In mammals, DNA methylation plays a crucial role in the regulation of gene expression, telomere length, cell differentiation, X chromosome inactivation, genomic imprinting and tumorigenesis1. DNA methylation patterns are established de novo by DNA methyltransferases (DNMTs) 3a and 3b, whereas DNMT1 maintains the parent-specific methylation from parental cells to their progeny2. After DNA replication, the new DNA strand is unmethylated. Thus with the mother methylated strand, the DNA is hemimethylated. The protein UHRF1 (ubiquitin-like, containing PHD and RING finger domains 1) recognizes and binds to the hemimethylated sites by its SRA domain. Then DNMT1 is recruited to the sites by the same domain, thereby it methylates the newly synthesized DNA strand3, 4. Increased DNMT1 abundance has been seen in many human cancers, and the roles of DNMT1 in tumorigenesis have been shown by some genetic knockout and RNA interference-mediated knockdown studies5, 6. Seeing that the mRNA abundance of DNMT1 contributes less to DNMT1 abundance, the stability of DNMT1 protein therefore plays an important role in human cancers7, 8.
Ubiquitin-proteasome pathway is significant in the stability of DNMT18, but ubiquitin-mediated protein degradation can be enhanced or attenuated by some modifications like acetylation/deacetylation, protein methylation/demethylation, phosphorylation and S-nitrosylation9, 10, 11. Estève et al demonstrated that SET7-mediated lysine methylation of DNMT1 decreased DNMT1 level by ubiquitin-mediated degradation10. Furthermore, an early study12 showed that HDAC inhibitors could induce degradation of DNMT1. These suggest that DNMT1-associated proteins may play key roles in DNMT1 stability. In a recent issue of Science Signaling, Du et al reported13 that some DNMT1-associated proteins can ubiquitinate/deubiquitinate and acetylate/deacetylate DNMT1. These modifications make the regulation of DNMT1 stability more coordinated (Figure 1).
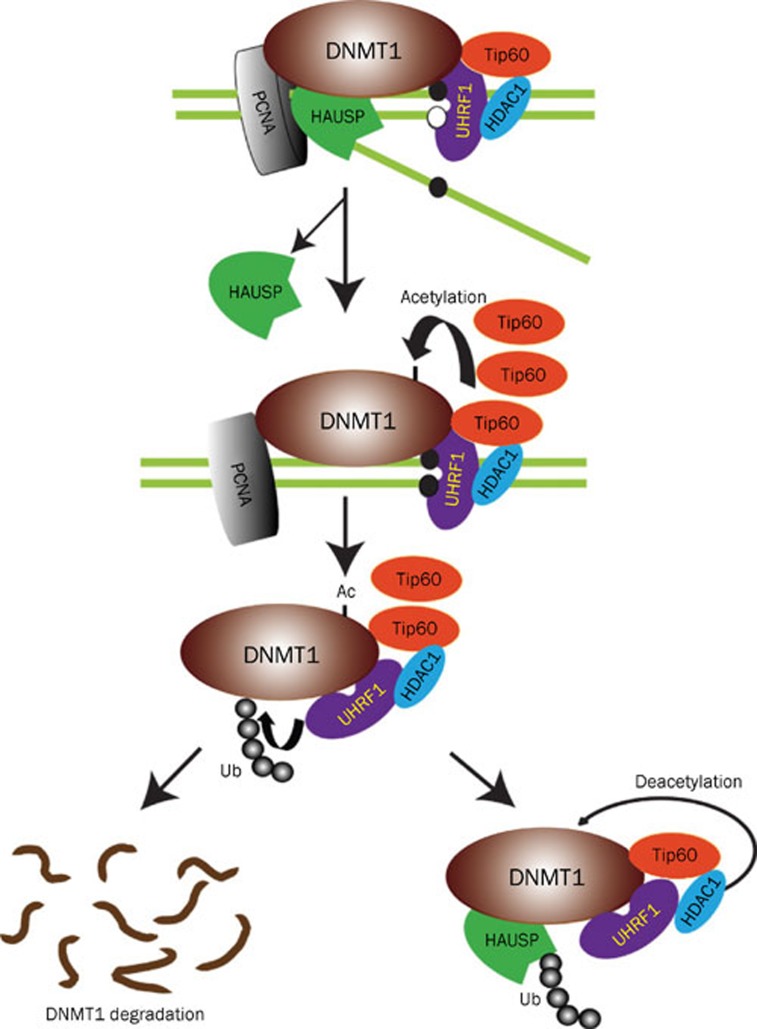
Figure 1.
A model of posttranslational regulation of DNMT1 stability13. DNMT1 physically interacts with HAUSP, Tip60, UHRF1, HDAC1, and PCNA on chromatin. After the completion of DNA methylation in S phase, HAUSP dissociates from DNMT1 to enable DNMT1 degradation. Moreover, increased abundance of Tip60 results in increased acetylation of DNMT1, which in turn triggers the ubiquitination of DNMT1 by UHRF1. This sequence of events results in proteasomal degradation of DNMT1. In contrast, HAUSP and HDAC1 protect DNMT1 from degradation through deubiquitination and deacetylation, respectively. Reprinted with permission from AAAS: Science Signaling, copyright 2010.
They identified deubiquitinase HAUSP (herpesvirus-associated ubiquitin specific protease) as a DNMT1-associated protein. Knockout or knockdown of HAUSP increased DNMT1 ubiquitination and reduced its abundance, then they demonstrated the direct deubiquitination of DNMT1 in vitro by purified HAUSP recombinant proteins. They also found that overexpression of DNMT1-associated E3 ligase UHRF1 led to increased ubiquitination of DNMT1 and decreased abundance of DNMT1 mutant lacking the HAUSP interaction domain, but not the full-length protein. These results show the coordination between ubiquitination of DNMT1 by UHRF1 and deubiquitination by HAUSP. Furthermore, they found that knockdown of HDAC1 increased DNMT1 acetylation, and reduced DNMT1 abundance. Additionally, acetyltransferase Tip60 which was found to acetylate DNMT1 promoted its ubiquitination, then destabilized it. At last, Tip60 and HAUSP were found to regulate DNMT1 protein stability during the cell cycle. The following clinical samples of human colon cancer also revealed the correlation between the abundance of HAUSP and the abundance of DNMT1.
Drugs targeting epigenetic modifications have been edging toward anticancer therapies. DNMT1 also provides a candidate anti-cancer target14. Although global genomic DNA hypomethylation and tumor suppressor genes hypermethylation have been frequently observed in different human cancers, this methylation change is not caused simply by increased levels of DNMT115. This paper shows that DNMT1, HAUSP, UHRF1, Tip60, HDAC1, and PCNA (proliferating cell nuclear antigen) can interact with each other, thus they regulate DNMT1 stability and activity by a more coordinated way. Consequently, the current or future epigenetic drugs such as 5-aza CdR (DNMT1 inhibitor), HDAC inhibitors, HAUSP inhibitors, and UHRF1 inhibitors may have a potential combination for effective cancer therapy. And equally, the drug interactions and side effects should be taken into account, as one of the inhibitors may affect the other targets.
References
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Ooi SK, Bestor TH. Cytosine methylation: remaining faithful. Curr Biol. 2008;18:R174–6. doi: 10.1016/j.cub.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Bostick M, Kim JK, Estève PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–4. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–12. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, et al. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33:61–5. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- Brown KD, Robertson KD. DNMT1 knockout delivers a strong blow to genome stability and cell viability. Nat Genet. 2007;39:289–90. doi: 10.1038/ng0307-289. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Yang ES, Veeraswamy R, Lin X, Nelson WG. Abnormal regulation of DNA methyltransferase expression during colorectal carcinogenesis. Cancer Res. 1999;59:3855–60. [PubMed] [Google Scholar]
- Agoston AT, Argani P, Yegnasubramanian S, De Marzo AM, Ansari-Lari MA, Hicks JL, et al. Increased protein stability causes DNA methyltransferase 1 dysregulation in breast cancer. J Biol Chem. 2005;280:18302–10. doi: 10.1074/jbc.M501675200. [DOI] [PubMed] [Google Scholar]
- Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–22. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estève PO, Chin HG, Benner J, Feehery GR, Samaranayake M, Horwitz GA, et al. Regulation of DNMT1 stability through SET7-mediated lysine methylation in mammalian cells. Proc Natl Acad Sci USA. 2009;106:5076–81. doi: 10.1073/pnas.0810362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad N, Iyer A, Vallyathan V, Wang L, Castranova V, Stehlik C, et al. Role of oxidative/nitrosative stress-mediated Bcl-2 regulation in apoptosis and malignant transformation. Ann N Y Acad Sci. 2010;1203:1–6. doi: 10.1111/j.1749-6632.2010.05608.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Agoston AT, Atadja P, Nelson WG, Davidson NE. Inhibition of histone deacetylases promotes ubiquitin-dependent proteasomal degradation of DNA methyltransferase 1 in human breast cancer cells. Mol Cancer Res. 2008;6:873–83. doi: 10.1158/1541-7786.MCR-07-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang S, et al. DNMT1 stability is regulated by proteins coordinating deubiquitination and acetylation-driven ubiquitination. Sci Signal. 2010;3:ra80. doi: 10.1126/scisignal.2001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karberg S. Switching on epigenetic therapy. Cell. 2009;139:1029–31. doi: 10.1016/j.cell.2009.11.038. [DOI] [PubMed] [Google Scholar]
- Szyf M. Towards a pharmacology of DNA methylation. Trends Pharmacol Sci. 2001;22:350–4. doi: 10.1016/s0165-6147(00)01713-2. [DOI] [PubMed] [Google Scholar]