Abstract
Adenoviral (Ad) vectors have emerged as a promising gene delivery platform for a variety of therapeutic and vaccine purposes during last two decades. However, the presence of preexisting Ad immunity and the rapid development of Ad vector immunity still pose significant challenges to the clinical use of these vectors. Innate inflammatory response following Ad vector administration may lead to systemic toxicity, drastically limit vector transduction efficiency and significantly abbreviate the duration of transgene expression. Currently, a number of approaches are being extensively pursued to overcome these drawbacks by strategies that target either the host or the Ad vector. In addition, significant progress has been made in the development of novel Ad vectors based on less prevalent human Ad serotypes and nonhuman Ad. This review provides an update on our current understanding of immune responses to Ad vectors and delineates various approaches for eluding Ad vector immunity. Approaches targeting the host and those targeting the vector are discussed in light of their promises and limitations.
1. BACKGROUND
The development of adenoviruses (Ad) as efficient gene delivery vehicles has been the focus of tremendous interest for the last two decades as indicated by more than 11,000 publications retrieved by searching PubMed using the keyword ‘adenovirus vectors’. The importance of Ad vectors for clinical gene delivery applications is also reflected by over 390 ongoing clinical trials based on these vectors (http://www.wiley.com/legacy/wileychi/genmed/clinical/) - more than any other gene delivery platform. The significance of using Ad vectors for gene delivery in cancer therapy, correction of metabolic disorders, and vaccine development is demonstrated in several animal studies and clinical trials which have produced very encouraging results.
As gene delivery vectors, Ad offer several advantages: (i) Many human and animal Ad are non-pathogenic for their natural hosts, (ii) a variety of both proliferating and quiescent cell types, such as epithelial cells, fibroblasts, hepatocytes, endothelial cells and stromal cells can be infected with Ad vectors, (iii) Ad vectors replicate to very high titers thereby providing enough vector particles to infect a large number of target cells, and (iv) replication-competent (e.g., early region (E) 3-deleted vectors), conditional replication-competent (e.g., vectors in which E1A is under the control of a tissue- or cancer antigen-specific promoter), replication-defective (e.g., E1, E1 & E3, E2, E4, E2 & E4, or E1, E2 & E4-deleted vectors) and helper-dependent (e.g., vectors in which the majority of Ad genome is deleted) Ad vectors can easily be generated. Moreover, the absence of germ-line transmission of Ad vectors in mice highlights one of the safety aspects of HAd vectors [1]. In this review we first discuss the current understanding of both innate and adaptive immune responses to Ad vectors and their significance in gene delivery applications. Then we will discuss the current state of various strategies to evade vector immunity.
2. ADENOVIRAL IMMUNITY
Although some serotypes are more prevalent than others, over fifty different serotypes of Ad are known to infect humans. During their lifetime, more than 80% of the human population has been exposed to at least one human Ad (HAd) serotype and has developed a serotype-specific immune response [2–4]. Preexisting immunity against a particular Ad serotype significantly reduces the vector uptake and shortens the duration of transgene expression. In addition to preexisting immunity, a leaky vector gene expression in the first generation Ad vectors (E1-deleted replication-defective Ad vectors) also stimulates cellular and humoral immune responses following vector inoculation and further abbreviates the transgene expression [5–9].
HAd-specific neutralizing antibodies are directed against the viral capsid components [10] and can significantly inhibit the virus uptake following readministration of the same Ad vector [11–15]. The preexisting anti-Ad antibodies worsen vector toxicity and lower the transgene expression following vector inoculation [16–18]. In addition, since anti-Ad antibodies mediate the interaction of Ad with dendritic cells (DC) [19] and macrophages (mφ) [20] through Fc receptors, Ad sequestration occurs resulting in poor transduction in the target tissues. It appears that the presence of preexisting anti-Ad antibodies stimulate stronger innate immune responses following vector inoculation [20] which could be of serious concern if a higher vector dose is used. Moreover, preexisting Ad-specific cellular immunity significantly impacts the efficacy of Ad-mediated gene delivery [8]. HAd5-specific CD8+ T cells with a specificity for hexon, penton, or fiber proteins are responsible for eliminating the target cells expressing the viral and transgene products, thus resulting in rapid loss of transgene expression [21–23].
In individuals that lack preexisting immunity against Ad, the first inoculation of Ad vectors will invoke cellular and humoral immune responses [7]. Repeat administration of the Ad vector in such individuals will be fraught with the above-mentioned challenges. Generally, multiple vector inoculations are necessary for most gene delivery applications to be clinically relevant. Therefore, it is important to develop strategies that could effectively elude immunity to the vector.
3. INDUCTION OF VECTOR-INDUCED INNATE IMMUNE RESPONSES AND TOXICITY
Systemic administration of Ad vectors results in an immediate activation of innate immune responses as manifested by the production of pro-inflammatory chemokines and cytokines, thrombocytopenia, coagulopathy, and liver damage [24]. The innate immune system is activated following Ad vector interactions with mediators of complement pathways [25–27], blood clotting factors [28–30], and toll-like receptors (TLRs) [24;31]. The Ad capsid interacts with the components of the classical, alternative, and common complement pathways [27;32] resulting in complement activation in either the presence or absence of preexisting antibodies. Ad seropositive individuals receiving a high dosage of Ad vectors are at a higher risk of strong complement activation which might lead to life threatening systemic responses [33]. Association of the Ad vector with the blood clotting factors VIII (FVIII), FIX, FX and protein C promotes hepatocyte and Kupffer cell transduction [29]. In addition, TLR2 and TLR9 recognize Ad-associated molecular patterns and thus activate MAPK and NF-κB pathways leading to the induction of a pro-inflammatory cytokine and chemokine response [24;34]. These inflammatory cytokines and chemokines which include interleukin (IL)-6, IL-8, IL-12, tumor necrosis factor (TNF)-α, interferon γ and λ, RANTES (Regulated upon Activation, Normal T-cell Expressed and Secreted), interferon-inducible protein 10 (IP-10), macrophage inflammatory protein (MIP)-1β, and MIP-2 are expressed in a dose-dependent manner following Ad vector administration [6;35] (Fig. 1).
Figure 1.
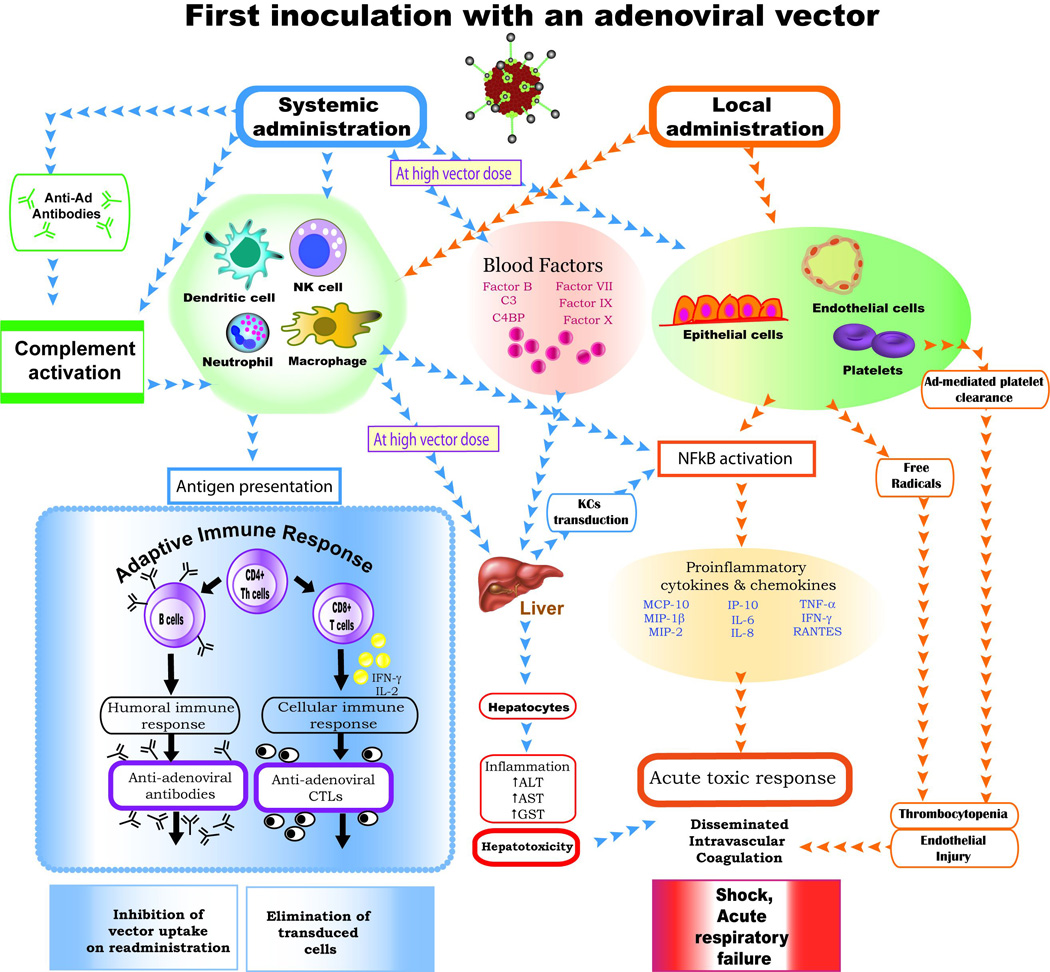
3.1. Implication of Route of Inoculation on Innate Immunity
3.1.1. Intravenous route
While Ad vectors can be delivered by various systemic or local routes depending on the purpose of the therapy, the chosen route will have an impact on the resultant innate immune response. Intravenous (i.v.) inoculation of Ad vectors results in a tri-phasic immune reaction. There are two phases of innate immune activation and a third phase of adaptive immune responses [36]. The first phase of innate response occurs from between thirty minutes to six hours post-inoculation as a result of the direct interaction of viral capsid proteins with innate immune sensors and is characterized by the induction of a proinflammatory cytokine/chemokine response [37;38]. The second phase, extending from few hours to several days post-inoculation, is manifested as thrombocytopenia, periportal polymorphonuclear leukocyte infiltration and elevated serum levels of liver enzymes reflecting liver damage. During this phase, the detection of the vector genome by TLRs results in the activation of immune cells and a second peak of the inflammatory cytokine/ chemokine response [36;39]. The third phase is characterized by the development of adaptive immune responses due to the expression of some of the viral genes and the antigen presentation by MHC class-I pathway which results in the generation of Ad-specific CD8+ T-Cells [36]. Subsequently the host will also elicit an Ad-specific humoral immune response.
Additionally, i.v. delivery of Ad vectors leads to the activation of endothelial cells as detected by the expression of phosphorylated Akt/PKB kinase, activated endothelial nitric oxide synthase (eNOS), and nitrotyrosine due to the interaction of viral particles with Kupffer cells [40]. Conserved arg-gly-asp (RGD) motifs of the Ad capsid appear to be important for efficient vector transduction and endothelial cell activation [37]. In rhesus monkeys, i.v. inoculation of a HAd vector induced thrombocytopenia as a result of platelet depletion [41]. Similarly, a baboon inoculated i.v. with an E1-deleted HAd vector developed acute clinical signs with a decrease in platelet counts, an increase in the liver enzymes, and injury to the vascular endothelium; it became moribund at 48 hours post-inoculation [42]. Collectively, these studies underscore the role of a strong innate immune response in mediating an acute inflammatory reaction following Ad vector administration.
Intraportal infusion of an E1-deleted HAd5 vector (7.5 × 1012 particles/kg) in nonhuman primates has resulted in acute activation of mφ and DC followed by a considerable apoptosis of splenocytes and hepatocytes due to the activation of innate immunity by viral capsid proteins [43]. However, vector doses of up to 5 × 1012 particles/kg usually lead to only limited hepatitis suggesting that vector toxicity could be diminished by lowering the vector dose per inoculation.
In another study, i.v. inoculation of mice with a HAd5 vector (2 × 1011 genomes/mouse) led to an acute inflammatory response characterized by high levels of IL-6 and IL-12 expression due to the preferential activation of mφ and DC [44]. In cirrhotic rats, the biodistribution of HAd vectors shifted from the liver to the lungs due to the presence of pulmonary intravascular mφ [45]. High doses of HAd vectors in cirrhotic rats not only upregulated TNF-α and IL-6 expression, but also led to markedly prolonged coagulation times resulting in fatal pulmonary hemorrhagic edema [46]. Cellular gene expression in response to wild type Ad, Ad vectors, or empty Ad particles was similar [47] suggesting the importance of the viral capsid proteins in mediating vector toxicity without viral gene expression.
3.1.2. Subcutaneous or intradermal route
In a subcutaneous mouse mammary tumor model, preimmunization with an HAd5 vector resulted in significantly reduced transgene expression in the tumor and normal tissues; however, the inhibition was more in the liver than in the mammary tumor [16;48]. Increasing the vector dose by 10- to 100-fold restored the level of transgene expression in preimmunized mice, but higher vector doses (2 × 1011 virus particles or more per inoculation) led to significantly higher hepatotoxicity compared to the naïve animals. Readministration of a second vector dose was associated with the same degree of toxicity as that with a single dose, but prompted a much more vigorous neutralizing antibody response [49;50]. Increased mortality was observed when pre-immunized mice were inoculated systemically with a high dose of Ad vector [18]. Intradermal inoculation of humans with an E1- and E3-deleted HAd5 vector resulted in a mild to moderate local lymphocyte infiltration and a minimal rise in systemic HAd5-specific lymphocytes [4] suggesting that this route of inoculation has the potential to elude systemic vector immunity.
3.1.3. Respiratory route
The respiratory route of Ad administration is preferred for the correction of lung disorders such as cystic fibrosis [51]. Broncho-specific delivery of a HAd5 based vector (1010 pfu) to non-human primates induced pro-inflammatory cytokines IL-1β and IL-8 in bronchi followed by infiltration by neutrophils, lymphocytes, and mononuclear cells suggesting the induction of innate immunity [52]. Similarly, intrapulmonary instillation of HAd5 in mice and cotton rats resulted in inflammation and pneumonia [53;54].
3.1.4. Intramuscular route
Intramuscular (i.m.) inoculation of HAd vectors resulted in prolonged and sustained transgene expression and an effective evasion of preexisting Ad immunity without the significant systemic toxicity detected after i.v. inoculation [55–57]. Cynomolgus macaques inoculated i.m. with 3 × 10 12 Ad vector particles/kg (200 times the clinical dose for humans) three times a week for up to eight weeks did not show significant toxicity, and the animals remained healthy throughout this twelve week study [55]. Significant abnormalities in hematology, blood chemistry or various tissues were not detected. Similar results were obtained in dogs receiving thirty times the proposed dose for humans for six days a week for three months [56]. These results suggest that repeated inoculations with Ad vectors do not lead to cumulative toxic effects, however, it is expected that the efficacy of the therapeutic effect will be compromised. In light of the data, the hypothesis to use different Ad vectors for sustaining the therapeutic effects is worth pursuing.
4. STRATEGIES FOR CIRCUMVENTION OF VECTOR IMMUNITY
The Ad based vectors are undoubtedly an indispensable tool for gene therapy and recombinant vaccine applications. To translate these attractive gene delivery vectors into clinically relevant therapies, it is imperative to refine existing strategies to evade vector immunity, to enhance target cell-specific transgene expression, to improve the duration of vector survival in the host, and to reduce their potential toxicity. A number of approaches have been pursued to meet these contradictory requirements for improving the efficacy of Ad-based gene transfer. These approaches can be broadly categorized into two categories: transient changes in the host (e.g. immunomodulation) and the modifications in the vector system (Fig.2)
Figure 2.
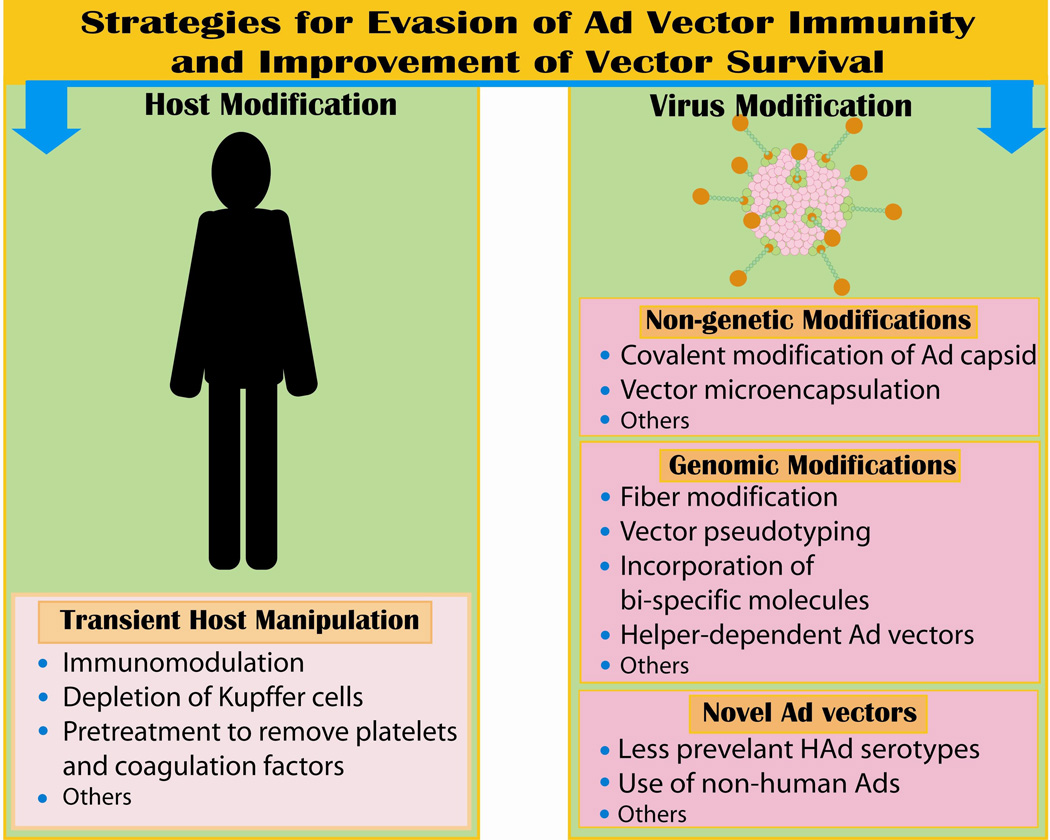
4.1. Strategies Targeted at the Host
4.1.1. Immunosuppression or Immunomodulation
Prolonged transgene expression has been demonstrated when Ad vectors are administered systemically after pre-treatment with immunosuppressive agents such as dexamethasone [58], cyclosporin, cyclophosphamide [59;60], deoxyspergualin [61], FK506 [62] and CTLA4Ig [63;64], the transient depletion of CD4 lymphocytes using an anti-CD4 monoclonal antibody [65], the use of an anti-CD40 ligand antibody to block CD40-CD40 ligand interactions [66], the combined use of anti-CD40 and CD86 antibodies to block co-stimulation by APCs [67] and oral tolerization to Ad proteins [68]. Depletion of B-cells with an anti-CD20 antibody along with T-cell inhibition allowed repeated liver gene transfer with homologous Ad vector [69]. These approaches assist in eluding both the humoral and/or cell-mediated immune responses to Ad. Passive immunization of immnosuppressed Syrian hamsters with anti-HAd5 neutralizing antibodies effectively prevented liver toxicity following i.v. injection of an extremely high dose of an oncolytic HAd5 [70]. It is unclear whether this approach of combining neutralizing antibodies with high doses of Ad vectors will have an adverse effect on the level and duration of transgene expression.
The cytokines TNF-α and IL-6 play important roles in mediating the innate immune response following Ad vector inoculation. Blockage of TNF-α through the Ad-encoded soluble TNF-α receptor caused a remarkable decrease in pro-inflammatory cytokines, reduced cellular infiltrates in the liver, and also prolonged transgene expression [71]. A TNF-α blockade in IL-6 mice markedly diminished the antibody responses to the Ad vector and the encoded transgene [72]. Similarly, pre-treatment with an anti-TNF-α drug etanercept or anti-TNF-α monoclonal antibody decreased Ad vector induced innate and adaptive immune responses [73].
TLR-9, a dsDNA sensor, contributed to acute toxicity following the systemic administration of Ad vectors. Ad dsDNA and TLR-9 interactions led to the activation of signal transduction pathways that eventually released the proinflammatory cytokines IL-6, TNF-α and MCP-1. Pre-treatment with a specific TLR-9 blocking oligonucleotide (ODN-2088) attenuated the acute inflammatory response resulting from the systemic administration of a helper-dependent Ad vector [34]. Co-administration of an Ad vector carrying a suppressor of cytokine signaling-1 (SOCS) gene suppressed serum levels of IL-6, MCP-1, RANTES, and TNF-α [74]. Similarly, the blocking of IL-1 signaling mitigated the pro-inflammatory response to i.v. injected Ad vectors [75].
The applicability of immunosuppressive or immunomodulatory approaches in clinical trials will depend upon their safety profile. In addition, combinatorial use of multiple strategies might improve the risk/benefit ratio of Ad vector- mediated gene delivery. Further understanding of the molecular mechanisms underlying Ad-induced innate and adaptive immune responses should uncover additional strategies to allow increased vector survival and prolonged transgene expression. There is a fine balance between the efficacy of Ad vector-based therapy and vector toxicity; care should be taken not to alter the host system to the level that may adversely affect the benefit of the approach.
4.1.2. Pharmacological Approaches
Uptake of systemically infused Ad vectors by mφ and DC in the liver and spleen results in activation of innate immune response and the consequent rise in serum inflammatory cytokines. Depletion of mφ and DC prior to Ad inoculation blocked the production of the inflammatory cytokines, IL-6, IL-12 and TNF-α, and also prevented an Ad-specific CTL response in mice [44]. Dichloromethylene bisphosphate [76], gadolinium chloride [77] and liposome encapsulated clodronate [78]-mediated mφ depletion prior to Ad inoculation resulted in longer vector genome persistence and transgene expression in mice. Further, Kupffer cell depletion also decreased the transgene-specific antibody response [78;79]. Interaction of the Ad capsid with coagulation factors IX and X was essential for Ad uptake by hepatocytes [29]. Pre-treatment with warfarin, which reduces plasma concentration of vitamin K-dependent coagulation factors, in combination with Kupffer cell depletion reduced hepatotoxicity and enhanced the efficacy of an oncolytic Ad vector [80].
The uptake of HAd5 by Kupffer cells is also thought to be mediated by the scavenger receptor A (SCA). Pre-administration of polyinosinic acid, which binds to SCA, was shown to reduce HAd5 clearance by Kupffer cells leading to a ten-fold increase in virus levels in the blood [81;82]. Another pathway for hepatic sequestration of HAd5 is via interaction with platelets and the subsequent retention of HAd5-platelet complexes in the liver sinusoids. Platelet depletion prior to HAd5 administration resulted in lesser sequestration of the virus in the liver, and it also led to lower levels of the cytokine/chemokine response [83].
4.2. Strategies Targeted at the Ad vector
In addition to the limited approaches available to modify the host’s immune system to evade Ad immunity, a number of strategies are being investigated to modify the vector itself. These include the physical, biochemical and genetic modifications discussed below.
4. 2.1. Covalent Modification of Ad Capsid
Induction of immune responses, acute toxicity and rapid vector clearance after the systemic infusion of Ad vectors result from interactions of the vector particles (capsids) with various blood factors, elements of immune system and preexisting anti-Ad antibodies. Therefore, masking of the immunodominant epitopes and capsid components required for these interactions should, in principle, reduce the Ad-induced toxicity and prolong the length of transgene expression. Covalent modification offers the advantage of producing high titer vector stocks which can be easily modified through well established protocols. Furthermore, a large number of amino acids of the capsid surface can be modified all at once which would not be feasible using a genetic approach (described later). However, substantial modifications in the vector genome to alter multiple amino acid residues on the capsid surface might adversely affect the virus assembly; genetically modified viruses are often difficult to propagate to levels comparable to unmodified vectors [84]. Indeed, several studies have successfully employed covalent modifications of Ad vectors utilizing polymers such as polyethylene glycol (PEG) [85–88], poly-N-(2-hydroxypropyl) methacrylamide [89] and large polysaccharide mannan [90].
FDA-approved PEG is commonly used for the covalent coupling of therapeutic proteins [84]. Up to 18,000 PEG molecules could be coupled to a single Ad particle thereby modifying the major capsid proteins, hexons, fibers and pentons which are also the targets for anti-HAd5 neutralizing antibodies (NAbs). PEGylation of Ad vectors can effectively overcome high titers of NAbs without losing biological activity [85;86;88;91;92]. This approach results in lower levels of Ad-specific adaptive immune responses and prolongs the duration of transgene expression [86;93].
Covalent capsid modifications are also expected to elude innate immunity since they will potentially mask the molecular patterns on the viral capsid with little or no effect on virus infectivity. Consistent with this, monomethoxypolyethylene glycol conjugation of Ad vectors led to reduced innate immunity and an improved therapeutic index in mice compared to the unmodified vector [94]. PEGylation of first generation and HD-Ad vectors did not affect vector transduction efficiencies in vivo, but did significantly reduce IL-6 response and vector uptake by mφ in vitro and Kupffer cells in vivo [95]. This is significant with regard to reducing innate immunity against Ad. Similarly, PEGylation of HD-Ad lowered serum levels of IL-6, IL-12 and TNF-α as compared to the unmodified HD-Ad vector [96]. Liver toxicity induced by E1-deleted Ad vectors remained unaffected by PEGylation, but a similar treatment of HD-Ad vectors successfully attenuated the liver damage [95]. However, in another study, Ad vectors PEGylated with a 20,000 MW PEG were efficiently detargeted from the liver after systemic delivery without affecting cellular and humoral immune responses against the encoded transgene [97]. Interestingly, Mok et. al. (2005) used much smaller PEG (5000 MW) suggesting there is a role of the molecular weight of PEG in determining the tropism of PEGylated Ad vectors. Furthermore, PEGylation can also be utilized to alter in vivo tropism of Ad vectors by employing a bifunctional PEG. One functional group is conjugated to the Ad capsid, leaving another functional group to be conjugated to a tissue-specific ligand or an antibody [98–100].
Ad vectors covalently shielded with poly-N-(2-hydroxypropyl) methacrylamide (pHPMA) were shown to evade preexisting Ad NAbs. Further, being a multivalent polymer, pHPMA coupled to tissue-specific receptors retargeted Ad vectors to respective tissues [89;101;102]. Importantly, systemically delivered pHPMA-Ad vectors transduced the liver at least 100-fold less efficiently than the unmodified vector demonstrating their potential for liver detargeting of an Ad vector [103]. Clearly, more understanding of the innate and adaptive immune system modulations by pHPMA-Ad vectors and their toxicity profiles are needed before the advancement of this approach to clinical studies.
4.2.2. Altering Native Ad Vector and Cell Surface Receptor Interactions
HAd5 attachment to a susceptible cell occurs via the interaction between the Ad fiber knob and Coxsackievirus-adenovirus receptor (CAR) on the host cell surface [104;105]. CAR is a member of the immunoglobulin superfamily and serves as a high-affinity receptor for many HAds in subgroup A, C, D, E, and F [104–106]. CD46 [107], receptor X [108], CD80 and CD86 [109] are receptors for the subgroup B HAds. In addition, major histocompatibility (MHC) class I α2 domain [110], heparin sulfate glycosaminoglycan [111] and sialic acid saccharide [112] may also serve as primary receptors for some HAd types. In addition to the primary receptors, host cell integrins serve as co-receptors for Ad entry [113]. The HAd penton base protein interacts with vitronectin-binding integrins, specifically αvβ3 and αvβ5, for virus uptake [113]. This process is facilitated by the arg-gly-asp (RGD) motif of the penton base. Interestingly, the RGD motif is also found in a number of adhesion molecules that are known to interact with integrins [114]. The interaction of HAd penton and αvβ1 integrins promotes actin cytoskeletal reorganization via the activation of several signaling molecules [115]. Some of strategies that have been developed to modify native tropism of Ad vectors are discussed below.
4.2.2a. Ad Fiber Knob Modifications
To ablate CAR binding of HAd5 vectors and retarget them to alternative receptors, a knob-specific neutralizing antibody could be complexed either to a specific ligand or a receptor-specific antibody [116]. This complex molecule will bind the Ad knob on one side and a specific cell surface molecule (receptor) on the other side. With this technology, a wide variety of HAd5 vectors have been successfully targeted to a number of receptors including folate, epidermal growth factor, fibroblast growth factor, epithelial cell adhesion molecule (EpCAM), tumor-associated glycoprotein (TAG)-67, and CD40 [116–120]. Similarly, pre-treatment of HAd5 vectors with a bi-specific adaptor molecule consisting of a CAR ectodomain and a single chain antibody against tissue-specific antigen has been used for tissue-specific transduction [121].
The HAd5 fiber knob induces DC activation and maturation [122]. Virus-induced maturation of DC was significantly reduced when knobless Ad particles were incubated with immature DC. Therefore, fiber knob modifications to incorporate cellular ligands with novel cell-binding capacity might confer targeting and decrease vector immunogenicity [123]. The fiber knob binding to coagulation factor IX and complement factor C4BP mediates transduction of hepatocytes and Kupffer cells and, as a result, enhances the innate immune response [30;124]. Intravenous inoculation of mice with an HAd5 vector that was mutated to ablate its interactions with CAR, factor IX and factor C4BP resulted in a fifty-fold decrease in the liver transduction implying that diminished interactions of Ad vectors with these blood factors will help in reducing liver toxicity.
4.2.2b. Vector Pseudotyping
A chimeric vector containing the HAd35 capsid and HAd5 fiber knob induced a stronger immune response in mice as compared to the unmodified HAd35 vector indicating the role of the fiber knob in the immunogenicity of HAd5 vectors [125]. The HAd5 vector carrying a chimeric human-bovine Ad fiber had a minimal hepatotoxicity and weaker inflammatory response upon i.v. injection in mice, and also partially evaded the preexisting humoral immunity [126]. Since virus NAbs are also directed to Ad hexon [127;128], a combination of fiber and hexon pseudotyping could be an effective approach to evade vector neutralizing antibodies.
As described above, the blood coagulation factors FVII, FX and protein C (PC) play a significant role in HAd5 transduction of hepatocytes. FX mediates hepatocyte transduction through interaction with a hypervariable region (HVR) on HAd5 hexons. Swapping of HAd5 HVR with HAd48 HVR which does not bind FX substantially decreased liver transduction [129] uncovering another potential pseudotyping approach to improve the safety profile of Ad vectors. Further, a HAd5 modification to ablate FX binding showed altered biodistribution, tropism and inflammatory profiles as compared to unmodified HAd5 [130].
Targeting of Ad vectors could also be achieved by fusing the extracellular domain of CAR to peptide-targeting ligands [131]. The genetic targeting of Ad vectors by engineering small peptides into the HAd fiber [132–137], protein IX [138;139] or by replacing the fiber protein with the phage T4 fibritin [140] has also been demonstrated, but the size of the peptide appears to be a limitation. Similarly, the use of bifunctional PEG molecules is useful in ablating the vector tropism by CAR-mediated interaction and providing specific vector targeting by incorporating a ligand for a particular receptor [87].
4.2.3. Vector Encapsulation
Encapsulation of Ad vectors with inert polymers such as PEG-cationic lipid [141] and poly (lactic-glycolic) acid (PLGA) copolymer encapsulation [142] has been shown to elude virus-neutralizing antibodies. However, the PLGA-encapsulated Ad particles are less stable due to harsh conditions during encapsulation and the acidic interior of PLGA microspheres. PEGylation of Ad prior to PLGA encapsulation enhanced the stability and transduction efficiency and decreased cytokine production in vitro [143]. Local administration of PLGA-encapsulated Ad showed significantly decreased virus dissemination to various organs including the liver [144] and also prolonged transgene expression in comparison to a non-encapsulated vector [145].
Liposomes, both cationic and anionic, have also been applied for encapsulation of Ad [146–148]. Recently, Ad encapsulated in anionic liposomes demonstrated high transduction efficiency in a CAR-deficient cell line, evaded vector-specific NAbs and elicited lesser cytotoxicity as compared to Ad complexed with cationic liposomes [149]. The systemic administration of Ad complexed to PEPGE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly-ethylene glycol)-2000), a cationic liposome, remarkably shifted the virus transduction from liver to other organs such as the lungs, heart, spleen and kidneys [146]. Use of bilamellar cationic liposomes to encapsulate HAd vectors also provided protection from preexisting humoral immune responses [150]. Similarly, microsphere-liposome complexes guard HAd vectors from NAbs and are capable of effectively transducing cells leading to successful transgene expression [151].
Sodium alginate-based biodegradable microparticles have been shown to encapsulate purified protein, bacteria, DNA or viruses and can be delivered to animals by various routes of inoculation [152–156]. Since alginate microspheres are biodegradable and are synthesized without the use of any harsh treatments or organic solvents, the viability of Ad vectors in these microparticles is usually very high. Encapsulation of a HAd5 vector into alginate microparticles could effectively evade the vector-specific immune response [14]. More than 70% of alginate microspheres are approximately 5–10 µm in size, and therefore, it is expected that the majority of them will be taken up by mφ and DC [157]. Alginate was found to activate NF-kB and stimulate IL-1β, IL-6 and TNF-α production in a murine mφ cell line [158]. However, in vivo innate immune response to alginate remains to be explored. It would be interesting to know how alginate encapsulation modulates the innate immune response to Ad vectors. Alginate encapsulation is likely to alter Ad tropism as it may block interactions with blood factors and Ad receptors. The Ad particles are expected to be released slowly from alginate microspheres thereby reducing liver toxicity [14].
4.2.4. Helper-Dependent Ad (HD-Ad) Vectors
E1-deleted Ad vectors have been further modified by creating deletions in the E2A, E2B and E4 regions with the anticipation that these additional deletions would lead to lower levels of Ad-specific adaptive immune responses, thereby significantly enhancing the levels and duration of transgene expression. These deletions, however, resulted in only limited improvements in the duration of transgene expression [7;9;159]. In order to further improve transgene expression by Ad vectors, HD-Ad vectors have been developed by deleting most of the viral genome except for the inverted terminal repeats and the packaging signal [160–163]. These HD-Ad vectors were a significant improvement over earlier Ad vectors since they resulted in improved safety and longer transgene expression [161–163]. The ability of preexisting antibodies to recognize epitopes on the capsid resulting in the vector clearance still remains a concern.
Initial studies showed that HD-Ad vectors had high cloning capacity, elicited limited cell-mediated immune responses, and produced long-term gene expression both in small laboratory animals [162;164] and nonhuman primates [162;165;166] without causing significant liver damage and toxicity. However, due to a natural propensity of Ad vectors for the liver, efficient transduction of extrahepatic target cells through systemic delivery requires high vector doses which in turn precipitate acute toxicity mediated by proinflammatory cytokines/chemokines released by activated innate immune cells Furthermore, it was shown that the HD-Ad vectors also induced a vector-specific immune response similar to that generated by an E1-deleted HAd [167]. Following the i.v. inoculation of HD-Ad vectors, an early expression of inflammatory cytokine and chemokine genes, including IP-10, MIP-2, and TNFα was induced in the liver in a pattern similar to that induced by first generation HAd vectors [168]. HD-Ad vectors also induced the recruitment of CD11b-positive leukocytes to the transduced liver cells within hours of administration. Importantly, while E1-deleted HAd vectors induced a second phase of liver inflammation, consisting of inflammatory gene expression and CD3-positive lymphocytic infiltrate at seven days post-transduction, these changes were not detected in the livers of mice receiving HD-Ad beyond 24 h post-transduction [168]. In addition, adaptive immune responses generated by HD-Ad vectors were also attenuated in comparison to that of E1-deleted HAd vectors. Systemic delivery of HD-Ad vectors has been shown to provide strong transgene expression for a prolonged period with minimal toxicity in the baboon, mouse, rat or canine models [166;169–173].
A method called pseudo-hydrodynamic injection employed balloon occlusion catheters to impede hepatic venous outflow with an increase in the intrahepatic pressure, thereby causing enlargement of hepatic fenestration [169]. Subsequent i.v. inoculation of HD-Ad significantly increased hepatocyte transduction with minimal systemic virus dissemination and innate immune response activation in mice and baboons. For therapeutic intervention requiring multiple inoculations, the vector-specific humoral immune response can be circumvented through sequential delivery of different HD-Ad vector serotypes [166]. Advances in the methods of generating HD-Ad vectors and their potential applications have been widely reviewed [39;51;174].
4.2.4.1. Long-term Transgene Expression with HD-Ad Vectors
In utero gene delivery of HD-Ad vectors could be used for long term transgene expression with genetic disorders such as Duchenne muscular dystrophy (DMD) [175]. Like the E1-deleted HAd vectors, HD-Ad vectors do not integrate into the host cell genome; therefore, the vector genome will be gradually diluted out in the dividing cells. In situations where long-term gene expression is desired, such as DMD, vector integration into the host genome will further improve the longevity of transgene expression. The hybrid HAd-adeno-associated virus (AAV) vectors could provide non-random integration of double-stranded DNA by ex vivo or in vivo gene delivery [176] thereby serving as a continuous source of transgene expression without potential systemic toxicity. Alternatively, long-term gene expression can be achieved by using a novel binary HD-Ad-Epstein-Barr virus (HDAd-EBV) hybrid system for the stable transfection of mammalian cells [177]. This system consists of a cre-recombinase expressing HD-Ad and a HD-Ad carrying an EBV episome and a transgene flanked by loxP sites. Another approach is based on an HD-Ad vector carrying the transgene cassette with attB, the ϕC31 integrase recognition site, flanked by Flp recombinase recognition sites [178].
HD-Ad vectors have also been investigated for their application in long-term neurological gene therapy. Pre-existing anti-HAd immunity drastically reduced transgene expression from E1-deleted Ad vectors, whereas HD-Ad vectors supported sustained long term therapeutic transgene expression levels in the brain [179–182]. Intracranial injection of a bicistronic HD-Ad vector carrying the thymidine kinase of HSV-1 and the human soluble Fms-like tyrosine kinase 3 ligand (Flt3L) gene cassette resulted in high therapeutic efficacy for up to one year in a rat model of glioblastoma multiforme (GBM) [183]. The anti-tumor efficacy of HD-Ad vectors was unaffected by anti-Ad immunity, and no systemic or neurological side effects were observed [184]. These results highlight the potential of local, intratumoral inoculation of HD-Ad vectors to avert the vector-specific immune response and systemic toxicity and may obviate the need to screen patients for pre-existing vector immunity. Furthermore, expression of two therapeutic genes in a single vector will bring down the vector dose required for efficient therapeutic effect, thereby reducing any untoward vector-associated toxicity.
4.2.5. Alternate Human Ad (HAd) Vectors (Serotype Switching)
Since more than fifty HAd serotypes exist and the neutralizing humoral immune response to Ad is serotype-specific, another strategy to overcome Ad vector immune response is serotype switching in vector construction [185–188]. HAd serotypes that have been developed as vectors include group B Ad (HAd7, HAd11, HAd34, HAd35 and HAd50), group C Ad (HAd2 and HAd5), group D Ad (HAd24, HAd26, HAd36, HAd48 and HAd49), group E Ad (HAd4), and group F Ad (HAd41) [189]. Subgroup B HAds utilize desmoglein 2 [190] and the membrane cofactor protein CD46 [107;191;192] as the primary receptor. Therefore, subgroup B HAds are potentially better candidates for gene delivery to those cells that are deficient in CAR, the primary receptor for group C Ad.
The low seroprevalence of HAd11 and HAd35 makes these promising vectors for in vivo applications. E1-deleted HAd35 vectors have efficiently transduced human cells and eluded preexisting HAd immunity [193–196]. Similarly, HAd35 based vaccine vectors have evaded preexisting HAd5 immunity in mice [197] as well as in rhesus monkeys [198]. HAd35 efficiently transduced various organs in cynomolgus monkeys following local injection, but not with i.v. inoculation, suggesting the importance of blood cells and/or other factors in the virus clearance [199;200]. Understanding the various pathways that HAd35 follows after systemic administration should pave the way for its further development as a gene delivery vector.
Other serotype-switching candidates are replication-defective HAd11 vectors which have efficiently transduced smooth muscle cells, synoviocytes, DC, cardiovascular cells and a number of tumor cell lines [201;202]. A significant fraction of HAd3 migrates to the lung; HAd31 migrates to tissues other than the liver, and HAd37 migrates to the spleen and liver [189]. Unfortunately, HAd serotypes 3, 4, 11 and 35 did not show a better safety profile than HAd5. Chimeric HAd5 vectors with a HAd11 or 35 fiber knob were found to be safer than HAd5 alone [203]. HAd49 vectors were grown to high titers on the HAd5 E1-complementing cell line, PER.C6, and induced transgene-specific immune response in the presence of high levels of anti-HAd5 immunity [204]. Further development of novel HAd vectors would require critical evaluation of their safety profile, biodistribution pattern and transgene expression. The combined use of HAd5 and alternative HAd serotypes and/or chimeric HAd5 vectors in a prime-boost regimen remains a possible effective strategy to overcome preexisting HAd immunity.
4.2.6. Nonhuman Ad Vectors
Several Ad originally isolated from nonhuman species have been developed as alternatives to HAd vectors for gene delivery applications. These Ad are species-specific and do not naturally infect humans, therefore, they evade preexisting HAd immunity. These nonhuman Ad include bovine Ad type 3 (BAd3) [205–207], canine Ad type 2 [208–210], ovine Ad [211;212], chimpanzee Ad (AdC) [213;214], and porcine Ad type 3 [2;215;216]. The construction and design of various non-human Ad vectors have been comprehensively reviewed [217]. Nonhuman Ad vectors were shown to infect human cells in culture and express transgene [2;205;210;213;218;219]. The sera of mice immunized with HAd serotypes 2, 4, 5, 7, and 12 did not neutralize chimpanzee Ad [213].
Neutralizing antibodies to chimpanzee origin Ad (AdC) are absent in the American and Asian populations, and only low titers are present in the African population [3]. AdC7 and AdC9 vectors encoding for T and B cell epitopes of a malaria antigen conferred protection in both the absence and presence of preexisting HAd5 immunity, although the level of protection decreased in the later case [220]. Heterologous administration of AdC6 and AdC7 vectors encoding gag protein of HIV-1 using a prime-boost regimen induced robust gag-specific CD4+ and CD8T+ T cells and high titers of anti-gag antibodies in rhesus macaques; priming with HAd5 did not affect the resultant immune response [221].
Similarly, HAd5-, BAd3- and PAd3-specific neutralizing antibodies did not cross-neutralize [13], and the species-specific cell-mediated immune responses were not significantly cross-reactive [222]. Therefore, sequential administration of HAd5, BAd3 and PAd3 is expected to evade the vector-specific neutralizing immune response. Remarkably, a BAd3 vector expressing the pandemic influenza hemagglutinin triggered exceptionally high levels of humoral and cell-mediated immune responses irrespective of anti-HAd5 immunity and conferred complete protection in immunized mice from a homologous lethal H5N1 virus challenge [223]. Similarly, a PAd3 vector expressing H5N1 hemagglutinin efficiently induced a quick and robust cellular immune response having a stronger neutralizing antibody response than a HAd5 vector and translating into a better survival rate following challenge with a lethal dose of H5N1 at one year post immunization[215]. BAd3 vectors showed relatively low liver tropism, longer vector genome persistence and significantly higher transgene expression than HAd5 vectors in the heart, kidney and lung following intravenous inoculation [224]. These reports underscore the potential of nonhuman Ad for the development of efficient gene delivery vehicles and evasion of vector immunity and toxicity.
5. CONCLUDING REMARKS
Inflammatory and toxic effects in response to Ad vector inoculation are mediated through innate and/or adaptive immune responses against the vector. Collectively, these effects may lead to systemic toxicity, reduced vector transduction and shortened duration of therapeutic transgene expression. Owing to the multifactorial nature of this problem, diverse strategies have been developed to overcome these limitations. The use of transgenic mice and nonhuman primates will be helpful in further evaluating the strategies for evading vector-induced innate and adaptive immune responses and toxicity. The critical analyses of the results obtained from animal studies are essential before any approach is tested in humans for the efficacy and safety.
Strategies targeted at modifying the host are based primarily on transient immunosuppression or the selective depletion of specific cell types using pharmacological approaches prior to Ad vector administration. With careful monitoring to ensure that the benefits outweigh the inherent risks, such strategies may benefit many patients. Strategies targeted at the vector are more diverse and extensively explored. A variety of physical and chemical methods to modify Ad vectors have enabled immune evasion and selective targeting with some degree of success. Genetic modification approaches have demonstrated the feasibility of incorporating immunomodulatory genes to evade vector immunity by inserting novel genes to specifically target or detarget (e.g. from liver) Ad vectors thereby endowing pseudotyped Ad vectors with immune evasion capability and novel tropism. Ongoing research efforts to refine these approaches will be instrumental in enhancing the efficacy and safety profiles of Ad vectors. HD-Ad vectors as well as vectors derived from novel human Ad types are continuously being explored for their usefulness in various preclinical models. Similarly, nonhuman Ad vectors have emerged as a novel gene transfer platform and, depending on the goal of gene transfer application, they provide attractive alternatives to HAd vectors.
In summary, exhaustive research efforts geared toward evading Ad vector immunity indicate the importance of innate and adaptive immunity in Ad vector-based gene delivery. Increased understanding of the molecular mechanisms of Ad immunity has prompted the development of novel strategies to overcome this serious limitation. It is hoped that further refinement of these strategies will soon offer investigators and clinicians multiple options for more efficient and safe gene transfer in patients. Initially, we may witness Ad-based therapy as one of the constituents of a combinational therapeutics.
Acknowledgements
This work was supported by Public Health Service grants AI059374 and CA110176 from the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, respectively. We thank Jane Kovach for her excellent secretarial assistance.
Reference List
- 1.Paielli DL, Wing MS, Rogulski KR, et al. Evaluation of the biodistribution, persistence, toxicity, and potential of germ-line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther. 2000;1:263–274. doi: 10.1006/mthe.2000.0037. [DOI] [PubMed] [Google Scholar]
- 2.Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Xiang Z, Li Y, Cun A, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey BG, Worgall S, Ely S, Leopold PL, Crystal RG. Cellular immune responses of healthy individuals to intradermal administration of an E1–E3- adenovirus gene transfer vector. Hum Gene Ther. 1999;10:2823–2837. doi: 10.1089/10430349950016555. [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Schwarz EM, Gu D, et al. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkon KB, Liu CC, Gall JG, et al. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kafri T, Morgan D, Krahl T, et al. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Nunes FA, Berencsi K, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toogood CI, Crompton J, Hay RT. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73(Pt 6):1429–1435. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- 11.Mittal SK, McDermott MR, Johnson DC, Prevec L, Graham FL. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993;28:67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- 12.Dong JY, Wang D, Van Ginkel FW, Pascual DW, Frizzell RA. Systematic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum Gene Ther. 1996;7:319–331. doi: 10.1089/hum.1996.7.3-319. [DOI] [PubMed] [Google Scholar]
- 13.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- 14.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter J, You Q, Hagstrom JN, Sands M, High KA. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc Natl Acad Sci USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlachaki MT, Hernandez-Garcia A, Ittmann M, et al. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol Ther. 2002;6:342–348. doi: 10.1006/mthe.2002.0669. [DOI] [PubMed] [Google Scholar]
- 17.Varnavski AN, Zhang Y, Schnell M, et al. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varnavski AN, Calcedo R, Bove M, Gao G, Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 19.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaiss AK, Vilaysane A, Cotter MJ, et al. Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J Immunol. 2009;182:7058–7068. doi: 10.4049/jimmunol.0804269. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Olive M, Pulmanausahakul R, et al. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology. 2006;350:312–322. doi: 10.1016/j.virol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 23.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;20(270):404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 24.Appledorn DM, McBride A, Seregin S, et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 2008;15:1606–1617. doi: 10.1038/gt.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian J, Xu Z, Smith JS, et al. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J Virol. 2009;83:5648–5658. doi: 10.1128/JVI.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiang A, Hartman ZC, Everett RS, et al. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol Ther. 2006;14:588–598. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Greig JA, Buckley SM, Waddington SN, et al. Influence of coagulation factor x on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Mol Ther. 2009;17:1683–1691. doi: 10.1038/mt.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 30.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee EG, Blattman JN, Kasturi SP, et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther. 2004;10:1140–1142. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Cichon G, Boeckh-Herwig S, Schmidt HH, et al. Complement activation by recombinant adenoviruses. Gene Therapy. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerullo V, Seiler MP, Mane V, et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol Ther. 2007;15:378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- 35.Zaiss AK, Liu Q, Bowen GP, et al. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segura MM, Alba R, Bosch A, Chillon M. Advances in helper-dependent adenoviral vector research. Current Gene Therapy. 2008;8:222–235. doi: 10.2174/156652308785160647. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Zaiss AK, Colarusso P, et al. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- 38.Brenner M. Gene transfer by adenovectors. Blood. 1999;94:3965–3967. [PubMed] [Google Scholar]
- 39.Seiler MP, Cerullo V, Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- 40.Schiedner G, Bloch W, Hertel S, et al. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum Gene Ther. 2003;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- 41.Wolins N, Lozier J, Eggerman TL, et al. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br J Haematol. 2003;123:903–905. doi: 10.1046/j.1365-2141.2003.04719.x. [DOI] [PubMed] [Google Scholar]
- 42.Morral N, O'Neal WK, Rice K, et al. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- 43.Schnell MA, Zhang Y, Tazelaar J, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Chirmule N, Gao GP, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 45.Smith JS, Tian J, Muller J, Byrnes AP. Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther. 2004;11:431–438. doi: 10.1038/sj.gt.3302149. [DOI] [PubMed] [Google Scholar]
- 46.Smith JS, Tian J, Lozier JN, Byrnes AP. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol Ther. 2004;9:932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Stilwell JL, McCarty DM, Negishi A, Superfine R, Samulski RJ. Development and characterization of novel empty adenovirus capsids and their impact on cellular gene expression. J Virol. 2003;77:12881–12885. doi: 10.1128/JVI.77.23.12881-12885.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bramson JL, Hitt M, Gauldie J, Graham FL. Pre-existing immunity to adenovirus does not prevent tumor regression following intratumoral administration of a vector expressing IL-12 but inhibits virus dissemination. Gene Ther. 1997;4:1069–1076. doi: 10.1038/sj.gt.3300508. [DOI] [PubMed] [Google Scholar]
- 49.Nagao S, Kuriyama S, Okuda H, et al. Adenovirus-mediated gene transfer into tumors: evaluation of direct readministration of an adenoviral vector into subcutaneous tumors of immunocompetent mice. Int J Oncol. 2001;18:57–65. [PubMed] [Google Scholar]
- 50.Nunes FA, Furth EE, Wilson JM, Raper SE. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- 51.Brunetti-Pierri N, Ng P. Progress towards liver and lung-directed gene therapy with helper-dependent adenoviral vectors. Curr Gene Ther. 2009;9:329–340. doi: 10.2174/156652309789753310. [DOI] [PubMed] [Google Scholar]
- 52.Wilmott RW, Amin RS, Perez CR, et al. Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum Gene Ther. 1996;7:301–318. doi: 10.1089/hum.1996.7.3-301. [DOI] [PubMed] [Google Scholar]
- 53.Ginsberg HS, Moldawer LL, Sehgal PB, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prince GA, Porter DD, Jenson AB, et al. Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus) J Virol. 1993;67:101–111. doi: 10.1128/jvi.67.1.101-111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Shao JY, Liu RY, et al. Evaluation of long-term toxicity of Ad/hIFN-gamma, an adenoviral vector encoding the human interferon-gamma gene, in nonhuman primates. Human Gene Therapy. 2008;19:827–839. doi: 10.1089/hum.2007.180. [DOI] [PubMed] [Google Scholar]
- 56.Huang BJ, Liu RY, Huang JL, et al. Long-term toxicity studies in canine of E10A, an adenoviral vector for human endostatin gene. Human Gene Therapy. 2007;18:207–221. doi: 10.1089/hum.2006.149. [DOI] [PubMed] [Google Scholar]
- 57.Maione D, Della RC, Giannetti P, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc Natl Acad Sci USA. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seregin SS, Appledorn DM, McBride AJ, et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas MA, Spencer JF, Toth K, et al. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith TA, White BD, Gardner JM, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 61.Kaplan JM, Smith AE. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther. 1997;8:1095–1104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- 62.Ilan Y, Jona VK, Sengupta K, et al. Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology. 1997;26:949–956. doi: 10.1002/hep.510260422. [DOI] [PubMed] [Google Scholar]
- 63.Guerette B, Vilquin JT, Gingras M, et al. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- 64.Jooss K, Turka LA, Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 65.Ye X, Robinson MB, Pabin C, Batshaw ML, Wilson JM. Transient depletion of CD4 lymphocyte improves efficacy of repeated administration of recombinant adenovirus in the ornithine transcarbamylase deficient sparse fur mouse. Gene Ther. 2000;7:1761–1767. doi: 10.1038/sj.gt.3301299. [DOI] [PubMed] [Google Scholar]
- 66.Chirmule N, Raper SE, Burkly L, et al. Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40-CD40 ligand interactions. J Virol. 2000;74:3345–3352. doi: 10.1128/jvi.74.7.3345-3352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haegel-Kronenberger H, Haanstra K, Ziller-Remy C, et al. Inhibition of costimulation allows for repeated systemic administration of adenoviral vector in rhesus monkeys. Gene Ther. 2004;11:241–252. doi: 10.1038/sj.gt.3302152. [DOI] [PubMed] [Google Scholar]
- 68.Ilan Y, Sauter B, Chowdhury NR, et al. Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology. 1998;27:1368–1376. doi: 10.1002/hep.510270525. [DOI] [PubMed] [Google Scholar]
- 69.Fontanellas A, Hervas-Stubbs S, Mauleon I, et al. Intensive pharmacological immunosuppression allows for repetitive liver gene transfer with recombinant adenovirus in nonhuman primates. Mol Ther. 2010;18:754–765. doi: 10.1038/mt.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhar D, Spencer JF, Toth K, Wold WS. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol Ther. 2009;17:1724–1732. doi: 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng Y, Trevejo J, Zhou J, et al. Inhibition of tumor necrosis factor alpha by an adenovirus-encoded soluble fusion protein extends transgene expression in the liver and lung. J Virol. 1999;73:5098–5109. doi: 10.1128/jvi.73.6.5098-5109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benihoud K, Esselin S, Descamps D, et al. Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice. Gene Ther. 2007;14:533–544. doi: 10.1038/sj.gt.3302885. [DOI] [PubMed] [Google Scholar]
- 73.Wilderman MJ, Kim S, Gillespie CT, et al. Blockade of TNF-alpha decreases both inflammation and efficacy of intrapulmonary Ad.IFNbeta immunotherapy in an orthotopic model of bronchogenic lung cancer. Mol Ther. 2006;13:910–917. doi: 10.1016/j.ymthe.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Sakurai H, Tashiro K, Kawabata K, et al. Adenoviral expression of suppressor of cytokine signaling-1 reduces adenovirus vector-induced innate immune responses. J Immunol. 2008;180:4931–4938. doi: 10.4049/jimmunol.180.7.4931. [DOI] [PubMed] [Google Scholar]
- 75.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J Immunol. 2005;174:7310–7319. doi: 10.4049/jimmunol.174.11.7310. [DOI] [PubMed] [Google Scholar]
- 76.Worgall S, Leopold PL, Wolff G, et al. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum Gene Ther. 1997;8:1675–1684. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- 77.Lieber A, He CY, Meuse L, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuzmin AI, Finegold MJ, Eisensmith RC. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Therapy. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 79.Schiedner G, Hertel S, Johnston M, et al. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 80.Shashkova EV, Doronin K, Senac JS, Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 81.Haisma HJ, Kamps JA, Kamps GK, et al. Polyinosinic acid enhances delivery of adenovirus vectors in vivo by preventing sequestration in liver macrophages. J Gen Virol. 2008;89:1097–1105. doi: 10.1099/vir.0.83495-0. [DOI] [PubMed] [Google Scholar]
- 82.Haisma HJ, Boesjes M, Beerens AM, et al. Scavenger receptor A: a new route for adenovirus 5. Mol Pharm. 2009;6:366–374. doi: 10.1021/mp8000974. [DOI] [PubMed] [Google Scholar]
- 83.Stone D, Liu Y, Shayakhmetov D, et al. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81:4866–4871. doi: 10.1128/JVI.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol Ther. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- 85.Croyle MA, Yu QC, Wilson JM. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther. 2000;11:1713–1722. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- 86.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- 87.Lanciotti J, Song A, Doukas J, et al. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Molecular Therapy. 2003;8:99–107. doi: 10.1016/s1525-0016(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 88.O'Riordan CR, Lachapelle A, Delgado C, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 89.Fisher KD, Stallwood Y, Green NK, et al. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 90.Espenlaub S, Wortmann A, Engler T, et al. Reductive amination as a strategy to reduce adenovirus vector promiscuity by chemical capsid modification with large polysaccharides. J Gene Med. 2008;10:1303–1314. doi: 10.1002/jgm.1262. [DOI] [PubMed] [Google Scholar]
- 91.Eto Y, Yoshioka Y, Ishida T, et al. Optimized PEGylated adenovirus vector reduces the anti-vector humoral immune response against adenovirus and induces a therapeutic effect against metastatic lung cancer. Biol Pharm Bull. 2010;33:1540–1544. doi: 10.1248/bpb.33.1540. [DOI] [PubMed] [Google Scholar]
- 92.Eto Y, Gao JQ, Sekiguchi F, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med. 2005;7:604–612. doi: 10.1002/jgm.699. [DOI] [PubMed] [Google Scholar]
- 93.Croyle MA, Chirmule N, Zhang Y, Wilson JM. "Stealth" adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geest BD, Snoeys J, Linthout SV, Lievens J, Collen D. Elimination of Innate Immune Responses and Liver Inflammation by PEGylation of Adenoviral Vectors and Methylprednisolone. Hum Gene Ther. 2005;16:1439–1451. doi: 10.1089/hum.2005.16.1439. [DOI] [PubMed] [Google Scholar]
- 95.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Molecular Therapy. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 96.Croyle MA, Le HT, Linse KD, et al. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 97.Wortmann A, Vohringer S, Engler T, et al. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol Ther. 2008;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
- 98.Park JW, Mok H, Park TG. Epidermal growth factor (EGF) receptor targeted delivery of PEGylated adenovirus. Biochem Biophys Res Commun. 2008;366:769–774. doi: 10.1016/j.bbrc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 99.Jung Y, Park HJ, Kim PH, et al. Retargeting of adenoviral gene delivery via Herceptin-PEG-adenovirus conjugates to breast cancer cells. J Control Release. 2007;123:164–171. doi: 10.1016/j.jconrel.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Ogawara K, Rots MG, Kok RJ, et al. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum Gene Ther. 2004;15:433–443. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- 101.Morrison J, Briggs SS, Green NK, et al. Cetuximab retargeting of adenovirus via the epidermal growth factor receptor for treatment of intraperitoneal ovarian cancer. Hum Gene Ther. 2009;20:239–251. doi: 10.1089/hum.2008.167. [DOI] [PubMed] [Google Scholar]
- 102.Green NK, Morrison J, Hale S, et al. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J Gene Med. 2008;10:280–289. doi: 10.1002/jgm.1121. [DOI] [PubMed] [Google Scholar]
- 103.Green NK, Herbert CW, Hale SJ, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 104.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 105.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roelvink PW, Lizonova A, Lee JG, et al. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 108.Tuve S, Wang H, Ware C, et al. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Short JJ, Pereboev AV, Kawakami Y, et al. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology. 2004;322:349–359. doi: 10.1016/j.virol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 110.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith KD, Andersen-Nissen E, Hayashi F, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 112.Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 113.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 114.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li E, Brown SL, Stupack DG, et al. Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol. 2001;75:5405–5409. doi: 10.1128/JVI.75.11.5405-5409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bilbao G, Gomez-Navarro J, Curiel DT. Targeted adenoviral vectors for cancer gene therapy. Adv Exp Med Biol. 1998;451:365–374. doi: 10.1007/978-1-4615-5357-1_57. 365–374. [DOI] [PubMed] [Google Scholar]
- 117.Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann N Y Acad Sci. 1999;886:158–171. doi: 10.1111/j.1749-6632.1999.tb09409.x. 158–71. [DOI] [PubMed] [Google Scholar]
- 118.Douglas JT, Rogers BE, Rosenfeld ME, et al. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 119.Gu DL, Gonzalez AM, Printz MA, et al. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Res. 1999;59:2608–2614. [PubMed] [Google Scholar]
- 120.Krasnykh V, Dmitriev I, Mikheeva G, et al. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li HJ, Everts M, Pereboeva L, et al. Adenovirus tumor targeting and hepatic untargeting by a coxsackie/adenovirus receptor ectodomain anti-carcinoembryonic antigen bispecific adapter. Cancer Res. 2007;67:5354–5361. doi: 10.1158/0008-5472.CAN-06-4679. [DOI] [PubMed] [Google Scholar]
- 122.Molinier-Frenkel V, Prevost-Blondel A, Hong SS, et al. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J Biol Chem. 2003;278:37175–37182. doi: 10.1074/jbc.M303496200. [DOI] [PubMed] [Google Scholar]
- 123.Myhre S, Henning P, Granio O, et al. Decreased immune reactivity towards a knobless, affibody-targeted adenovirus type 5 vector. Gene Ther. 2007;14:376–381. doi: 10.1038/sj.gt.3302875. [DOI] [PubMed] [Google Scholar]
- 124.Schoggins JW, Nociari M, Philpott N, Falck-Pedersen E. Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J Virol. 2005;79:11627–11637. doi: 10.1128/JVI.79.18.11627-11637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nanda A, Lynch DM, Goudsmit J, et al. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol. 2005;79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rogee S, Grellier E, Bernard C, et al. Influence of chimeric human-bovine fibers on adenoviral uptake by liver cells and the antiviral immune response. Gene Ther. 2010;17:880–891. doi: 10.1038/gt.2010.37. [DOI] [PubMed] [Google Scholar]
- 127.Ostapchuk P, Hearing P. Pseudopackaging of adenovirus type 5 genomes into capsids containing the hexon proteins of adenovirus serotypes B, D, or E. J Virol. 2001;75:45–51. doi: 10.1128/JVI.75.1.45-51.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sumida SM, Truitt DM, Lemckert AA, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 129.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 130.Alba R, Bradshaw AC, Coughlan L, et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood. 2010;116:2656–2664. doi: 10.1182/blood-2009-12-260026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim J, Smith T, Idamakanti N, et al. Targeting adenoviral vectors by using the extracellular domain of the coxsackie-adenovirus receptor: improved potency via trimerization. J Virol. 2002;76:1892–1903. doi: 10.1128/JVI.76.4.1892-1903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aoki Y, Hosaka S, Kawa S, Kiyosawa K. Potential tumor-targeting peptide vector of histidylated oligolysine conjugated to a tumor-homing RGD motif. Cancer Gene Ther. 2001;8:783–787. doi: 10.1038/sj.cgt.7700362. [DOI] [PubMed] [Google Scholar]
- 133.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biermann V, Volpers C, Hussmann S, et al. Targeting of high-capacity adenoviral vectors. Hum Gene Ther. 2001;12:1757–1769. doi: 10.1089/104303401750476258. [DOI] [PubMed] [Google Scholar]
- 135.Douglas JT, Miller CR, Kim M, et al. A system for the propagation of adenoviral vectors with genetically modified receptor specificities. Nat Biotechnol. 1999;17:470–475. doi: 10.1038/8647. [DOI] [PubMed] [Google Scholar]
- 136.Mizuguchi H, Koizumi N, Hosono T, et al. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther. 2001;8:730–735. doi: 10.1038/sj.gt.3301453. [DOI] [PubMed] [Google Scholar]
- 137.Nicklin SA, Von Seggern DJ, Work LM, et al. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol Ther. 2001;4:534–542. doi: 10.1006/mthe.2001.0489. [DOI] [PubMed] [Google Scholar]
- 138.Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J Virol. 2002;76:6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zakhartchouk A, Connors W, van KA, Tikoo SK. Bovine adenovirus type 3 containing heterologous protein in the C-terminus of minor capsid protein IX. Virology. 2004;320:291–300. doi: 10.1016/j.virol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 140.Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5:995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- 142.Beer SJ, Matthews CB, Stein CS, et al. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5:740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- 143.Mok H, Park JW, Park TG. Microencapsulation of PEGylated adenovirus within PLGA microspheres for enhanced stability and gene transfection efficiency. Pharm Res. 2007;24:2263–2269. doi: 10.1007/s11095-007-9441-y. [DOI] [PubMed] [Google Scholar]
- 144.Wang D, Molavi O, Lutsiak ME, et al. Poly(D,L-lactic-co-glycolic acid) microsphere delivery of adenovirus for vaccination. J Pharm Pharm Sci. 2007;10:217–230. [PubMed] [Google Scholar]
- 145.Garcia del BG, Hendry J, Renedo MJ, Irache JM, Novo FJ. In vivo sustained release of adenoviral vectors from poly(D,L-lactic-co-glycolic) acid microparticles prepared by TROMS. J Control Release. 2004;94:229–235. doi: 10.1016/j.jconrel.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 146.Han SY, Lee YJ, Jung HI, et al. Gene transfer using liposome-complexed adenovirus seems to overcome limitations due to coxsackievirus and adenovirus receptor-deficiency of cancer cells, both in vitro and in vivo. Exp Mol Med. 2008;40:427–434. doi: 10.3858/emm.2008.40.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fukuhara H, Hayashi Y, Yamamoto N, et al. Improvement of transduction efficiency of recombinant adenovirus vector conjugated with cationic liposome for human oral squamous cell carcinoma cell lines. Oral Oncol. 2003;39:601–609. doi: 10.1016/s1368-8375(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 148.Cao M, Deng HX, Zhao J, et al. Antitumour activity of cationic-liposome-conjugated adenovirus containing the CCL19 [chemokine (C-C motif) ligand 19] gene. Biotechnol Appl Biochem. 2007;48:109–116. doi: 10.1042/ba20070038. [DOI] [PubMed] [Google Scholar]
- 149.Zhong Z, Shi S, Han J, Zhang Z, Sun X. Anionic liposomes increase the efficiency of adenovirus-mediated gene transfer to coxsackie-adenovirus receptor deficient cells. Mol Pharm. 2010;7:105–115. doi: 10.1021/mp900151k. [DOI] [PubMed] [Google Scholar]
- 150.Yotnda P, Chen DH, Chiu W, et al. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol Ther. 2002;5:233–241. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- 151.Steel JC, Cavanagh HM, Burton MA, Kalle WH. Microsphere-liposome complexes protect adenoviral vectors from neutralising antibody without losses in transfection efficiency, in-vitro. J Pharm Pharmacol. 2004;56:1371–1378. doi: 10.1211/0022357044643. [DOI] [PubMed] [Google Scholar]
- 152.Aggarwal N, HogenEsch H, Guo P, et al. Biodegradable alginate microspheres as a delivery system for naked DNA. Can J Vet Res. 1999;63:148–152. [PMC free article] [PubMed] [Google Scholar]
- 153.Bowerstock TL, HogenEsch H, Suckow M, et al. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17:1804–1811. doi: 10.1016/s0264-410x(98)00437-x. [DOI] [PubMed] [Google Scholar]
- 154.Hilbert AK, Fritzsche U, Kissel T. Biodegradable microspheres containing influenza A vaccine: immune response in mice. Vaccine. 1999;17:1065–1073. doi: 10.1016/s0264-410x(98)00323-5. [DOI] [PubMed] [Google Scholar]
- 155.Mittal SK, Aggarwal N, Sailaja G, et al. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine. 2000;19:253–263. doi: 10.1016/s0264-410x(00)00170-5. [DOI] [PubMed] [Google Scholar]
- 156.Periwal SB, Speaker TJ, Cebra JJ. Orally administered microencapsulated reovirus can bypass suckled, neutralizing maternal antibody that inhibits active immunization of neonates. J Virol. 1997;71:2844–2850. doi: 10.1128/jvi.71.4.2844-2850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lomotan EA, Brown KA, Speaker TJ, Offita PA. Aqueous-based microcapsules are detected primarily in gut-associated dendritic cells after oral inoculation of mice. Vaccine. 1997;15:1959–1962. doi: 10.1016/s0264-410x(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 158.Yang D, Jones KS. Effect of alginate on innate immune activation of macrophages. Journal of Biomedical Materials Research Part A. 2009;90A:411–418. doi: 10.1002/jbm.a.32096. [DOI] [PubMed] [Google Scholar]
- 159.Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fisher KJ, Choi H, Burda J, Chen SJ, Wilson JM. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 161.Kochanek S, Clemens PR, Mitani K, et al. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morsy MA, Gu M, Motzel S, et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Parks R, Chen L, Anton M, et al. A new helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schiedner G, Morral N, Parks RJ, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 165.Morral N, Parks RJ, Zhou H, et al. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 166.Morral N, O'Neal W, Rice K, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roth MD, Cheng Q, Harui A, et al. Helper-dependent adenoviral vectors efficiently express transgenes in human dendritic cells but still stimulate antiviral immune responses. J Immunol. 2002;169:4651–4656. doi: 10.4049/jimmunol.169.8.4651. [DOI] [PubMed] [Google Scholar]
- 168.Muruve DA, Cotter MJ, Zaiss AK, et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brunetti-Pierri N, Ng T, Iannitti DA, et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- 170.Brunetti-Pierri N, Nichols TC, McCorquodale S, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- 171.Toietta G, Mane VP, Norona WS, et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci U S A. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brown BD, Shi CX, Powell S, et al. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–810. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- 173.Kim IH, Jozkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Palmer DJ, Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- 175.Bilbao R, Reay DP, Wu E, et al. Comparison of high-capacity and first-generation adenoviral vector gene delivery to murine muscle in utero. Gene Ther. 2005;12:39–47. doi: 10.1038/sj.gt.3302392. [DOI] [PubMed] [Google Scholar]
- 176.Recchia A, Perani L, Sartori D, Olgiati C, Mavilio F. Site-specific integration of functional transgenes into the human genome by adeno/AAV hybrid vectors. Mol Ther. 2004;10:660–670. doi: 10.1016/j.ymthe.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 177.Dorigo O, Gil JS, Gallaher SD, et al. Development of a novel helper-dependent adenovirus-Epstein-Barr virus hybrid system for the stable transformation of mammalian cells. J Virol. 2004;78:6556–6566. doi: 10.1128/JVI.78.12.6556-6566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ehrhardt A, Yant SR, Giering JC, et al. Somatic integration from an adenoviral hybrid vector into a hot spot in mouse liver results in persistent transgene expression levels in vivo. Mol Ther. 2007;15:146–156. doi: 10.1038/sj.mt.6300011. [DOI] [PubMed] [Google Scholar]
- 179.King GD, Muhammad AK, Xiong W, et al. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J Virol. 2008;82:4680–4684. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Barcia C, Jimenez-Dalmaroni M, Kroeger KM, et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xiong W, Goverdhana S, Sciascia SA, et al. Regulatable Gutless Adenovirus Vectors Sustain Inducible Transgene Expression in the Brain in the Presence of an Immune Response against Adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Puntel M, Muhammad AK, Candolfi M, et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol. 2010;84:6007–6017. doi: 10.1128/JVI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muhammad AK, Puntel M, Candolfi M, et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther. 2010;88:204–213. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 186.Mack CA, Song WR, Carpenter H, et al. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 187.Mastrangeli A, Harvey BG, Yao J, et al. "Sero-switch" adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 188.Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 189.Appledorn DM, Kiang A, McBride A, et al. Wild-type adenoviruses from groups A–F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 2008;15:885–901. doi: 10.1038/gt.2008.18. [DOI] [PubMed] [Google Scholar]
- 190.Wang H, Li ZY, Liu Y, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Segerman A, Atkinson JP, Marttila M, et al. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sirena D, Lilienfeld B, Eisenhut M, et al. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gao W, Tamin A, Soloff A, et al. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reddy PS, Ganesh S, Limbach MP, et al. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 195.Sakurai F, Mizuguchi H, Hayakawa T. Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Ther. 2003;10:1041–1048. doi: 10.1038/sj.gt.3301959. [DOI] [PubMed] [Google Scholar]
- 196.Vogels R, Zuijdgeest D, van RR, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Barouch DH, Pau MG, Custers JH, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 198.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 199.Sakurai F, Nakamura S, Akitomo K, et al. Transduction properties of adenovirus serotype 35 vectors after intravenous administration into nonhuman primates. Mol Ther. 2008;16:726–733. doi: 10.1038/mt.2008.19. [DOI] [PubMed] [Google Scholar]
- 200.Sakurai F, Nakamura SI, Akitomo K, et al. Adenovirus serotype 35 vector-mediated transduction following direct administration into organs of nonhuman primates. Gene Ther. 2009;16:297–302. doi: 10.1038/gt.2008.154. [DOI] [PubMed] [Google Scholar]
- 201.Holterman L, Vogels R, van dV, et al. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stone D, Ni S, Li ZY, et al. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stone D, Liu Y, Li ZY, et al. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther. 2007;15:2146–2153. doi: 10.1038/sj.mt.6300319. [DOI] [PubMed] [Google Scholar]
- 204.Lemckert AA, Grimbergen J, Smits S, et al. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J Gen Virol. 2006;87:2891–2899. doi: 10.1099/vir.0.82079-0. [DOI] [PubMed] [Google Scholar]
- 205.Mittal SK, Bett AJ, Prevec L, Graham FL. Foreign gene expression by human adenovirus type 5-based vectors studied using firefly luciferase and bacterial beta-galactosidase genes as reporters. Virology. 1995;210:226–230. doi: 10.1006/viro.1995.1337. [DOI] [PubMed] [Google Scholar]
- 206.Reddy PS, Idamakanti N, Hyun BH, Tikoo SK, Babiuk LA. Development of porcine adenovirus-3 as an expression vector. J Gen Virol. 1999;80:563–570. doi: 10.1099/0022-1317-80-3-563. [DOI] [PubMed] [Google Scholar]
- 207.van Olphen AL, Tikoo SK, Mittal SK. Characterization of bovine adenovirus type 3 E1 proteins and isolation of E1-expressing cell lines. Virology. 2002;295:108–118. doi: 10.1006/viro.2002.1389. [DOI] [PubMed] [Google Scholar]
- 208.Bru T, Salinas S, Kremer EJ. An Update on Canine Adenovirus Type 2 and Its Vectors. Viruses-Basel. 2010;2:2134–2153. doi: 10.3390/v2092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hemminki A, Kanerva A, Kremer EJ, et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 210.Klonjkowski B, Gilardi-Hebenstreit P, Hadchouel J, et al. A recombinant E1-deleted canine adenoviral vector capable of transduction and expression of a transgene in human-derived cells and in vivo. Hum Gene Ther. 1997;8:2103–2115. doi: 10.1089/hum.1997.8.17-2103. [DOI] [PubMed] [Google Scholar]
- 211.Bridgeman A, Roshorm Y, Lockett LJ, et al. Ovine atadenovirus, a novel and highly immunogenic vector in prime-boost studies of a candidate HIV-1 vaccine. Vaccine. 2009;28:474–483. doi: 10.1016/j.vaccine.2009.09.136. [DOI] [PubMed] [Google Scholar]
- 212.Hofmann C, Loser P, Cichon G, et al. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Farina SF, Gao GP, Xiang ZQ, et al. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xiang Z, Gao G, Reyes-Sandoval A, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Patel A, Tikoo S, Kobinger G. A porcine adenovirus with low human seroprevalence is a promising alternative vaccine vector to human adenovirus 5 in an H5N1 virus disease model. PLoS One. 2010;5:e15301. doi: 10.1371/journal.pone.0015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reddy PS, Idamakanti N, Chen Y, et al. Replication-defective bovine adenovirus type 3 as an expression vector. J Virol. 1999;73:9137–9144. doi: 10.1128/jvi.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 219.Rasmussen UB, Benchaibi M, Meyer V, Schlesinger Y, Schughart K. Novel human gene transfer vectors: evaluation of wild-type and recombinant animal adenoviruses in human-derived cells. Hum Gene Ther. 1999;10:2587–2599. doi: 10.1089/10430349950016636. [DOI] [PubMed] [Google Scholar]
- 220.Reyes-Sandoval A, Sridhar S, Berthoud T, et al. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol. 2008;38:732–741. doi: 10.1002/eji.200737672. [DOI] [PubMed] [Google Scholar]
- 221.Tatsis N, Lasaro MO, Lin SW, et al. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J Immunol. 2009;182:6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sharma A, Tandon M, Ahi YS, et al. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010;17:634–642. doi: 10.1038/gt.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther. 2008;16:965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sharma A, Li X, Bangari DS, Mittal SK. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009;143:184–194. doi: 10.1016/j.virusres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


