Abstract
Aim:
To study the effects of 3-n-butylphthalide (NBP) on the TREK-1 channel expressed in Chinese hamster ovary (CHO) cells.
Methods:
Whole-cell patch-clamp recording was used to record TREK-1 channel currents. The effects of varying doses of l-NBP on TREK-1 currents were also observed. Current-clamp recordings were performed to measure the resting membrane potential in TREK-1-transfected CHO (TREK-1/CHO) and wild-type CHO (Wt/CHO) cells.
Results:
l-NBP (0.01–10 μmol/L) showed concentration-dependent inhibition on TREK-1 currents (IC50=0.06±0.03 μmol/L), with a maximum current reduction of 70% at a concentration of 10 μmol/L. l-NBP showed a more potent inhibition on TREK-1 current than d-NBP or dl-NBP. This effect was partially reversed upon washout and was not voltage-dependent. l-NBP 10 μmol/L elevated the membrane potential in TREK-1/CHO cells from -55.3 mV to -42.9 mV. However, it had no effect on the membrane potential of Wt/CHO cells.
Conclusion:
l-NBP potently inhibited TREK-1 current and elevated the membrane potential, which may contribute to its neuroprotective activity.
Keywords: Tandem-pore-domain potassium channel, TREK-1 channel, whole-cell patch-clamp recording, chiral 3-n-butylphthalide, membrane potential, neuroprotection
Introduction
Two-pore-domain potassium (K2P) channels are a novel family of potassium channels with four transmembrane segments and two pore-forming domains located in tandem1, 2. These channels control neuronal excitability through their influence on resting membrane potential (RMP). Thus, they are classified as background potassium channels or leak potassium channels3, 4. To date, 17 human K2P channel subunits have been identified according to their amino acid sequence identity and regulatory mechanisms. They can be divided into six subfamilies: TWIK, THIK, TASK, TALK, TREK, and TRESK5, 6.
TREK-1 is one of the most important members of the K2P channel family and is expressed throughout the central nervous system (CNS)4, 7. In addition to its unusual gating properties, such as background channel activity and sensitivity to membrane stretch, the TREK-1 channel can be modulated by many different intracellular and extracellular chemical agents. For example, TREK-1 is activated by increased temperature, membrane stretch and internal acidosis and is also sensitive to the presence of some polyunsaturated fatty acids [such as arachidonic acid (AA)] and gaseous general anesthetics (such as halothane and nitrous oxide) 8, 9, 10, 11. It has been recently reported that the TREK-1 channel is also modulated by neuroprotective agents such as riluzole and plays an important role in neuroprotection12, 13. In our previous studies, we showed that the expression of TREK-1 mRNA and protein significantly increased after acute and chronic cerebral ischemia, suggesting that the TREK-1 channel may be closely linked to pathological conditions such as cerebral ischemia14, 15.
3-n-Butylphthalide (NBP) is a potent neuroprotectant that was approved by the State Food and Drug Administration (SFDA) of China at the end of 2002 as a new drug for the treatment of ischemic stroke16. Pre-clinical and clinical studies have demonstrated that racemic NBP (dl-NBP) is a promising drug for the treatment of ischemic stroke. This neuroprotectant influences several pathophysiological processes such as improving rat brain microcirculation, inhibiting platelet aggregation, preventing oxidative damage from ischemia and reducing neuronal apoptosis 17, 18, 19, 20, 21. However, the molecular mechanisms underlying the actions of dl-NBP remain unclear. Recently, we have found that the optical isomer l-NBP is more potent in terms of neuronal protection against ischemic stroke than dl-NBP16. This study aimed to compare the effect of dl-NBP and its optical isomers on the TREK-1 channel and to further elucidate the mechanism of the protective effects of l-NBP against ischemia.
Materials and methods
Materials
l-NBP, dl-NBP, and d-NBP (purity >99%) (Figure 1A) were provided by the Department of Medical Synthetic Chemistry, Institute of Materia Medica. HEPES, EGTA, Na2ATP, AA, penicillin G, and streptomycin sulfate were purchased from Sigma Chemical Co (St Louis, MO, USA); Dulbecco's modified Eagle's medium (DMEM), trypsin and G418 were purchased from GibcoBRL (Gaithersburg, MD, USA). Other reagents were provided by Beijing Chemical Company (Beijing, China).
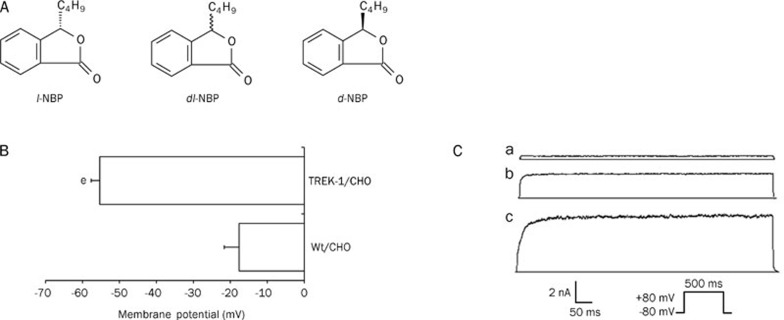
Figure 1.
Chemical structures of NBP and basic properties of the TREK-1 channel. (A) Chemical structures of racemic dl-NBP and its optical isomers. (B) The transfection of CHO cells with TREK-1 channels hyperpolarized the membrane potential, from -17.6±4.0 mV (Wt/CHO, n=7) to -55.3±2.4 mV (TREK-1/CHO, n=25). Values are expressed as the mean±SEM; eP<0.05 vs Wt/CHO cells. (C) Electrophysiological verification of the presence of TREK-1 channels in transfected CHO cells. a: Current elicited from Wt/CHO cells after being depolarized to +80 mV from a holding potential of -80 mV. b: Current elicited from TREK-1/CHO cells. c: Activation of TREK-1 currents by AA.
Cell culture
A stable cell line of wild-type Chinese hamster ovary (Wt/CHO) cells expressing rat TREK-1 channels was maintained in culture medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 μg/mL penicillin G and 100 μg/mL streptomycin sulfate in a humidified incubator with an atmosphere of 95% air and 5% CO2 at 37 °C. G418 was added into the culture medium to select for transfected cells. When the cells were 80% confluent, they were split and plated onto 35-mm culture dishes. The cells were assayed 24 h later.
Electrophysiology and drug application
Membrane currents were recorded using a whole-cell voltage-clamp configuration. Recording glass pipettes had a resistance of 3–5 MΩ. The external solution contained the following (in mmol/L): NaCl, 150; KCl, 5.4; MgCl2, 2; CaCl2, 1.2; glucose, 15; and HEPES, 5 (titrated to pH 7.4 with NaOH). The patch-pipette solution contained the following (in mmol/L): KCl, 140; MgCl2, 0.5; EGTA, 10; and HEPES, 10 (titrated to pH 7.2 with KOH). Currents were evoked in response to voltage ramps, and voltage steps were generated using an EPC-10 patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany), filtered at 2.9 kHz, digitized at 10 kHz and stored on a computer. Data were analyzed using Pulse 8.6 software (HEKA Electronics, Lambrecht, Germany). Before seal formation, the voltage offset between the patch electrode and the bath solution was adjusted to produce zero current. After seal formation (≥1 GΩ) and membrane rupturing, the cells were allowed to stabilize for approximately 5 min. The holding potential during experiments was set at -80 mV. All electrophysiological measurements were carried out at room temperature (23–25 °C).
Data analysis and statistics
All data were analyzed using Pulsefit 8.6 (HEKA Electronics, Lambrecht, Germany) and MicroCal Origin software and are expressed as means±SEM. For dose-response experiments, current amplitudes at +60 mV in the presence and absence of NBP were measured by evoking the currents with a ramp pulse protocol from -80 mV to +60 mV over 400 ms. To obtain concentration-response curves, the percent inhibition of the current by NBP was quantified at various test concentrations according to the following equation: percent inhibition=100(1–Idrug/Icontrol). The current density of TREK-1 was calculated by dividing the current by the whole-cell capacitance (expressed in pA/pF). Significant differences between groups were assessed by unpaired Student's t-test and one-way analysis of variance (ANOVA). The criterion for significance was P<0.05 in all analyses. n values indicate the number of experiments performed.
Results
Electrophysiological properties of TREK-1 channels
Under the current-clamp configuration, the RMPs of Wt/CHO and TREK-1/CHO cells were -17.6±4.0 mV (n=7) and -55.3±2.4 mV (n=25), respectively (Figure 1B). A large, depolarizing voltage step from -80 mV to +80 mV evoked a dramatic, outward, non-inactivating current in TREK-1/CHO cells but not in Wt/CHO cells (Figure 1C). We also found that 10 μmol/L AA increased TREK-1 current by 60.0%±3.6% (n=6) (Figure 1Cc). These results are consistent with previous reports regarding the properties of TREK-1 channels22.
Effect of NBP isomers on TREK-1 channel currents
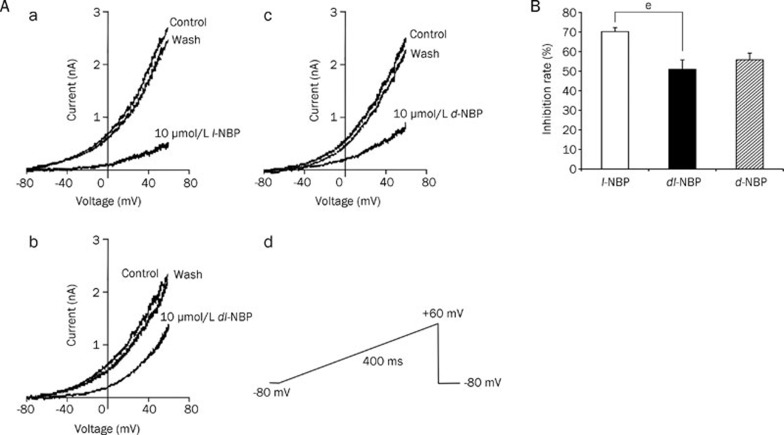
To investigate whether dl-NBP isoforms modulate transfected TREK-1 channels, we exposed TREK-1/CHO cells to dl-NBP and its optical isomers after a 5-min control period and then washed out the drugs with the control solution. TREK-1 currents were evoked by a ramp protocol from a holding potential of -80 mV to +60 mV over 400 ms. A typical control current is shown in Figure 2A, and a comparison of the inhibitory effects of NBP isoforms at +60 mV is shown in Figure 2B (P<0.05). We found that 10 μmol/L of l-NBP, dl-NBP, and d-NBP inhibited the current by 70.0%±2.0% (n=8), 50.9%±4.8% (n=9) and 55.8%±3.4% (n=9), respectively. Current inhibition was not caused by rundown, which was less than 10% over a 20-min period. This result indicates that l-NBP is much more potent than dl- and d-NBP in the inhibition of TREK-1 currents. Therefore, we focused on l-NBP alone for the remainder of the study. The inhibitory effects of NBP isomers on TREK-1 currents were partially reversed upon washout.
Figure 2.
Effect of NBP isomers on TREK-1 channel currents. (A) Whole-cell ramp currents as a function of membrane potential before, during and after application of (a) 10 μmol/L l-NBP (n=8), (b) 10 μmol/L dl-NBP (n=9) and (c) 10 μmol/L d-NBP (n=9). (d) Whole-cell ramp current recording protocol. The currents were evoked from a holding potential of -80 mV by ramping the membrane potential from -80 mV to +60 mV over 400 ms. (B) Comparison of the inhibition rates of TREK-1 currents by NBP isoforms (l-NBP n=8, dl-NBP n=9 and d-NBP n=9) measured at +60 mV using TREK-1/CHO cells. The currents were evoked from a holding potential of -80 mV, and the membrane was ramped from -80 mV to +60 mV over 400 ms. Values are expressed as percentages of the control (means±SEM); eP<0.05 vs dl-NBP group.
l-NBP inhibited TREK-1 channel currents in a dose-dependent manner
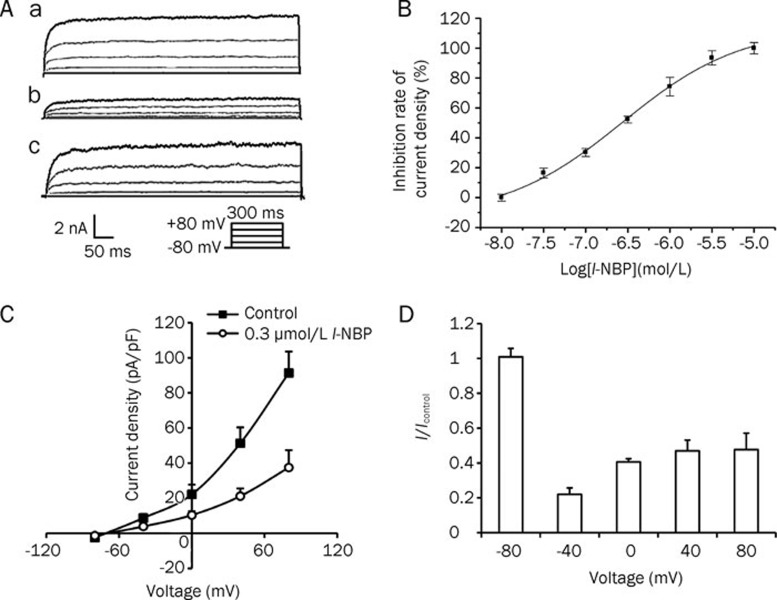
We elicited TREK-1 currents with depolarizing voltage steps from a holding potential of -80 mV (Figure 3A). l-NBP-mediated inhibition of TREK-1 currents was partially reversed upon washout. This inhibition was concentration dependent over the range of 0.01 to 10 μmol/L. The maximum inhibition of TREK-1 current by l-NBP (70.0%±2.0%, n=8) was observed at a concentration of 10 μmol/L (Figure 3B). This dose-dependent response was well fitted to the Hill equation, with an IC50 of 0.06±0.03 μmol/L and a Hill coefficient of 0.54±0.13. Figure 3C shows the current-voltage relationship (I–V) curve for the inhibition of TREK-1 channels by 0.3 μmol/L l-NBP. The inhibition was gradual and usually reached a peak 3–5 min after l-NBP exposure. Whole-cell current density was normalized to control currents, and the voltage dependence of the blockade by 10 μmol/L l-NBP was calculated (Figure 3D). The inhibition did not change substantially between -80 mV and +80 mV, indicating a lack of voltage dependence for the effect of l-NBP. This effect was partially reversed upon washout.
Figure 3.
l-NBP inhibited TREK-1 channel currents in a concentration-dependent manner. (A) The inhibition of TREK-1 currents by l-NBP. Representative current evoked by 300-ms voltage pulses from -80 mV to +80 mV in 40 mV increments. (a) Currents in TREK-1/CHO cells. (b) Inhibition of TREK-1 currents by 10 μmol/L l-NBP. (c) The TREK-1 currents returned to near the control level after washout. (B) Concentration-response curve for the inhibition of TREK-1 channels by l-NBP measured at +80 mV from the holding potential -80 mV at the end of a 300-ms pulse. Data are expressed as means±SEM from at least six cells. The IC50 was calculated as 0.06±0.03 μmol/L. (C) The I–V curve for the inhibition of TREK-1 channels by 0.3 μmol/L l-NBP was measured at +80 mV from the holding potential of -80 mV at the end of a 300-ms pulse. Data are expressed as means±SEM. (D) Voltage-independent inhibition of TREK-1 currents by l-NBP (10 μmol/L). Whole-cell current densities were normalized to control currents (lcontrol). The normalized current density for l-NBP-treated cells did not change significantly.
Effects of l-NBP on the membrane potential of TREK-1/CHO cells
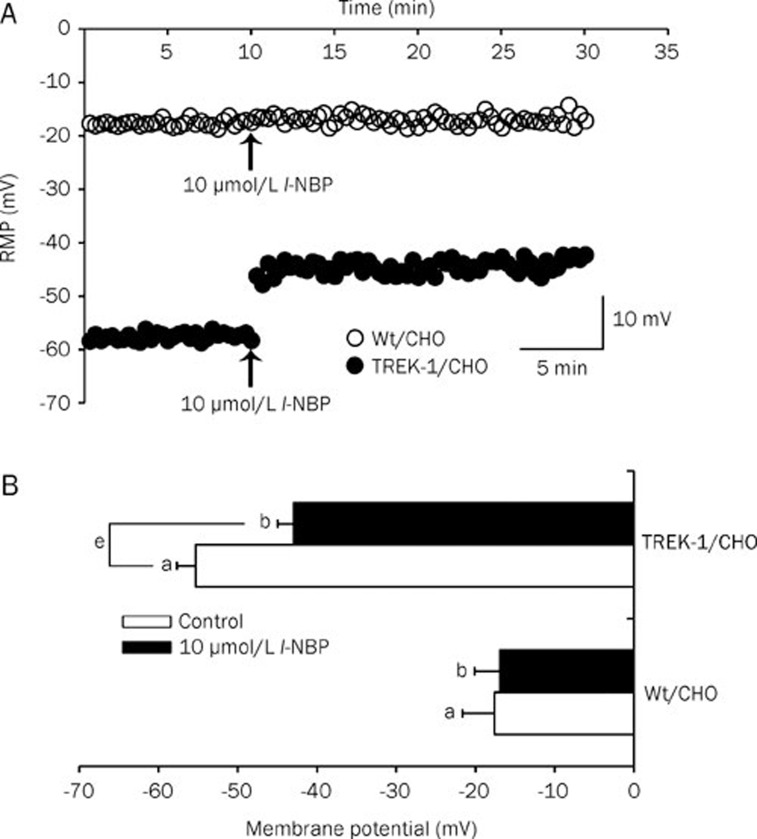
Inhibition of TREK-1 channels has been reported to depolarize the cell membrane7, 12. Therefore, we compared the effects of l-NBP on the RMPs of Wt/CHO and TREK-1/CHO cells in current-clamp mode. The results show that 10 μmol/L l-NBP shifted the RMP from -55.3±2.4 mV to -42.9±2.1 mV (n=25, Figure 4, P<0.05) in TREK-1/CHO cells but not in Wt/CHO cells (n=7), confirming the role of this channel in the maintenance of the RMP.
Figure 4.
l-NBP depolarized the membrane potential of TREK-1/CHO cells but not Wt/CHO cells. (A) Effects of 10 μmol/L l-NBP on the RMPs of Wt/CHO cells and TREK-1/CHO cells. Under a current clamp, the RMPs of the two cell lines were measured similarly every 20 s for 30 min. The RMPs for control cells and cells treated with 10 μmol/L l-NBP were monitored at 10 and 20 min, respectively. (B) Summary the RMP changes in Wt/CHO and TREK-1/CHO cells before and after exposure to 10 μmol/L l-NBP. Bar a shows the control RMPs of Wt/CHO cells and TREK-1/CHO cells. The effects of 10 μmol/L l-NBP on the RMP of the two cell lines are shown in bar b. The results are presented in pA/pF as means±SEM (Wt/CHO, n=7; TREK-1/CHO, n=25); eP<0.05 vs control.
Discussion
Two-pore-domain potassium channels form a novel class of K+ channels identified in various types of neurons23, 24. They are open when membrane potentials are in the physiological range and are therefore likely to contribute to background or leak currents. They are also crucial in shaping neuronal excitability by regulating the RMP. The TREK-1 channel is an important member of the K2P family and is expressed throughout the CNS. The TREK-1 channel is voltage independent and is not inactivated25.
TREK-1 has been previously reported to play an important role in neuroprotection against acid pathological conditions4, 7, 12, 26. In electrophysiological studies, some lipids substantially increase the probability of these K2P channels being open, thus hyperpolarizing the membrane potential and reducing neuronal excitability 12, 27, 28, 29, 30. This action on the part of lipids would be predicted to counteract the neuronal damage that arises from the increased membrane excitability that often accompanies CNS insults such as ischemia. A link between TREK-1 and neuroprotection, although a highly attractive hypothesis, has not been unequivocally demonstrated, mainly due to the lack of selective K2P antagonists13, 31.
In this study, we demonstrated that NBP, a neuroprotective agent, potently inhibited the TREK-1 channel expressed in CHO cells in a concentration-dependent manner. This study is the first description of the inhibition of TREK-1 by NBP, and this property may underlie its beneficial neuroprotective activity31. Moreover, the inhibition of TREK-1 currents by l-NBP was more significant than that by d- and dl-NBP. Therefore, the optical activity of NBP may have a close relationship with its biological activities.
Our data stand somewhat in contrast to the neuroprotection reported for TREK-1 facilitators, such as unsaturated fatty acids, riluzole and volatile anesthetics. Thus, although TREK-1 potentiation may be neuroprotective, our data also suggest that the inhibition of these channels may yield significant cell protection, similar to the effects of the neuroprotective agent sipatrigine on TREK-1 channels31.
It is well known that K2P channels can modulate the RMP and that their activity may regulate cell excitability. K2P antagonism would produce depolarization and increased membrane excitability, which may induce or enhance neuronal damage. However, recent studies have shown that the regulation of K2P channels may be complex under pathological conditions12, 32. As reported by Meadows et al31, TREK-1 antagonism produced greater changes in the excitability of inhibitory neurons than their excitatory counterparts. An increase in inhibitory tone could represent a neuroprotective mechanism. Furthermore, some immunohistochemical studies indicate that TREK-1 is predominantly expressed in GABAergic interneurons of the hippocampus, isocortex, thalamus and cerebellum7, 28, 33, 34.
Decreasing glutamate release is a widely accepted neuroprotective strategy and is also a previously well-demonstrated activity of NBP18, 31. Thus, one potential mechanism of K2P inhibition-related neuroprotection would occur through an increase in glutamate uptake by astrocytes. It has been demonstrated that TREK-1 is expressed in astrocytes, and the activators of TREK-1, including arachidonic acid and chloroform, significantly attenuate glutamate uptake by astrocytes35, 36, 37. Therefore, l-NBP-mediated inhibition of TREK-1 channels may help to maintain or increase the function of glutamate uptake by astrocytes during brain ischemia. Furthermore, the TREK-1 channel is known to be an O2-sensitive K+ channel, and acute hypoxia can occlude its activation by AA and other activators38, 39. This finding suggests that TREK-1 may not be activated during systemic hypoxia (as occurs during cerebral ischemia). Therefore, it is difficult to explain the role of TREK-1 in neuroprotection. One possibility is that this property may depend on the expression pattern of TREK-131, 38, 40, 41. Further investigation into the role of TREK-1 in neuronal damage/protection is needed.
In summary, this study demonstrated that NBP (and especially l-NBP), a novel neuroprotective agent, potently inhibits TREK-1 channels. The inhibition of TREK-1 channels results in the depolarization of the cell membrane. We suggest that the effects of NBP on TREK-1 channels are closely related to its neuroprotective role. TREK-1 channels may represent a target for NBP in treatment of cerebral ischemia and neurodegenerative diseases. However, the mechanism of protection of neurons via TREK-1 inhibition by NBP requires further study.
Author contribution
Xiao-liang WANG and Xin-cai JI designed the research; Xin-cai JI, Wan-hong ZHAO, Dong-xu CAO, and Qiao-qiao SHI performed the research; Xin-cai JI and Qiao-qiao SHI analyzed the data; Xin-cai JI and Xiao-liang WANG wrote the paper.
Acknowledgments
Our study was supported by the National Science Foundation of China (No 90913019) and a grant from the Ministry of Science and Technology of China (No 2009Zx09303-003).
We thank Dr Grilles Martin for expert technical and language assistance.
References
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–62. [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–84. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–46. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Tsai SJ. Sipatrigine could have therapeutic potential for major depression and bipolar depression through antagonism of the two-pore-domain K+ channel TREK-1. Med Hypotheses. 2008;70:548–50. doi: 10.1016/j.mehy.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, et al. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278:27406–12. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–76. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Honore E, Lazdunski M, Patel AJ. Molecular basis of the voltage-dependent gating of TREK-1, a mechano-sensitive K+ channel. Biochem Biophys Res Commun. 2002;292:339–46. doi: 10.1006/bbrc.2002.6674. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–91. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–6. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–61. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–12. [PubMed] [Google Scholar]
- Li ZB, Zhang HX, Li LL, Wang XL. Enhanced expressions of arachidonic acid-sensitive tandem-pore domain potassium channels in rat experimental acute cerebral ischemia. Biochem Biophys Res Commun. 2005;327:1163–9. doi: 10.1016/j.bbrc.2004.12.124. [DOI] [PubMed] [Google Scholar]
- Xu X, Pan Y, Wang X. Alterations in the expression of lipid and mechano-gated two-pore domain potassium channel genes in rat brain following chronic cerebral ischemia. Brain Res Mol Brain Res. 2004;120:205–9. doi: 10.1016/j.molbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Ma S, Xu S, Liu B, Li J, Feng N, Wang L, Wang X. Long-term treatment of l-3-n-butylphthalide attenuated neurodegenerative changes in aged rats. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:565–74. doi: 10.1007/s00210-009-0398-8. [DOI] [PubMed] [Google Scholar]
- Peng Y, Xing C, Lemere CA, Chen G, Wang L, Feng Y, et al. l-3-n-Butylphthalide ameliorates beta-amyloid-induced neuronal toxicity in cultured neuronal cells. Neurosci Lett. 2008;434:224–9. doi: 10.1016/j.neulet.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zeng X, Feng Y, Wang X. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol. 2004;43:876–81. doi: 10.1097/00005344-200406000-00018. [DOI] [PubMed] [Google Scholar]
- Deng W, Feng Y. Effect of dl-3-n-butylphthalide on brain edema in rats subjected to focal cerebral ischemia. Chin Med Sci J. 1997;12:102–6. [PubMed] [Google Scholar]
- Dong GX, Feng YP. Effects of 3-n-butylphthalide on cortical calcineurin and calpain activities in focal cerebral ischemia rats. Yao Xue Xue Bao. 2000;35:790–2. [PubMed] [Google Scholar]
- Lin JF, Feng YP. Effect of dl-3-n-butylphthalide on delayed neuronal damage after focal cerebral ischemia and intrasynaptosomes calcium in rats. Yao Xue Xue Bao. 1996;31:166–70. [PubMed] [Google Scholar]
- Punke MA, Licher T, Pongs O, Friederich P. Inhibition of human TREK-1 channels by bupivacaine. Anesth Analg. 2003;96:1665–73. doi: 10.1213/01.ANE.0000062524.90936.1F. [DOI] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–36. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–18. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- Liu H, Enyeart JA, Enyeart JJ. Potent inhibition of native TREK-1 K+ channels by selected dihydropyridine Ca2+ channel antagonists. J Pharmacol Exp Ther. 2007;323:39–48. doi: 10.1124/jpet.107.125245. [DOI] [PubMed] [Google Scholar]
- Segal-Hayoun Y, Cohen A, Zilberberg N. Molecular mechanisms underlying membrane-potential-mediated regulation of neuronal K2P2.1 channels. Mol Cell Neurosci. 2010;43:117–26. doi: 10.1016/j.mcn.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–93. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–33. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Laigle C, Blondeau N, Jarretou G, Lazdunski M. Alpha-linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience. 2006;137:241–51. doi: 10.1016/j.neuroscience.2005.08.083. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Chapman CG, Duckworth DM, Kelsell RE, Murdock PR, Nasir S, et al. The neuroprotective agent sipatrigine (BW619C89) potently inhibits the human tandem pore-domain K+ channels TREK-1 and TRAAK. Brain Res. 2001;892:94–101. doi: 10.1016/s0006-8993(00)03239-x. [DOI] [PubMed] [Google Scholar]
- Lawson K, McKay NG. Modulation of potassium channels as a therapeutic approach. Curr Pharm Des. 2006;12:459–70. doi: 10.2174/138161206775474477. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD, et al. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, et al. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001;86:101–14. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, et al. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci. 2009;29:8551–64. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, et al. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci. 2009;29:7474–88. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Racagni G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Mol Pharmacol. 1994;46:986–92. [PubMed] [Google Scholar]
- Miller P, Kemp PJ, Lewis A, Chapman CG, Meadows HJ, Peers C. Acute hypoxia occludes hTREK-1 modulation: re-evaluation of the potential role of tandem P domain K+ channels in central neuroprotection. J Physiol. 2003;548:31–7. doi: 10.1113/jphysiol.2003.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P, Peers C, Kemp PJ. Polymodal regulation of hTREK1 by pH, arachidonic acid, and hypoxia: physiological impact in acidosis and alkalosis. Am J Physiol Cell Physiol. 2004;286:C272–82. doi: 10.1152/ajpcell.00334.2003. [DOI] [PubMed] [Google Scholar]
- Kemp PJ, Peers C, Lewis A, Miller P. Regulation of recombinant human brain tandem P domain K+ channels by hypoxia: a role for O2 in the control of neuronal excitability. J Cell Mol Med. 2004;8:38–44. doi: 10.1111/j.1582-4934.2004.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caley AJ, Gruss M, Franks NP. The effects of hypoxia on the modulation of human TREK-1 potassium channels. J Physiol. 2005;562:205–12. doi: 10.1113/jphysiol.2004.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]