Abstract
Aim:
To assess whether systemic delivery of kynurenic acid improves the outcomes of heatstroke in rats.
Methods:
Anesthetized rats were divided into 2 major groups and given vehicle solution (isotonic saline 0.3 mL/kg rat weight) or kynurenic acid (30–100 mg in 0.3 mL saline/kg) 4 h before the start of thermal experiments. They were exposed to an ambient temperature of 43 °C for 68 min to induce heatstroke. Another group of rats were exposed to room temperature (26 °C) and used as normothermic controls. Their core temperatures, mean arterial pressures, serum levels of systemic inflammatory response molecules, hypothalamic values of apoptotic cells and neuronal damage scores, and spleen, liver, kidney and lung values of apoptotic cells were determined.
Results:
The survival time values during heatstroke for vehicle-treated rats were decreased from the control values of 475–485 min to new values of 83–95 min. Treatment with KYNA (30–100 mg/kg, iv) 4 h before the start of heat stress significantly and dose-dependently decreased the survival time to new values of 152–356 min (P<0.05). Vehicle-treated heatstroke rats displayed hypotension, hypothalamic neuronal degeneration and apoptosis, increased serum levels of tunor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and interleukin-10 (IL-10), and spleen, liver, kidney, and lung apoptosis. KYNA preconditioning protected against hypotension but not hyperthermia and attenuated hypothalamic neuronal degeneration and apoptosis during heatstroke. KYNA preconditioning attenuated spleen, kidney, liver, and lung apoptosis and up-regulated serum IL-10 levels but down-regulated serum TNF-α and ICAM-1 levels during heatstroke.
Conclusion:
Our results suggest that systemic delivery of kynurenic acid may attenuate multiorgan dysfunction in rats after heatstroke.
Keywords: heatstroke, tumor necrosis factor-α, interleukin-10, intercellular adhesion molecule-1, N-methyl-D-aspartate receptor, kynurenic acid, hypothalamus, spleen, kidney, liver
Introduction
Heatstroke is defined as a form of excessive hyperthermia (>40 °C) associated with a systemic inflammatory response that leads to multi-organ dysfunction in which central nervous system (CNS) disorders such as delusion, convulsion and coma predominate1. Our recent results have demonstrated that heatstroke rodents display hypotension, systemic inflammatory responses, hypothalamic ischemia and neuronal damage, and multi-organ dysfunction2, 3, 4.
Kynurenic acid (KYNA) or its metabolic precursor L-kynurenine may be of therapeutic value in neurodegenerative diseased models5, 6. For example, systemically administered high doses of KYNA had a neuroprotective effect in the gerbil model of global ischemia7. Both the homocysteine-induced impairment of endothelial cells8 and the motility and inflammatory activation in the early phase of acute experimental colitis in the rat9 were significantly reduced by administration of KYNA.
The aim of this study was to investigate whether the heatstroke induced hypotension, systemic inflammatory responses, hypothalamic ischemia and damage, and multi-organ dysfunction could be attenuated by KYNA preconditioning. Accordingly, the temporal profiles of the apoptotic cell numbers of spleen, kidney, liver, lung, and hypothalamus, the serum levels of systemic inflammatory response molecules (eg, tumor necrosis factor-α [TNF-α], intracellular adhesion molecule-1 [ICAM-1], and interleukin-10 [IL-10]), body core temperature (Tco), and mean arterial pressure (MAP) during heatstroke were assessed in rats treated with or without KYNA (3–30 mg/kg of body weight, intravenously, 4 h before the start of thermal experiments).
Materials and methods
Animals
Adult male Sprague-Dawley rats (weight 263±15 g) were obtained from the Animal Resource Center of the National Science Council of China (Taipei, Taiwan). The animals were housed 4 in a group at an ambient temperature of 22±1 °C, with a 12-h light/dark cycle. Pellet rat chow and tap water were available ad libitum. All protocols were approved by the Animal Ethics Committee of the Chi Mei Medical Center (Tainan, Taiwan, China) in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, as well as the guidelines of the Animal Welfare Act. Adequate anesthesia was maintained to abolish the corneal reflex and pain reflexes induced by tail pinching throughout all experiments (approximately 6 h) by a single intraperitoneal dose of urethane (1.4 g/kg body weight). At the end of the experiments, control rats that had survived heatstroke were killed with an overdose of urethane.
Surgery and physiological parameter monitoring
The right femoral artery and vein of rats were cannulated with polyethylene tubing (PE50), under urethane anesthesia, for blood pressure monitoring and drug administration. The Tco was monitored continuously by a thermocouple, while MAP and heart rate (HR) were monitored continuously with a pressure transducer.
Induction of heatstroke
The Tco of the anesthetized animals were maintained at about 37 °C with an infrared light lamp, except during the heat stress experiments. Heatstroke was induced by placing the animals in a folded heating pad maintained at 43 °C by circulating hot water. Survival time values (interval between the start of heat stress and animal death) were determined.
Experimental groups
Animals were assigned randomly to one of three groups. One group of rats, treated with an intravenous dose of vehicle solution (0.3 mL normal saline per rat) was exposed to 26 oC for up to 480 min (or to the end of the experiments). This group of rats was used as normothermic controls. The second group was treated with the same dose of vehicle solution 4 h before the initiation of heat exposure (43 °C for 68 min) and was used as vehicle-treated heatstroke animals. The third group of rats was intravenously treated with KYNA (30–100 mg/kg of body weight in 0.3 mL saline; Sigma Chemical Co, St Louis, MO, USA) 4 h before the start of thermal experiments. The last two groups of rats were exposed to heat stress (43 °C) for exactly 68 min to induce heatstroke and were then allowed to recover at 26 °C. Physiological parameters and survival time were observed for up to 480 min (or to the end of the experiments).
In Experiment 1, the survival time values for normothermic rats, vehicle-treated heatstroke rats, and KYNA-treated heatstroke rats were determined randomly.
In Experiment 2, values of both Tco and MAP for normothermic rats (NT), vehicle-treated heatstroke rats (VEH+HS), and KYNA-treated heatstroke rats (KYNA+HS) were determined at 0 min, 68 min, or 85 min after the initiation of heat exposure in heatstroke rats or the equivalent times in NT group. All heatstroke groups had heat exposure (43 °C) withdrawn exactly at 68 min and were then allowed to recover at 26 °C temperature.
In Experiment 3, values of neuronal damage score, apoptolic cell numbers of hypothalamus, spleen, kidney, liver, and lung, and serum levels of TNF-α, ICAM-1, and IL-10 were determined at 85 min after the initiation of heat exposure in heatstroke rats or the equivalent time in normothermic controls.
Neuronal damage score
Eighty-five minutes after the start of heat stress, animals were killed by an overdose of urethane, and the brains were fixed in 10% neutral-buffered formalin for at least 24 h. The brain was removed and embedded in paraffin blocks. Serial sections (10 μm thick) through the hypothalamus were stained with hematoxylin and eosin for microscopic evaluation. The extent of neuronal damage was scored on a scale of 0–3, modified from the grading system of Pulsinelli et al10, in which 0 is normal, 1 indicates approximately 30% of the neurons are damaged, 2 indicates that approximately 60% of the neurons are damaged, and 3 indicates that 100% of the neurons are damaged. Each hemisphere was evaluated independently by an examiner blinded to the experimental conditions.
The terminal deoxynucleotidyl transferase-mediated and dUTP-biotin nickened-labeling, TUNEL, staining
The TUNEL assay was performed using the apoptotic cells in different tissue samples including the spleen, kidney, liver, lung and hypothalamus. Color was developed using 3,3′-diaminobenzidine tetrachloride (Sigma chemical Co, St Louis, MO, USA). Sections were treated with xylene and ethanol to remove paraffin and for dehydration. They were then washed with phosphate buffered saline (PBS) and incubated in 3% hydrogen peroxide solution for 20 min. The sections were treated with 5 μg/mL proteinase k for 2 min at room temperature, and rewashed in PBS (0.1 mol/L, pH 7.4). The sections were then treated with a TUNEL reaction mixture (terminal deoxynucleotidyl transferase nucleotide mixture, Roche, Mannheim, Germany) at 37 oC for 1 h, and the sections were washed with distilled water. They were then incubated in anti-fluorescein antibody conjugated with horseradish peroxidase at room temperature for 30 min, washed and visualized using the avadinbiotin complex technique and 0.05% 3,3′-diaminobenzidine tetrachloride as a chromogen. The numbers of TUNEL-positive cells were counted by a pathologist at 200× magnification, 30 fields per section. Blinding was performed for the pathologist's grading of results.
Measurement of serum TNF-α, ICAM-1, and IL-10 levels
Eighty-five minutes after the start of heat stress, blood samples were collected, immediately separated, and stored at -80 °C until they could be assayed. We used commercially available ELISA kits for the determination of serum TNF-α, ICAM-1, and IL-10 levels (Quantikine, R&D Systems Inc, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
All data are expressed as means±standard deviation. One-way analysis of variance with Tukey's multiple comparisons test was used for serum markers, Tco, MAP, survival time, and apoptotic cell numbers. The Wilcoxon test was used for histological assessment. Significant differences were established at P<0.05. For all statistical analyses, SPSS software version 10.0 (SPSS Inc, Chicago, IL, USA) was used.
Results
KYNA prolonged survival time values during heatstroke
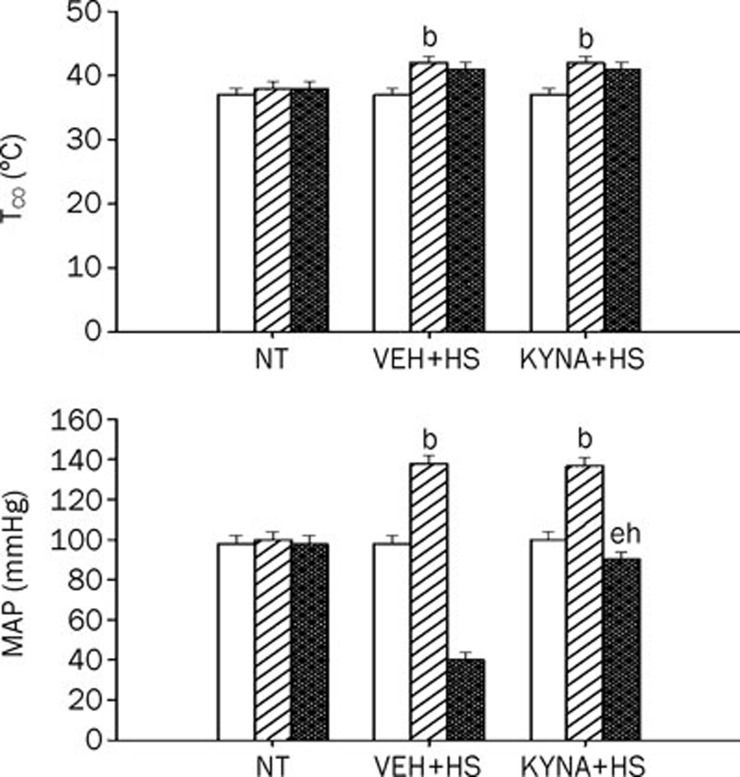
As depicted in Figure 1, body Tco and MAP increased to values of about 41 °C and about 140 mmHg, respectively at 68 min after the start of heat exposure (43 °C). However, 85 min after the start of heat stress, the Tco and MAP reached new values of about 41 °C and about 40 mmHg, respectively. The instant (85 min after heat exposure) in which Tco rose above 41 °C and MAP dropped about 40 mmHg was arbitrarily defined as the time point for the onset of heatstroke3, 11, 12. At 68 min, the heating pad was removed, and the animals were allowed to recover at room temperature (26 °C). The survival time values during heatstroke for vehicle-treated rats were decreased from the control values of 475–485 min (n=6) to new values of 83-95 min (n=6). Treatment with KYNA (30–100 mg per kg of body weight, intravenously) 4 h before the start of heat stress significantly (P<0.05) and dose-dependently decreased the survival time to new values of 152–356 min compared with normothermic rats (Table 1).
Figure 1.
Values of both core temperature (Tco) and mean arterial pressure (MAP) for normothermic controls (NT), heatstroke (HS) rats treated with vehicle solution (VEH+HS) and HS rats treated with KYNA (KYNA+HS). The values were obtained at 0 (empty bar), 68 (diagonally shaded bar), or 85 (cross-hatched bar) min after the initiation of heat exposure in heatstroke rats or the equivalent times in NT. bP<0.05 in comparison with NT group; eP<0.05 in comparison with time “0” group; hP<0.05 in comparison with (VEH+HS) group. All HS groups had heat exposure (43 °C) withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD of six rats for each group.
Table 1. The survival time values for normothermic rats, vehicle-treated rats, and KYNA-treated rats. Data were mean±SD. n=6. bP<0.05, in comparison with group 1; eP<0.05, in comparison with group 2.
| Treatment groups | Survival time (min) |
|---|---|
| Normothermic rats | 480±5 |
| Vehicle-treated heatstroke rats | 89±6b |
| KYNA (10 mg/kg, iv)-treated heatstroke rats | 152±7e |
| KYNA (30 mg/kg, iv)-treated heatstroke rats | 191±9e |
| KYNA (100 mg/kg, iv)-treated heatstroke rats | 356±17e |
All heatstroke rats which had heat exposure (43 °C) were withdrawn exactly at 68 min and then allowed to recover at room temperature (26 °C). Heatstroke rats were killed 85 min after the start of heat stress, where as the normothermic rats were killed about 480 min after the start of experiment (or at the experiment end). Vehicle or KYNA was injected 4 h before the start of experiments.
KYNA protected against hypotension but not hyperthermia during heatstroke
As shown in this Figure 1, heat stress induced significant (P<0.05) increase in both Tco and MAP in vehicle-treated heatstroke rats at 68 min. However, 17 min after termination of heat stress (or 85 min after the initiation of heat exposure), the values of MAP but not Tco were significantly (P<0.05) lower than those of the normothermic rats (40 mmHg vs 98 mmHg; Figure 1). The heat-induced hypotension, but not hyperthermia, was significantly (P<0.05) reduced by KYNA preconditioning.
KYNA attenuated hypothalamic neuronal degeneration and apoptosis during heatstroke
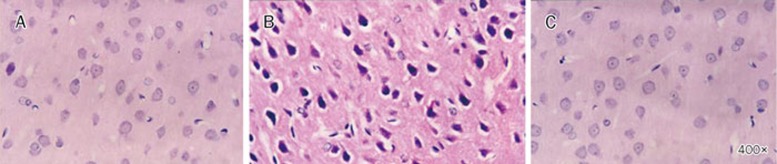
As shown in Table 2, after the onset of heatstroke, the hypothalamic neuronal damage scores were higher in animals treated vehicle compared with the normothermic controls. Histopathologic verification revealed that heatstroke caused cell body shrinkage, pyknosis of the nucleus, loss of Nissl substance, and disappearance of the nucleus in the hypothalamus of vehicle-treated rats (Figure 2). The figure also showed that the heat-induced hypothalamic neuronal degeneration were greatly reduced in KYNA-treated heatstroke rats (P<0.05).
Table 2. The neuronal damage scores for normothermic rats, vehiclet-reated heatstroke rats, and KYNA-treated heatstroke rats. bP<0.05, compared with group 1; eP<0.05, compared with group 2.
| Treatment groups | Neuronal damage score (0–3) |
|---|---|
| Normothermic rats | 0 (0, 0) |
| Vehicle-treated heatstroke rats | 2 (2, 2)b |
| KYNA (30 mg/kg, iv)-treated heatstroke rats | 1 (0.25, 0.75)e |
| KYNA (100 mg/kg, iv)-treated heatstroke rats | 0 (0, 0)e |
Values represented the median with the first and third quartile in parentheses of 6 rats per group. For determination of neuronal damage score, heatstroke rats were killed 85 min after the start of heat stress, whereas the normothermic rats were killed 480 min after the start of heat exposure (or at the experiment end). All heatstroke rats which had heat exposure (43 °C) were withdrawn exactly at 68 min and then allowed to recover at room temperature (26 °C). The data were evaluated by a Wilcoxon signed rank test followed by the Duncan test when appropriate.
Figure 2.
Histological examination of neuronal damage. The photomicrograph of the hypothalamus for a normothermic rat (A), a heatstroke rats treated with vehicle solution (B), or a heatstroke rat treated with KYNA (C). Heatstroke rats were killed 85 min after the start of heat stress, whereas the normothermic rats were killed at the equivalent time period. The hypothalamus of the vehicle-treated rats showed cell body shrinkage, pyknosis of the nucleus, loss of Nissl substance, and disappearance of the nucleolus (B). However, heat-induced neuronal damage was reduced in group “C”. Magnification ×400
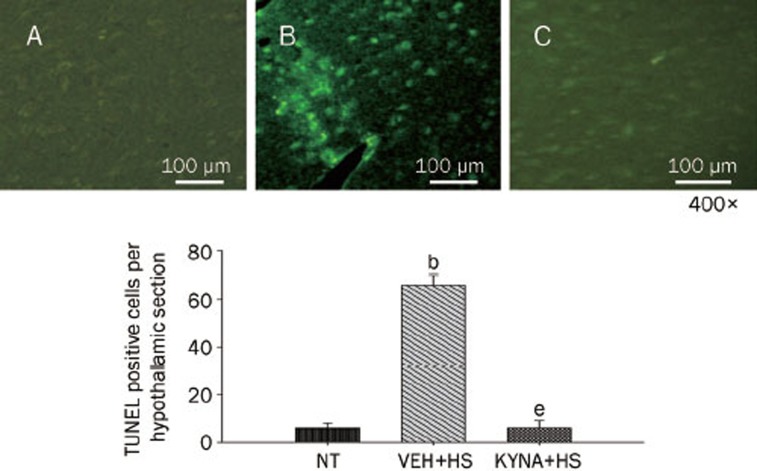
Figure 3 showed the effects of heat exposure on the number of TUNEL-positive cells in the hypothalamus of normothermic controls, vehicle-treated heatstroke rats, and KYNA-treated heatstroke rats. After the onset of heatstroke (or 85 min after the start of heat stress), the number of TUNEL-positive cells of the hypothalamus was greater (P<0.05) in vehicle-treated heatstroke rats than in the normothermic controls. However, increase of TUNEL-positive cells in the hypothalamus of heatstroke rats was greatly attenuated by KYNA. A typical example of TUNEL staining of the hypothalamus was shown in the top panel of Figure 3.
Figure 3.
Mean number of TUNEL-positive cells per hypothalamic section for normothermic controls (NT), vehicle-treated heatstroke rats (VEH+HS), and KYNA-treated heatstroke rats (KYNA+HS). The values were obtained 85 min after the start of heat exposure for heatstroke groups or the equivalent time period for normothermic group. bP<0.05, in comparison with NT group. eP<0.05, in comparison with (VEH+HS) group. All heatstroke groups had heat exposure (43 °C) withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD or six rats per group. Top panels showed the hypothalamic staining for a NT rat (A), a (VEH+HS) rat (B), and a (KYNA+HS) rat (C).
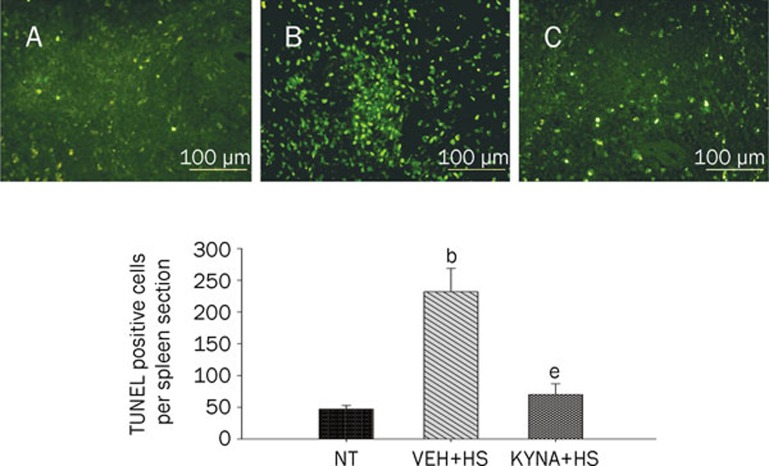
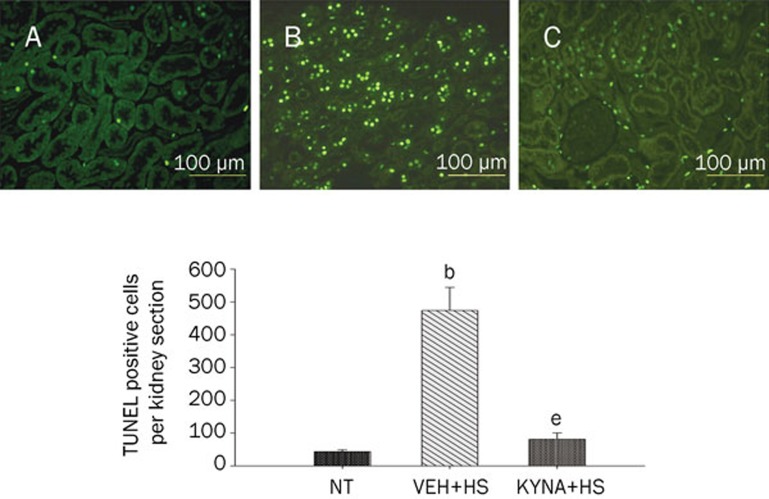
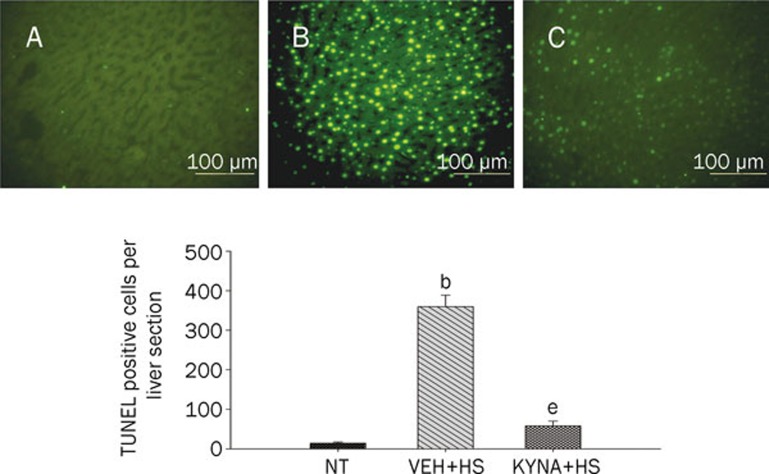
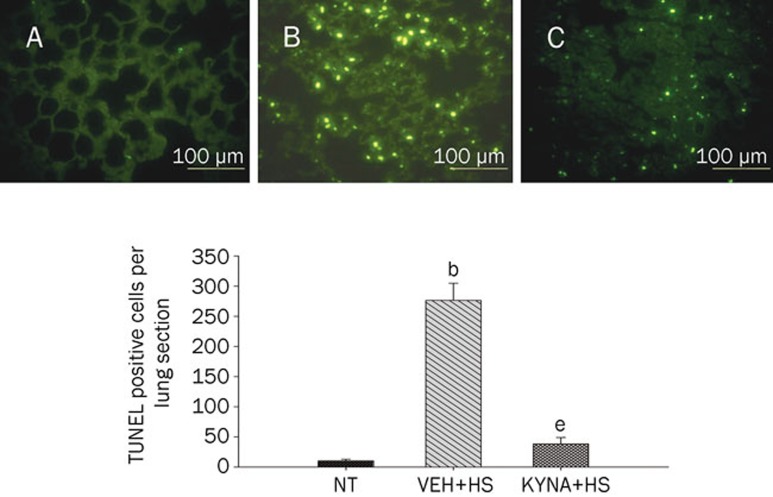
KYNA attenuated spleen, kidney, liver, and lung apoptosis during heatstroke
Figures 4, 5, 6, 7 summarized the effects of heat exposure on the number of TUNEL-positive cells in the spleen, the kidney, the liver, and the lung, respectively, of normothermic controls, vehicle-treated heatstroke rats, and KYNA-treated heatstroke rats. After the onset of heatstroke, the number of TUNEL-positive cells of these organs were greater (P<0.05) in vehicle-treated heatstroke rats than in the normothermic controls. However, increase of TUNEL-positive cells in these organs of heatstroke rats was greatly (P<0.05) attenuated by KYNA preconditioning. The typical examples of TUNEL stainings of these organs were shown in the top panel of Figures 4, 5, 6, 7, respectively.
Figure 4.
Values of renal TUNEL-positive cells for NT rats, (VEH+HS) rats, and (KYNA+HS) rats. The values were obtained at 85 min after the initiation of heat exposure in heatstroke rats or the equivalent times in NTs. bP<0.05 in comparison with (NT) group; eP<0.05 in comparison with (VEH+HS) group. All HS groups had heat exposure (43 °C) withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD of six rats for each group. Top panels depicted the kidney TUNEL-positive cells for a NT rat (A), a (VEH+HS) rat (B), and a (KYNA+HS) rat (C).
Figure 5.
Values of spleen TUNEL-positive cells for NT rats, (VEH+HS) rats, and (KYNA+HS) rats. The values were obtained at 85 min after the initiation of heat exposure in heatstroke rats or the equivalent times in NTs. bP<0.05 in comparison with (NT) group; eP<0.05 in comparison with (VEH+HS) group. All HS group had heat withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD of six rats for each group. Top panels depicted the TUNEL stainings for a NT rat (A), a (VEH+HS) rat (B), and a (KYNA+HS) rats (C).
Figure 6.
Values of liver TUNEL-positive cells for NT rats, (VEH+HS) rats, and (KYNA+HS) rats. The values were obtained at 85 min after the initiation of heat exposure in heatstroke rats or the equivalent times in NTs. bP<0.05 in comparison with NT group; eP<0.05 in comparison with (VEH+HS) group. All HS groups had heat withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD of six rats for each group. Top panels depicted the TUNEL stainings for a NT rat (A), a (VEH+HS) rat (B), and a (KYNA+HS) rat (C).
Figure 7.
Values of lung TUNEL-positive cells for NT rats, (VEH+HS) rats, and (KYNA+HS) rats. The values were obtained at 85 min after the initiation of heat exposure in heatstroke rats or the equivalent times in NTs. bP<0.05 in comparison with NT group; eP<0.05 in comparison with (VEH+HS) group. All HS groups had heat withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD of six rats for each group. Top panels depicted the TUNEL stainings for a NT rat (A), a (VEH+HS) rat (B), and a (KYNA+HS) rat (C).
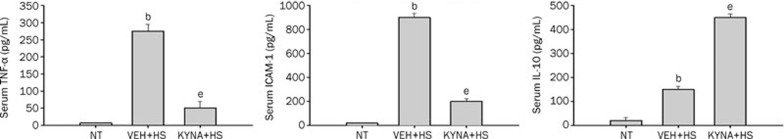
KYNA up-regulated serum IL-10 levels but down-regulated serum TNF-α and ICAM-1 levels during heatstroke
Figure 8 showed the serum levels of ICAM-1, TNF-α, and IL-10 among the three experimental groups. Compared with the normothermic controls, vehicle-treated heatstroke rats had higher levels (P<0.05) of ICAM-1 and TNF-α after the onset of heatstroke. The increase in the serum levels of these two markers caused by heatstroke were significantly reduced by KYNA preconditioning. However, compared with the vehicle-treated rats, KYNA-treated rats had higher (P<0.05) serum levels of IL-10 after the onset of heatstroke.
Figure 8.
Serum levels of tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and interleukin-10 (IL-10) for NT rats, (VEH+HS) rats, and (KYNA+HS) rats. The samples were obtained at 85 min after the initiation of heat exposure in heatstroke rats or the equivalent times in NTs group. bP<0.05, in comparison with NT group; eP<0.05 in comparison with (VEH+HS) group. All heatstroke group had heat exposure (43 °C) withdrawn exactly at 68 min and were then allowed to recover at room temperature (26 °C). Bars were the mean±SD of six rats for each group.
Discussion
A hypothesis of how heat stress leads to multi-organ dysfunction has been proposed2, 13. Heat stress stimulates metabolism and progressively reduces blood flow to critical splanchnic and brain tissues. Increased metabolic demand coupled with reduced splanchnic and brain blood flow generates cellular hypoxia, compromises cellular energy production, and produces derangements in intracellular Ca2+ homeostasis. As heat stress continues, hypoxic cells can produce multiorgan dysfunction and inflammation. Although the severity of heatstroke depends on the degree of hyperthermia and its duration14, normal volunteers can passively endure a core temperature of about 42 °C with none or minimal tissue injury15, 16. Indeed, as demonstrated in the present results, KYNA treatment significantly prevented the occurrence of heat-induced multi-organ damage and inflammation without affecting the induced hyperthermia. It should be mentioned that, in the present study, all these heatstroke animals were under the general anesthesia of urethane. Although the survival time values of these heatstroke animals were prolonged by KYNA preconditioning, but all the animals died. To determine whether there was durable improvement in survival, unanesthetized and unrestrained animals with or without KYNA should be exposed to heat stress in future studies.
Recent findings have documented that unanesthetized, unrestrained rodents display thermoregulatory deficits (eg, the heatstroke animals showed hypothermia when exposed to room temperature, 26 °C) 4 h after the initiation of heat stress17, 18. The heatstroke-induced thermoregulatory deficits may have resulted from neuronal apoptosis and cell degeneration in the hypothalamus (as demonstrated in the current study). The current results further showed that the hypothalamic apoptosis and neuronal degeneration that occurred during heatstroke in rats could be significantly ameliorated by KYNA treatment.
KYNA is a tryptophan metabolite formed as part of the kynurenine pathway. It has been shown that KYNA is an antagonist of the glycine site of NMDA and of the α-7 nicotinic acetylcholine receptors19, 20 as well as a ligand for the orphan G-protein coupled receptor, GPR 3521. Excessive or persistent activation of glutamate receptors in the mammalian central nervous system results in neuronal death, contributing to brain damage in stroke, trauma, epilepsy22 or other neurodegenerative disorders23. Most glutamate effects are mediated by the N-methyl-D-aspartate (NMDA) receptor, although α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) or kainite24 receptors, and metabotropic G-protein-coupled receptors25 may also be involved. Only high doses of KYNA proved to be neuroprotective in neonatal rats by reducing anoxia-26 or hypoxia-ischemia-induced27 brain edema and in adult rats28 and gerbils7 given before ischemia induction. For example, KYNA exerted a neuroprotective effect at very high doses (1000–1200 mg/kg, ip), which induced a marked increase in the whole brain concentration of KYNA in gerbils subjected to transient forebrain ischemia7. Based on these observations together, KYNA might cause the attenuation of heat-induced multiple organ dysfunction via the mechanisms of the glycine site of NMDA and of the α-7 nicotinic acetylcholine receptors as well as a ligand for the orphan G-protein coupled receptor, GPR35. Altered blood-brain-barrier (BBB) permeability and brain injury occurred during heatstroke in rats29. In addition, human umbilical cord derived CD34+ cells were found to be able to pass into the hyperthermic brain to attenuate heat-induced brain injury and damage in rats30. It could be derived from the foregoing statements that systemic delivery of KYNA might ameliorate multiorgan dysfunction that occurred during heatstroke via both the central and peripheral components of inhibition. In particular, KYNA might have achieved its central action via passing through the disrupted blood-brain-barrier.
It is generally believed that activation of the excitatory amino acid receptors plays an important role in neuronal death in stroke31, as well as in the grey matter ischemia32. Kynurenic acid is one of the few known endogenous NMDA receptor inhibitors33. Our data and theoretical consideration suggest that KYNA or its metabolic precursor L-kynurenine may be of therapeutic value in heatstroke syndromes. However, the use of KYNA as a neuroprotective agent can be excluded because it is barely able to cross the BBB, whereas L-kynurenine is transported much more readily across the BBB34.
In additional to inducing neuronal damage to brain tissues, heatstroke caused severe damage to the lung, kidney, liver, and spleen2, 3, 4. The current results further demonstrated that heat-induced multi-organ damage could be significantly ameliorated by KYNA preconditioning in the rat. In fact, the glutamate receptors are also present in pancreas, gut, kidney, liver, lung, spleen, and testis35, 36. Experiments performed on bovine aorta endothelial cell cultures showed that KYNA exerted a protective activity against the homocysteine-induced impairment of endothelial cells8. The addition of KYNA significantly increased endothelial cell migration and proliferation, which was diminished by homocysteine. KYNA also protected cells against homocysteine-induced cytotoxicity. KYNA was also shown to decrease motility and inflammatory activation in the early phase of acute experimental colitis in the rat9.
A high affinity glutamate/aspartate transport system exists in pancreatic islets of langerhans and that this system contributes to a glutamatergic signaling pathway that can modulate glucose-inducible insulin secretion35. Our previous results37 showed that, after the onset of heatstroke in the rat, hypotension accompanied by no change in blood levels of glucose were observed.
It has been documented that the pathophysiological responses exerted during heatstroke are the results of a systemic inflammatory response that ensues following thermal injury rather than a direct effect of hyperthermia1. The serum TNF-α and ICAM-1 levels can be regarded as markers for the systemic inflammatory response as they indirectly reflect the whole body production of both TNF-α and ICAM-1 in various organs38, 39, 40, 41. The expression of adhesion molecules can be induced by TNF-α38. The adhesion molecule ICAM-1 mediates firm adhesion between leukocytes and endothelial cells and contributes to the migration of leukocytes from post-capillary venues into the reperfused tissue42, 43. ICAM-1 initiates adhesion and transendothelial migration of circulating leukocytes42. Evidence has also suggested that IL-10 may have a therapeutic potential in acute and chronic inflammatory disease44. Exogenous administration of recombinant IL-10 protects mice from lethal endotoxemia by reducing TNF-α release44. In the present study, we showed that KYNA preconditioning increased the serum levels of IL-10 and decreased the serum levels of both TNF-α and ICAM-1, and prolonged the survival time during heatstroke. These observations suggested that KYNA might improve survival of heatstroke rats via reducing systemic inflammatory response in the rat.
In summary, the present data indicated that KYNA might exert a protective role on multiple organs during heatstroke through the following mechanisms: (A) Anti-inflammation: KYNA inhibited the up-regulation of systemic inflammatory response molecules such as TNF-α and ICAM-1 but enhanced the up-regulation of IL-10: (B) Anti-hypotension: KYNA reduced heat-induced splanchnic and brain ischemia; (C) Anti-apoptosis: KYNA inhibited heat-induced hypothalamic neuronal apoptosis and degeneration and multi-organ dysfunction.
Author contribution
Yi-chang HSIEH and Sheng-hsien CHEN designed research; Yi-chang HSIEH and Ruei-feng CHEN performed research; Yi-shian YEH contributed new analytical tools and reagents; Sheng-hsien CHEN and Jui-hsiang HSIEH analyzed data; Mao-tsun LIN and Sheng-hsien CHEN wrote the paper.
Acknowledgments
This work was supported in part by the National Science Council (Taipei, Taiwan, China) NSC96-2314-B-384-002 and NSC96-2314-B-384-003-MY3 and DOH99-TD-B-111-003, Center of Excellence for Clinical Trail and Research in Neuroscience.
References
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Chang CK, Chang CP, Chiu WT, Lin MT. Prevention and repair of circulatory shock and cerebral ischemia/injury by various agents in experimental heatstroke. Curr Med Chem. 2006;13:3145–54. doi: 10.2174/092986706778742945. [DOI] [PubMed] [Google Scholar]
- Liu WS, Chen CT, Foo NH, Huang HR, Wang JJ, Chen SH, et al. Human umbilical cord blood cells protect against hypothalamic apoptosis and systemic inflammation response during heatstroke in rats. Pediatr Neonatol. 2009;50:208–16. doi: 10.1016/S1875-9572(09)60065-6. [DOI] [PubMed] [Google Scholar]
- Yang HH, Chang CP, Cheng JT, Lin MT.Inhibition of acute lung inflammation and injury is a target of brain cooling after heatstroke injury J Trauma 2010. in press. [DOI] [PubMed]
- Nemeth H, Robotka H, Toldi J, Vecsei L. Kynurenines in the central nervous system: recent developments. Centr Nerv Sys Agents Med Chem. 2007;7:45–56. [Google Scholar]
- Salvati P, Ukmar G, Dho L, Rosa B, Cini M, Marconi M, et al. Brain concentrations of kynurenic acid after a systemic neuroprotective dose in the gerbil model of global ischemia. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:741–52. doi: 10.1016/s0278-5846(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Wejksza K, Rzeski W, Turski WA. Kynurenic acid protects against the homocysteine-induced impairment of endothelial cells. Pharmacol Rep. 2009;61:751–6. doi: 10.1016/s1734-1140(09)70130-6. [DOI] [PubMed] [Google Scholar]
- Varga G, Erces D, Fazekas B, Fulop M, Kovacs T, Kaszaki J, et al. N-Methyl-D-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol Motil. 2010;22:217–25. doi: 10.1111/j.1365-2982.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Duffy TE. Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol. 1982;11:499–509. doi: 10.1002/ana.410110510. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Tsai YC, Huang KF, Lin MT. Resuscitation from experimental heatstroke by transplantation of human umbilical cord blood cells. Crit Care Med. 2005;33:1377–83. doi: 10.1097/01.ccm.0000165966.28936.89. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Tsai YC, Huang KF, Lin CL, Lin MT. Infusion of human umbilical cord blood cells protect against cerebral ischemia and damage during heatstroke in the rat. Exp Neurol. 2006;199:67–76. doi: 10.1016/j.expneurol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol. 2001;280:H509–21. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, et al. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173–81. doi: 10.7326/0003-4819-129-3-199808010-00001. [DOI] [PubMed] [Google Scholar]
- Bynum GD, Pandolf KB, Schuette WH, Goldman RF, Lees DE, Whang-Peng J, et al. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. Am J Physiol. 1978;235:R228–36. doi: 10.1152/ajpregu.1978.235.5.R228. [DOI] [PubMed] [Google Scholar]
- Pettigrew RT, Galt JM, Ludgate CM, Horn DB, Smith AN. Circulatory and biochemical effects of whole body hyperthermia. Br J Surg. 1974;61:727–30. doi: 10.1002/bjs.1800610914. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Premachandran S, Bagewadikar RS, Bhattacharya S, Chattopadhyay S, Poduval TB. Arginine metabolic pathways determine its therapeutic benefit in experimental heatstroke: role of Th1/Th2 cytokine balance. Nitric Oxide. 2006;15:408–16. doi: 10.1016/j.niox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Shen KH, Lin CH, Chang HK, Chen WC, Chen SH. Premarin can act via estrogen receptors to rescue mice from heatstroke-induced lethality. Shock. 2008;30:668–74. doi: 10.1097/SHK.0b013e31817538cb. [DOI] [PubMed] [Google Scholar]
- Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154:85–7. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–8. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Prass K, Dirnagl U. Glutamate antagonists in therapy of stroke. Restor Neurol Neurosci. 1998;13:3–10. [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Simon RP, Young RS, Stout S, Cheng J. Inhibition of excitatory neurotransmission with kynurenate reduces brain edema in neonatal anoxia. Neurosci Lett. 1986;71:361–4. doi: 10.1016/0304-3940(86)90648-8. [DOI] [PubMed] [Google Scholar]
- Andine P, Lehmann A, Ellren K, Wennberg E, Kjellmer I, Nielsen T, et al. The excitatory amino acid antagonist kynurenic acid administered after hypoxic-ischemia in neonatal rats offers neuroprotection. Neurosci Lett. 1988;90:208–12. doi: 10.1016/0304-3940(88)90813-0. [DOI] [PubMed] [Google Scholar]
- Germano IM, Pitts LH, Meldrum BS, Bartkowski HM, Simon RP. Kynurenate inhibition of cell excitation decreases stroke size and deficits. Ann Neurol. 1987;22:730–4. doi: 10.1002/ana.410220609. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Westman J, Cervos-Navarro J, Nyberg F. Role of neurochemicals in brain edema and cell changes following hyperthermic brain injury in the rat. Acta Neurochir Suppl. 1997;70:269–74. doi: 10.1007/978-3-7091-6837-0_84. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Chang HK, Chen WC, Huang KF, Lin MT. Human umbilical cord blood-derived CD34+ cells cause attenuation of multiorgan dysfunction during experimental heatstroke. Shock. 2007;27:663–71. doi: 10.1097/01.shk.0000248593.71388.40. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–4. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Kumura E, Rosner G, Heiss WD, Graf R. Extracellular correlates of glutamate toxicity in short-term cerebral ischemia and reperfusion: a direct in vivo comparison between white and gray matter. Brain Res. 2005;1037:43–51. doi: 10.1016/j.brainres.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci. 1990;10:2965–73. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Gundersen V, Verdoorn TA. A high affinity glutamate/aspartate transport system in pancreatic islets of Langerhans modulates glucose-stimulated insulin secretion. J Biol Chem. 1998;273:1647–53. doi: 10.1074/jbc.273.3.1647. [DOI] [PubMed] [Google Scholar]
- Gill SS, Mueller RW, McGuire PF, Pulido OM. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol Pathol. 2000;28:277–84. doi: 10.1177/019262330002800207. [DOI] [PubMed] [Google Scholar]
- Chou YT, Lai ST, Lee CC, Lin MT. Hypothermia attenuates circulatory shock and cerebral ischemia in experimental heatstroke. Shock. 2003;19:388–93. doi: 10.1097/00024382-200304000-00016. [DOI] [PubMed] [Google Scholar]
- Wyble CW, Desai TR, Clark ET, Hynes KL, Gewertz BL. Physiologic concentrations of TNFalpha and IL-1beta released from reperfused human intestine upregulate E-selectin and ICAM-1. J Surg Res. 1996;63:333–8. doi: 10.1006/jsre.1996.0271. [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–81. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Kuzu MA, Koksoy C, Kuzu I, Gurhan I, Ergun H, Demirpence E. Role of integrins and intracellular adhesion molecule-1 in lung injury after intestinal ischemia-reperfusion. Am J Surg. 2002;183:70–4. doi: 10.1016/s0002-9610(01)00846-7. [DOI] [PubMed] [Google Scholar]
- Olanders K, Sun Z, Borjesson A, Dib M, Andersson E, Lasson A, et al. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86–92. doi: 10.1097/00024382-200207000-00016. [DOI] [PubMed] [Google Scholar]
- Menger MD, Vollmar B. Adhesion molecules as determinants of disease: from molecular biology to surgical research. Br J Surg. 1996;83:588–601. doi: 10.1002/bjs.1800830506. [DOI] [PubMed] [Google Scholar]
- Hogg N, Bates PA, Harvey J. Structure and function of intercellular adhesion molecule-1. Chem Immunol. 1991;50:98–115. [PubMed] [Google Scholar]
- Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–47. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–50. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]