Abstract
Aim:
To investigate whether high glucose stimulates the expression of inflammatory cytokines and the possible mechanisms involved.
Methods:
ELISA and real-time PCR were used to determine the expression of the inflammatory factors, and a chemiluminescence assay was used to measure the production of reactive oxygen species (ROS).
Results:
Compared to low glucose (10 mmol/L), treatment with high glucose (35 mmol/L) increased the secretion of tumor necrosis factor (TNF)α and monocyte chemotactic protein-1 (MCP-1), but not interleukin (IL)-1β and IL-6, in a time-dependent manner in primary cultured rat microglia. The mRNA expression of TNFα and MCP-1 also increased in response to high glucose. This upregulation was specific to high glucose because it was not observed in the osmotic control. High-glucose treatment stimulated the formation of ROS. Furthermore, treatment with the ROS scavenger NAC significantly reduced the high glucose-induced TNFα and MCP-1 secretion. In addition, the nuclear factor kappa B (NF-κB) inhibitors MG132 and PDTC completely blocked the high glucose-induced TNFα and MCP-1 secretion.
Conclusion:
We found that high glucose induces TNFα and MCP-1 secretion as well as mRNA expression in rat microglia in vitro, and this effect is mediated by the ROS and NF-κB pathways.
Keywords: high glucose, microglia, tumor necrosis factor α, monocyte chemotactic protein-1, reactive oxygen species, NF-κB
Introduction
Inflammation plays a key role in central nervous system diseases. As the immune cells in the brain, microglia play an important role in the pathogenesis of central nervous system. In 2000, using nonradioactive in situ hybridization, Gregersen reported that microglia were one of the major sources of TNF after the induction of stroke in mice1. Glutamate was released by the oxygen and glucose deprivation-stressed neuron-astrocyte cultures, and then activated microglia by mGluRIIs to produce and release tumor necrosis factor (TNF)α, which induced neurotoxicity in this stroke model2. These results show that cytokines, such as TNFα, released by microglia contribute to the development of neuronal damage during a stroke. Other studies have suggested that microglia might be involved in the pathogenesis of Parkinson's disease. In the mesencephalic neuron-glia cultures, extracellular aggregated human α-synuclein activated microglia, and the activation of microglia enhanced dopaminergic neurodegeneration3. A loss of α-synuclein expression altered the morphology and inflammatory response of microglia and impaired microglial phagocytic ability4. Increased TNFα production from amyloid β-stimulated microglia led to elevated iNOS in astrocytes and neuronal apoptosis, illustrating that an increase in microglia-derived TNFα in response to amyloid β may be a contributor to neuron loss in Alzheimer's disease5. Moreover, the suppression of microglial derived TNFα and interleukin (IL)-1β by copolymer-1 diminished neurodegeneration in HIV-1 encephalitis6. Thus, inflammation induced by activated microglia is one of the major contributors to the pathogenesis of brain related diseases.
Diabetes causes many complications, including retinopathy, nephropathy, and peripheral neuropathy. In addition, diabetes has a central nervous system complication, diabetic encephalopathy, which leads to direct neuronal damage7, 8. Apoptosis-induced neuronal loss and damage in type 1 diabetes is associated with cognitive impairment9. Although the mechanisms of neuronal apoptosis in diabetic encephalopathy have not been clearly studied, hyperglycemia is thought to be one of the most important factors10, 11, 12. An increased level of glucose in the brain induced by hyperglycemia leads to neuronal apoptosis and impaired cognition9, 12, 13, 14, 15. However, the effect of hyperglycemia on the activation of microglia and the role of inflammation in the pathogenesis of diabetic encephalopathy is still unclear. We previously reported that high glucose can activate microglia and significantly increase the secretion and mRNA expression of growth-regulated oncogene (GRO), a member of the IL-8 family, in rat microglia in vitro16. This finding suggests that in the central nervous system of patients with diabetes mellitus, high concentrations of glucose may induce microglial activation and the secretion of IL-8, thus contributing to the development of diabetic encephalopathy. In this study, we investigated the effect of high glucose on the inflammatory function of microglia. We found that compared with low glucose (10 mmol/L), high glucose (35 mmol/L) increased the secretion and mRNA expression of TNFα and monocyte chemoattract protein-1 (MCP-1) in rat microglia in vitro. Reactive oxygen species (ROS) and nuclear factor kappa B (NF-κB) pathways were involved in this process. However, there were no effects of high glucose on the secretion of IL-1β and IL-6.
Materials and methods
Reagents
The OX-42 antibody was purchased from Serotec (Oxford, UK). The MCP-1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The glial fibrillary acidic protein (GFAP) antibody was purchased from Zhongshan Goldenbridge Biotechnology (Beijing, China). Poly-L-lysine, N-acetyl-L-cysteine (NAC), MG132 and pyrrolidine dithiocarbamate (PDTC) were purchased from Sigma Chemical Co (St Louis, MO, USA). Culture medium RPMI-1640 was obtained from Hyclone (Logan, UT, USA). D-glucose was obtained from Beihua Fine Chemicals (Beijing, China). Mannitol was obtained from DCPC (Beijing, China). A TNFα ELISA kit was obtained from Jingmei Biotech Co (Beijing, China).
Rat microglia primary culture
The treatment of laboratory animals and experimental protocols adhered to the guidelines of the Health Science Center of Peking University and were approved by the Institutional Authority for Laboratory Animal Care. Rat microglial cells were isolated as previously described16. In brief, cortical tissue was carefully freed from the blood vessels and meninges, minced, mechanically agitated, and washed. Single cortical cells were cultured in RPMI-1640 containing 10% fetal bovine serum (Invitrogen, Karlsruhe, Germany), 2 mmol/L glutamine, 100 U/mL penicillin G sodium and 100 μg/mL streptomycin sulfate (medium was changed every 4 d). After 14 d, the microglia were separated from the underlying astrocytic monolayer by gentle agitation and spun down. The cell pellet was resuspended and plated on uncoated Costar culture dishes. Non-adherent cells were removed after 30–60 min by changing the medium, and the adherent microglia were incubated for 24 h in culture medium before use. The purity of the microglial cultures was assessed by fluorescence immunocytochemistry with antibodies against OX-42 (microglial marker) and GFAP (astrocyte marker). This procedure produced pure primary microglial cultures that were more than 95% microglia (data not shown).
Cytokine and chemokine secretion analysis
After being seeded for 24 h, the cells were stimulated with low glucose (LG; 10 mmol/L) or high glucose (HG; 35 mmol/L). The treatment was stopped at different times, and samples of the supernatants were collected and frozen at -40 °C. MCP-1 secreted into the medium was detected using ELISA, as previously described16. TNFα and IL-6 were measured with an enzyme-linked immunosorbent assay (ELISA) kit (Jingmei Biotech Co, Beijing, China). IL-1β was quantified with a commercial kit (R&D Systems, Minneapolis, MN, USA).
Real-time quantitative RT-PCR
Total RNA was isolated using RNAtrip from Applygene (Beijing, China) and converted into first-strand cDNA with AMV reverse transcriptase, 40 U/μL of RNase inhibitor and 500 ng/μL Oligo (dT)15 primer (Promega, Madison, WI, USA). Real-time PCR was performed using 1–3 μL of the reverse transcription product, 0.5 μL HotStart Taq DNA Polymerase (TIANGEN, China), SYBR Green I (Molecular Probes, Eugene, OR, USA), 1 mmol/L dNTPs, and 0.2 μmol/L of each primer. Cycling conditions were 5 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 30 s at 58 °C, and 30 s at 72 °C, and a final incubation for 5 min at 72 °C. The primers for TNFα were 5'-TCC CAA CAA GGA GGA GAA GT-3' and 5'-TGG TAT GAA GTG GCA AAT CG-3'. The primers for MCP-1 were 5'-GGC CTG TTG TTC ACA GTT GCT-3' and 5'-TCT CAC TTG GTT CTG GTC CAG T-3'. The β-actin primers were 5'-GAG ACC TTC AAC ACC CCA GCC-3' and 5'-TCG GGG CAT CGG AAC CGC TCA-3'. To assure the specificity of the primers, the amplicons underwent melting-curve analysis. Quantification of the PCR product was carried out using the relative quantification method. Results are expressed as arbitrary units and normalized against β-actin mRNA expression, as previously described16.
Detection of reactive oxygen species
The production of ROS, especially H2O2, in rat microglia was detected with luminol plus horseradish peroxide-derived chemiluminescence in a light tight box using a luminescence analyzer (BPCL Ultra-weak, Beijing, China) at 37 °C17, 18. Photon counts were integrated over 1 s and digitized onto a computer monitor. Preparation of the reaction buffer was described previously18. Briefly, 10 mg/mL horseradish peroxide, 0.5 mmol/L luminol and HG or NG were dissolved in a total volume of 2 mL.
Statistical analyses
The results are expressed as means±SD. The differences between two groups were analyzed by Student's t-test. Multiple comparisons were made using a one-way ANOVA as appropriate with Bonferroni post-hoc test. Statistical analysis was carried out using the Prism program from GraphPad Software.
Results
High glucose induces the inflammatory reaction of rat microglia
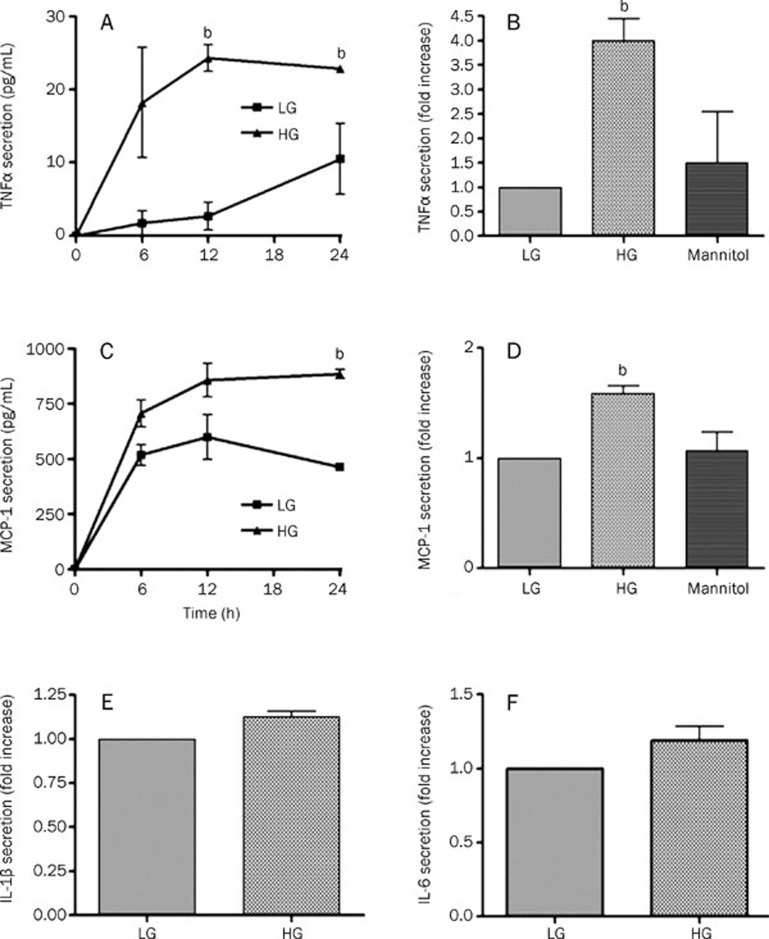
We used ELISA to examine the level of TNFα in the culture medium. Treatment with high glucose increased TNFα secretion from rat microglia in a time-dependent manner beginning at 6 h and continuing to 24 h. The final concentration was 2-fold higher than that observed in cells treated with low glucose (Figure 1A). Incubation with mannitol for 24 h had no effect on TNFα secretion (Figure 1B). These results demonstrate that the high glucose-induced TNFα secretion was not due to osmotic pressure.
Figure 1.
High glucose induces an inflammatory reaction in rat microglia. (A) Time course of TNFα secretion stimulated by high-glucose treatment. Rat microglia were cultured under low glucose (LG; 10 mmol/L) or high glucose (HG; 35 mmol/L), and the secretion of TNFα in the supernatant was measured with ELISA at the indicated time points. (B) The high-glucose treatment increased TNFα secretion independent of osmotic pressure. Rat microglia were cultured for 24 h in LG, HG, or mannitol (25 mmol/L+10 mmol/L glucose). (C) Time course of MCP-1 secretion stimulated by the high-glucose treatment. (D) The high-glucose treatment increased MCP-1 secretion independent of osmotic pressure. (E, F) High glucose had no effect on IL-1β and IL-6 secretion. The data represent the mean±SD of three independent experiments. bP<0.05 vs LG.
The level of MCP-1 in the culture medium was also determined by ELISA. MCP-1 secretion from high glucose-treated rat microglia increased in a time-dependent manner, with a final 1.5-fold increase compared to treatment with low glucose (Figure 1C). Incubation with mannitol for 24 h had no effect on MCP-1 secretion (Figure 1D). However, treatment with high glucose for 24 h had no effect on either IL-1β or IL-6 (Figure 1E, 1F) secretion compared with low glucose.
High glucose elevates TNFα and MCP-1 mRNA expression
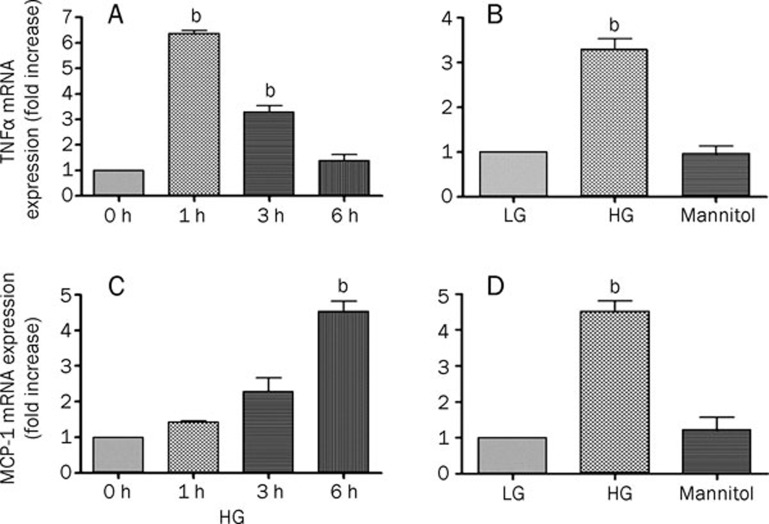
We also investigated whether high-glucose treatment could upregulate the mRNA expression of TNFα. Using real-time PCR, we found that high glucose treatment induced a 6-fold increase in TNFα mRNA expression after 1 h and a 3-fold increase after 3 h compared with low glucose (Figure 2A). However, there was no difference in TNFα mRNA expression between low glucose and high glucose treatment after 6 h. Similarly, the induction of TNFα mRNA by high glucose incubation was independent of hyperosmolality, since equimolar amounts of mannitol had no effect (Figure 2B).
Figure 2.
High-glucose treatment promotes the expression of TNFα and MCP-1 mRNA in rat microglia. (A) Real-time PCR analysis of the upregulation of TNFα mRNA by HG at different time points. bP<0.05 vs 0 h. (B) Treatment with high glucose elevated TNFα mRNA independent of osmotic pressure. Rat microglia was treated with LG, HG or mannitol for 3 h. bP<0.05 vs LG. (C) Real-time PCR analysis of the upregulation of MCP-1 mRNA by HG at different time points. bP<0.05 vs 0 h. (D) Treatment with high glucose increased MCP-1 mRNA independent of osmotic pressure. Rat microglia was treated with LG, HG or mannitol for 6 h. The data represent the mean±SD of three individual experiments. bP<0.05 vs LG.
Treatment with high glucose also induced MCP-1 mRNA expression in a time-dependent manner (Figure 2C). Rat microglia treated with high glucose for 3 h showed a 2-fold increase in MCP-1 mRNA expression compared to low glucose and an even higher increase after 6 h. Equimolar amounts of mannitol did not increase MCP-1 mRNA expression; thus, the high glucose-induced MCP-1 mRNA expression was also independent of osmotic pressure (Figure 2D).
Reactive oxygen species are involved in high glucose-induced secretion of TNFα and MCP-1
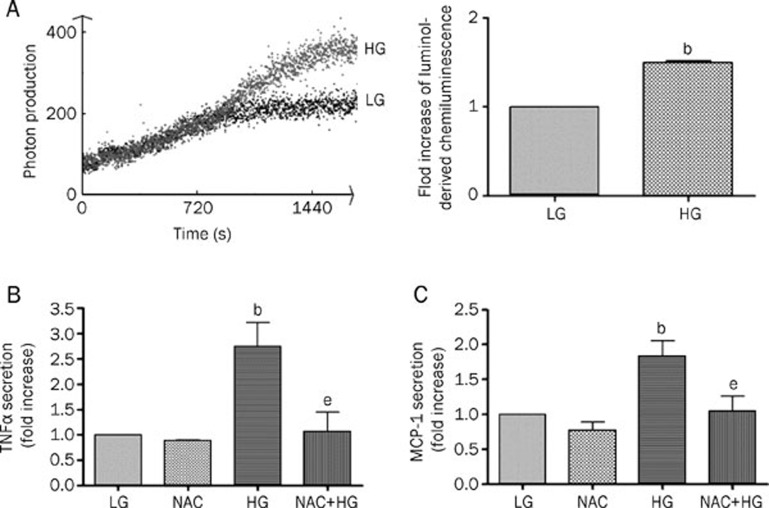
The ROS pathway is one of the most important contributors to hyperglycemia-induced cell damage19. To examine whether high-glucose treatment induces ROS production in cultured rat microglia, we measured ROS levels using chemiluminescence. After incubation with low glucose and high glucose for 20 min, we observed ROS production. Compared with low glucose, high glucose stimulated the chemiluminescence photon production and the photon-signal peak for high glucose-treated cells occurred at 1500 s (Figure 3A). To detect the involvement of ROS in the TNFα and MCP-1 secretion induced by high glucose, we tested the effect of a non-specific ROS scavenger, NAC. Microglia were pretreated with 2 mmol/L NAC for 60 min and then exposed to high glucose treatment for 24 h. NAC significantly prevented the high glucose-induced secretion of TNFα and MCP-1 (Figure 3B, 3C), but NAC alone had no effect on TNFα and MCP-1 secretion.
Figure 3.
ROS play a key role in the high glucose-induced secretion of TNFα and MCP-1 in rat microglia. (A) Effect of LG and HG on cellular ROS level. Microglia was stimulated with LG and HG for 20 min followed by a luminol-derived chemiluminescent assay. Cellular ROS levels were quantified. (B) The role of ROS in high glucose-induced TNFα secretion. (C) The role of ROS in high glucose-induced MCP-1 secretion. After treatment with 2 mmol/L of the ROS scavenger NAC for 1 h, microglia was cultured in LG or HG for 24 h. The medium was collected and the levels of TNFα and MCP-1 were analyzed by ELISA. Data represent the mean±SD of three experiments. bP<0.05 vs LG. eP<0.05 vs HG.
NF-κB plays a key role in high glucose-induced TNFα and MCP-1 secretion from rat microglia
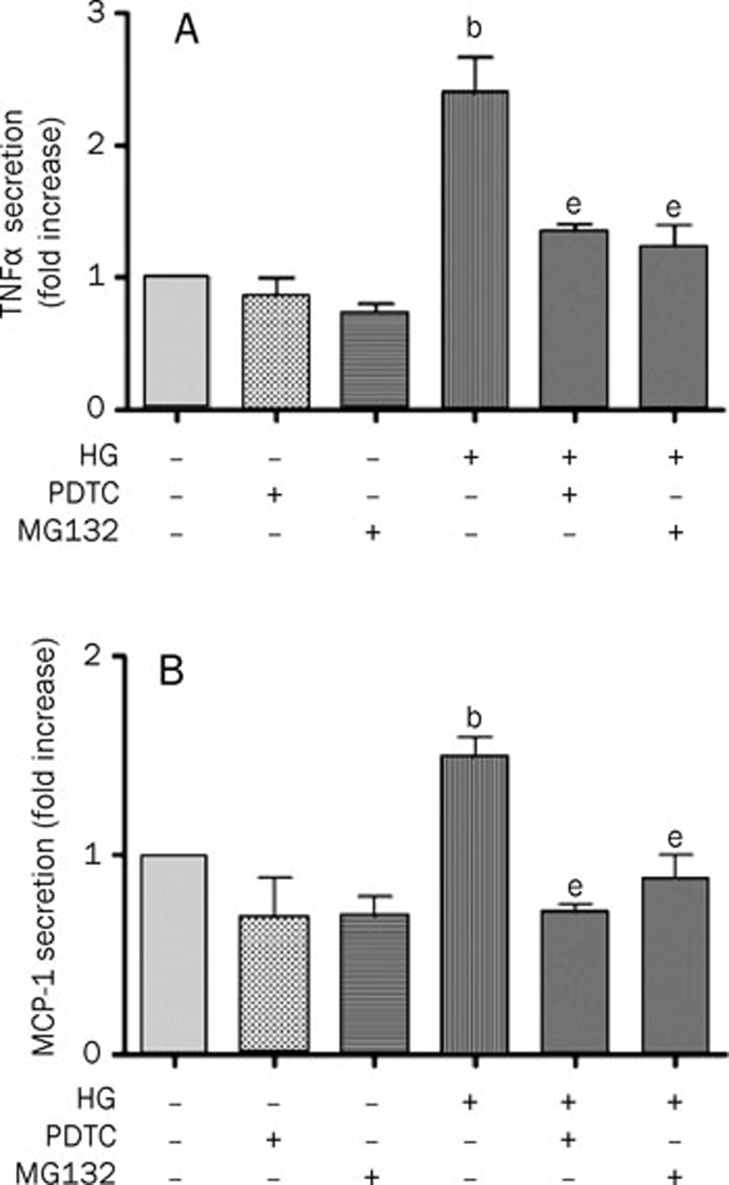
NF-κB is one of the most important transcription factors in the expression of inflammatory genes20 and is associated with the gene expression of TNFα and MCP-120, 21. High glucose incubation has been shown to significantly enhance NF-κB activation by increasing its DNA binding activity16. We investigated the role of NF-κB in high glucose-induced TNFα and IL-6 secretion at the transcriptional level. We pretreated rat microglia with the NF-κB inhibitors MG132 (10 μmol/L) or PDTC (5 μmol/L) for 60 min and then exposed them to low or high glucose for 24 h. Both MG132 and PDTC completely blocked the high glucose-induced TNFα and IL-6 secretion (Figure 4A and 4B), but neither had an effect on TNFα and IL-6 secretion alone.
Figure 4.
NF-κB is essential for high glucose-induced TNFα and MCP-1 secretion in rat microglia. After treatment with 10 μmol/L MG132 or 5 μmol/L PDTC for 1 h, microglia was cultured in LG or HG for 24 h. The medium was collected and the secretion of TNFα and MCP-1 was measured by ELISA. (A) Inhibition of the NF-κB pathway blocked high glucose-mediated TNFα secretion. (B) Inhibition of the NF-κB pathway blocked high glucose-mediated MCP-1 secretion. Data represent the mean±SD of three experiments. bP<0.05 vs control. eP<0.05 vs HG.
Discussion
We have shown that high glucose increases the secretion and mRNA expression of TNFα and MCP-1 in rat microglia. These effects are specific to high glucose rather than hyperosmolality. ROS and NF-κB pathways mediate the high glucose-induced secretion of TNFα and MCP-1. However, high glucose has no effect on the secretion of IL-1β and IL-6 compared to low glucose.
TNFα, a proinflammatory cytokine, is synthesized and released by microglia and can activate the caspase pathway, leading to neural apoptosis through the TNF receptor22, 23. It has been reported that fewer sympathetic and sensory neurons died during the phase of naturally occurring neuronal death in TNF-deficient embryos, and neurons from these embryos also survived in the culture better than wild-type neurons24. In addition to its role in apoptotic events, TNFα acts as a regulator of synaptic plasticity. Using TNFα knockout mice, Golan et al showed that TNFα may specifically suppress hippocampal function in adult mice through the suppression of NGF expression and dentate gyrus development25. MCP-1, one of the most important chemokines, plays a critical role in the migration of bone marrow derived and resident cells to sites of inflammation26. Huang et al found that MCP-1 null mice exhibited markedly reduced clinical and histological autoimmune encephalomyelitis (EAE) compared with wild-type animals. These results suggest that MCP-1 plays a crucial role in Th1 immune responses during EAE induction27. MCP-1 has been shown to inhibit HIV-tat and NMDA-induced apoptosis in mixed cultures of human neurons and astrocytes by reducing the extracellular levels of glutamate and regulating the intracellular trafficking of tat and NMDA receptor 1 expression in neurons. This finding suggests that MCP-1 may play a novel role as a protective agent against the toxic effects of glutamate and HIV-tat28. In the CNS of diabetic patients, high concentrations of glucose may induce microglial activation and the secretion of TNFα and MCP-1, thus contributing to the development of diabetic encephalopathy.
Oxidative injury induced by high glucose concentrations plays a central role in the pathogenesis of diabetic complications29. Recent observations suggest that hyperglycemia-triggered ROS contribute to the development of diabetic nephropathy30, 31. High glucose-induced ROS activated NF-κB and increased MCP-1 production, which may cause macrophage recruitment and activation, resulting in diabetic renal injury32. In addition, the formation of oxygen-derived radicals led to the activation of NF-κB, which increased the expression of vascular cell adhesion molecule 1 and MCP-1 in human aortic endothelial cells. Thus, ROS-mediated NF-κB activation may play a role in the onset of atherosclerosis in diabetic patients33. The involvement of ROS and NF-κB in high glucose-induced apoptosis has also been demonstrated in cultured human endothelial cells34. We found that treatment with a high concentration of glucose induced ROS formation and that the ROS scavenger NAC significantly reduced the high glucose-induced secretion of TNFα and MCP-1. Furthermore, the NF-κB inhibitors MG132 and PDTC completely prevented the high glucose-induced TNFα and MCP-1 secretion. Thus, ROS and NF-κB pathways mediate high glucose-induced secretion of TNFα and MCP-1 from rat microglia.
In conclusion, in our diabetic model, microglia are activated by high glucose treatment to produce ROS, which, in turn, activate the transcription factor NF-κB. Activation of NF-κB leads to the transcription of TNFα and MCP-1, resulting in an increased expression of TNFα and MCP-1. The release of inflammatory TNFα and MCP-1 by microglia may be involved in the pathogenesis of neuronal injury in diabetic encephalopathy. Overall, our study demonstrates that microglia might be a new target for the treatment of diabetic encephalopathy.
Author contribution
Most experiments were done by Yi QUAN and she also wrote the paper. Chang-tao JIANG, Bing XUE, and Shi-gong ZHU did some of the experiments. Xian WANG and Yi QUAN designed the experiments together.
Acknowledgments
This study was supported by the Major National Basic Research Program of China (No 2006CB503802) and the Program for Changjiang Scholars and Innovative Research Team in Universities, as well as the National Natural Science Foundation of China (No 30330250) awarded to Xian WANG.
References
- Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28:2221–30. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb J. 2005;19:533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–63. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–24. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Liu J, Sneller H, Dou H, Holguin A, Smith L, et al. Copolymer-1 induces adaptive immune anti-inflammatory glial and neuroprotective responses in a murine model of HIV-1 encephalitis. J Immunol. 2007;179:4345–56. doi: 10.4049/jimmunol.179.7.4345. [DOI] [PubMed] [Google Scholar]
- Li ZG, Sima AA. C-peptide and central nervous system complications in diabetes. Exp Diabesity Res. 2004;5:79–90. doi: 10.1080/15438600490424550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AA, Kamiya H, Li ZG. Insulin, C-peptide, hyperglycemia, and central nervous system complications in diabetes. Eur J Pharmacol. 2004;490:187–97. doi: 10.1016/j.ejphar.2004.02.056. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–31. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Quatraro A, Giugliano D. Diabetes mellitus and hypertension: the possible role of hyperglycaemia through oxidative stress. Diabetologia. 1993;36:265–6. doi: 10.1007/BF00399961. [DOI] [PubMed] [Google Scholar]
- Sima AA. New insights into the metabolic and molecular basis for diabetic neuropathy. Cell Mol Life Sci. 2003;60:2445–64. doi: 10.1007/s00018-003-3084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimattila S, Malmberg-Ceder K, Hakkinen AM, Vuori K, Salonen O, Summanen P, et al. Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab. 2004;24:1393–9. doi: 10.1097/01.WCB.0000143700.15489.B2. [DOI] [PubMed] [Google Scholar]
- Knudsen GM, Jakobsen J, Barry DI, Compton AM, Tomlinson DR. Myo-inositol normalizes decreased sodium permeability of the blood-brain barrier in streptozotocin diabetes. Neuroscience. 1989;29:773–7. doi: 10.1016/0306-4522(89)90148-6. [DOI] [PubMed] [Google Scholar]
- Sredy J, Sawicki DR, Notvest RR. Polyol pathway activity in nervous tissues of diabetic and galactose-fed rats: effect of dietary galactose withdrawal or tolrestat intervention therapy. J Diabet Complications. 1991;5:42–7. doi: 10.1016/0891-6632(91)90010-m. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA. C-peptide enhances insulin-mediated cell growth and protection against high glucose-induced apoptosis in SH-SY5Y cells. Diabetes Metab Res Rev. 2003;19:375–85. doi: 10.1002/dmrr.389. [DOI] [PubMed] [Google Scholar]
- Quan Y, Du J, Wang X. High glucose stimulates GRO secretion from rat microglia via ROS, PKC, and NF-kappaB pathways. J Neurosci Res. 2007;85:3150–9. doi: 10.1002/jnr.21421. [DOI] [PubMed] [Google Scholar]
- Allen RC. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–93. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- Chang L, Xu J, Yu F, Zhao J, Tang X, Tang C. Taurine protected myocardial mitochondria injury induced by hyperhomocysteinemia in rats. Amino Acids. 2004;27:37–48. doi: 10.1007/s00726-004-0096-2. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ Res. 2003;93:311–20. doi: 10.1161/01.RES.0000087642.01082.E4. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Lefebvre d'Hellencourt C, McPherson CA, Funk JA, Aoyama M, Wine RN. Tumor necrosis factor p55 and p75 receptors are involved in chemical-induced apoptosis of dentate granule neurons. J Neurochem. 2008;106:281–98. doi: 10.1111/j.1471-4159.2008.05382.x. [DOI] [PubMed] [Google Scholar]
- Bertazza L, Mocellin S. Tumor necrosis factor (TNF) biology and cell death. Front Biosci. 2008;13:2736–43. doi: 10.2741/2881. [DOI] [PubMed] [Google Scholar]
- Barker V, Middleton G, Davey F, Davies AM. TNFalpha contributes to the death of NGF-dependent neurons during development. Nat Neurosci. 2001;4:1194–8. doi: 10.1038/nn755. [DOI] [PubMed] [Google Scholar]
- Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex. 2004;14:97–105. doi: 10.1093/cercor/bhg108. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–26. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee HB. Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology (Carlton) 2005;10:S7–10. doi: 10.1111/j.1440-1797.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee HB. Reactive oxygen species and matrix remodeling in diabetic kidney. J Am Soc Nephrol. 2003;14:S246–9. doi: 10.1097/01.asn.0000077411.98742.54. [DOI] [PubMed] [Google Scholar]
- Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB. Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol. 2002;13:894–902. doi: 10.1681/ASN.V134894. [DOI] [PubMed] [Google Scholar]
- Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–34. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–45. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]