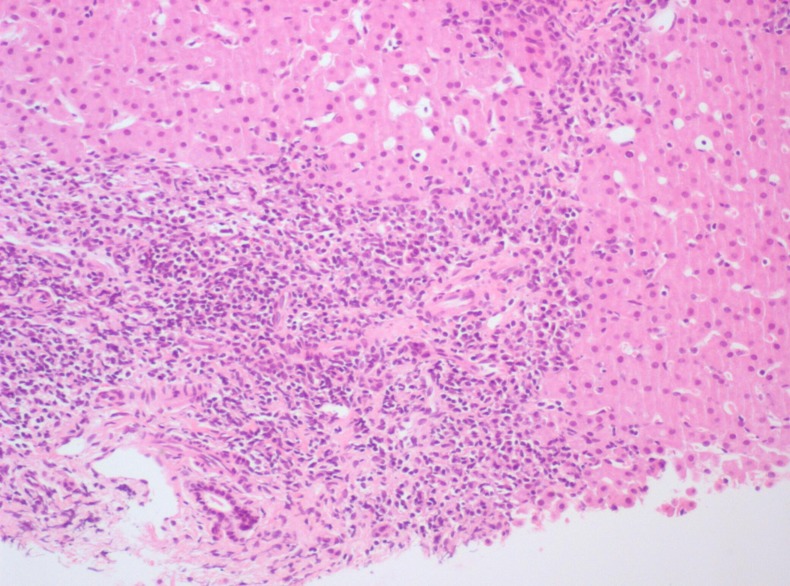
Inflammatory lymphoplasmacytic infiltrate in portal areas and severe interface hepatitis.
Treatment
Treatment was initiated with prednisolone (1 mg/kg/day) for 1 month. Then, azathioprine (1.5 mg/kg/day) was added and prednisolone was gradually decreased to a maintenance dose of 5 mg/day. After 10 months of combined treatment, steroids were stopped and the patient was treated with azathioprine alone.
Outcome and follow-up
Aminotransferases and serum IgG returned to normal after 4 and 8 weeks of treatment, respectively. Seventeen months later, relapse occurred due to non-adherence and steroids were reintroduced. After 2 years of treatment, the patient is on azathioprine and steroids (5 mg/day) with normal transaminases and IgG levels.
Discussion
The widespread use of routine biochemical assays has probably increased incidental findings of abnormal liver chemistry tests. Persistent elevated levels of AST and ALT should be investigated to provide early diagnosis in the absence of typical clinical signs. In fact, even mild aminotransferase elevations may indicate the presence of chronic diseases, including genetic and metabolic diseases (eg, α-1-antitrypsin deficiency, Wilson disease and haemochromatosis).
Clinical presentation variability makes AIH diagnosis extremely difficult and a high index of suspicion is necessary. Acute hepatitis and insidious onset with progressive jaundice, headache, anorexia and weight loss are the most frequent presentations. However, asymptomatic appearance or severe complications like portal hypertension may also be found in children. AIH seems to be a relatively silent disease, with poor clinical manifestations contrasting histological severity.3 5 6
Classification of AIH is based on serum autoantibody profiles. Type 1 is characterised by the presence of ASMA and/or ANA; type 2 presents antibodies to anti-LKM1 and/or anti-LC1. Severity and prognosis appear to be similar in the two forms,6 7 but anti-LKM1 positive patients present more frequently with early fulminant hepatic failure, than those with AIH type 1.3 7
Early diagnosis and treatment are essential to reduce liver inflammation and improve prognosis. Initial treatment varies between centres. Steroids and azathioprine have been proposed as first-line treatment of patients but recent reviews have underlined that this combination seems to be far from ideal.5 Immunosuppressive therapy duration is undefined but is recommended in AIH type 1 to ensure histological resolution and at least 3 years of biochemical remission before beginning a close and gradual withdrawal.
In AIH type 2, usually a long-term therapy is needed and rarely stopped.4 7
Learning points.
A serious approach towards persistent elevated levels of aspartate aminotransferase and alanine aminotransferase is essential to provide early diagnosis in the absence of typical clinical signs.
All children who are diagnosed with autoimmune hepatitis (AIH) should be treated. Delays in diagnosis and treatment adversely affect the long-term outcome.
Relapse during treatment is common. Optimal duration of immunosuppressive treatment for AIH is unknown but long-term treatment is required.
Acknowledgments
The authors are grateful for additional advice, critical revision and support from Dra Florbela Cunha.
Footnotes
Contributors: All authors have contributed for conception and design, drafting the manuscript, revision and final approval of this version.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Iorio R, Sepe A, Giannattasio A, et al. Hypertransaminasemia in childhood as a marker of genetic liver disorders. J Gastroenterol 2005;40:820–6 [DOI] [PubMed] [Google Scholar]
- 2.American Gastroenterological Association Medical Position Statement. Evaluation of liver chemistry tests. Gastroenterology 2002;123:1364–6 [DOI] [PubMed] [Google Scholar]
- 3.Vajro P, Paolella G. Autoimmune hepatitis: current knowledge. Clin Res Hepatol Gastroenterol 2012;36:284–6 [DOI] [PubMed] [Google Scholar]
- 4.Della Corte C, Sartorelli MR, Sindoni CD, et al. Autoimmune hepatitis in children: an overview of the disease focusing on current therapies. Eur J Gastroenterol Hepatol 2012;24:739–46 [DOI] [PubMed] [Google Scholar]
- 5.Zachou K, Muratori P, Koukoulis GK, et al. Review article: autoimmune hepatitis—current management and challenges. Aliment Pharmacol Ther 2013;38:887–913 [DOI] [PubMed] [Google Scholar]
- 6.Vitfell-Pedersen J, Jørgensen MH, Müller K, et al. Autoimmune hepatitis in children in East Denmark. J Pediatr Gastroenterol Nutr 2012;55:376–9 [DOI] [PubMed] [Google Scholar]
- 7.Mieli-Vergani G, Vergani D. Autoimmune hepatitis in children: what is different from adult AIH? Semin Liver Dis 2009;29:297–306 [DOI] [PubMed] [Google Scholar]


