Abstract
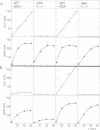
The complex of DnaA protein with its 9 bp consensus binding site, the dnaA box 5'-TT(A/T)T(A/C)CA(A/C)A, blocks transcribing RNA polymerase. In a model system, the rate of transcription was monitored distal to the dnaA box 5'-TTTTCCACA by the expression of a reporter gene. DnaA-dependent transcription termination occurred irrespective of whether the dnaA box region was or was not translated. Only the dnaA box orientation 5'-TTTTCCACA on the non-coding strand, but not the reverse orientation, was active in termination. This suggests that DnaA protein contacts only one strand of the DNA duplex. Oligonucleotide-directed mutation of a dnaA box present within the dnaA coding region resulted in increased expression of dnaA. This demonstrates that DnaA protein-directed transcription termination is an element of the autoregulation of the dnaA gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. E., Ptashne M., Harrison S. C. Structure of the repressor-operator complex of bacteriophage 434. 1987 Apr 30-May 6Nature. 326(6116):846–852. doi: 10.1038/326846a0. [DOI] [PubMed] [Google Scholar]
- Atlung T. Allele-specific suppression of dnaA(Ts) mutations by rpoB mutations in Escherichia coli. Mol Gen Genet. 1984;197(1):125–128. doi: 10.1007/BF00327932. [DOI] [PubMed] [Google Scholar]
- Atlung T., Clausen E. S., Hansen F. G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol Gen Genet. 1985;200(3):442–450. doi: 10.1007/BF00425729. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Braun R. E., O'Day K., Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985 Jan;40(1):159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- Braun R. E., O'Day K., Wright A. Cloning and characterization of dnaA(Cs), a mutation which leads to overinitiation of DNA replication in Escherichia coli K-12. J Bacteriol. 1987 Sep;169(9):3898–3903. doi: 10.1128/jb.169.9.3898-3903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., Wright A. DNA methylation differentially enhances the expression of one of the two E. coli dnaA promoters in vivo and in vitro. Mol Gen Genet. 1986 Feb;202(2):246–250. doi: 10.1007/BF00331644. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Buhk H. J., Messer W. The replication origin region of Escherichia coli: nucleotide sequence and functional units. Gene. 1983 Oct;24(2-3):265–279. doi: 10.1016/0378-1119(83)90087-2. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Gentz R., Bujard H. lac Repressor blocks transcribing RNA polymerase and terminates transcription. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4134–4137. doi: 10.1073/pnas.83.12.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Gielow A., Kücherer C., Kölling R., Messer W. Transcription in the region of the replication origin, oriC, of Escherichia coli: termination of asnC transcripts. Mol Gen Genet. 1988 Nov;214(3):474–481. doi: 10.1007/BF00330483. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Hansen F. G., von Meyenburg K. The nucleotide sequence of the dnaA gene and the first part of the dnaN gene of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 25;10(22):7373–7385. doi: 10.1093/nar/10.22.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1982;1(9):1043–1048. doi: 10.1002/j.1460-2075.1982.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Kellenberger-Gujer G., Podhajska A. J., Caro L. A cold sensitive dnaA mutant of E. coli which overinitiates chromosome replication at low temperature. Mol Gen Genet. 1978 Jun 1;162(1):9–16. doi: 10.1007/BF00333845. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kücherer C., Lother H., Kölling R., Schauzu M. A., Messer W. Regulation of transcription of the chromosomal dnaA gene of Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):115–121. doi: 10.1007/BF02428040. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landick R., Carey J., Yanofsky C. Translation activates the paused transcription complex and restores transcription of the trp operon leader region. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4663–4667. doi: 10.1073/pnas.82.14.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A., Atlung T., Rasmussen K. V. Stability and replication control of Escherichia coli minichromosomes. J Bacteriol. 1987 Jun;169(6):2835–2842. doi: 10.1128/jb.169.6.2835-2842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Messer W., Seufert W., Schaefer C., Gielow A., Hartmann H., Wende M. Functions of the DnaA protein of Escherichia coli in replication and transcription. Biochim Biophys Acta. 1988 Dec 20;951(2-3):351–358. doi: 10.1016/0167-4781(88)90106-6. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C., Messer W. Termination of the Escherichia coli asnC transcript. The DnaA protein/dnaA box complex blocks transcribing RNA polymerase. Gene. 1988 Dec 20;73(2):347–354. doi: 10.1016/0378-1119(88)90499-4. [DOI] [PubMed] [Google Scholar]
- Schauzu M. A., Kücherer C., Kölling R., Messer W., Lother H. Transcripts within the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1987 Mar 25;15(6):2479–2497. doi: 10.1093/nar/15.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. Sequential early stages in the in vitro initiation of replication at the origin of the Escherichia coli chromosome. J Biol Chem. 1988 May 25;263(15):7124–7130. [PubMed] [Google Scholar]
- Sekimizu K., Yung B. Y., Kornberg A. The dnaA protein of Escherichia coli. Abundance, improved purification, and membrane binding. J Biol Chem. 1988 May 25;263(15):7136–7140. [PubMed] [Google Scholar]
- Seufert W., Dobrinski B., Lurz R., Messer W. Functionality of the dnaA protein binding site in DNA replication is orientation-dependent. J Biol Chem. 1988 Feb 25;263(6):2719–2723. [PubMed] [Google Scholar]
- Stuitje A. R., de Wind N., van der Spek J. C., Pors T. H., Meijer M. Dissection of promoter sequences involved in transcriptional activation of the Escherichia coli replication origin. Nucleic Acids Res. 1986 Mar 11;14(5):2333–2344. doi: 10.1093/nar/14.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. Transcriptional repression of the dnaA gene of Escherichia coli by dnaA protein. Mol Gen Genet. 1987 Oct;209(3):518–525. doi: 10.1007/BF00331158. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Slippery runs, shifty stops, backward steps, and forward hops: -2, -1, +1, +2, +5, and +6 ribosomal frameshifting. Cold Spring Harb Symp Quant Biol. 1987;52:687–693. doi: 10.1101/sqb.1987.052.01.078. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]