Abstract
Aim:
To investigate the role of glutamate and N-methyl-D-aspartate (NMDA) receptors in central sensitization following peripheral inflammation in the arcuate nucleus (ARC) of the mediobasal hypothalamus.
Methods:
Mediobasal hypothalamic slices were prepared from rats undergoing peripheral inflammation, which was induced by a unilateral injection of complete Freund's adjuvant (CFA) into hind paw. Neuronal activation levels in the ARC were monitored by recording extracellular unit discharges. The NMDA receptor NR1 subunit (NR1) was measured using Western blot analysis.
Results:
Enhanced NR1 phosphorylation was observed in the ARC of CFA-inflamed rats. Compared with the control rats, the firing rate of spontaneous discharges in ARC neurons of inflamed rats was significantly higher, and it was significantly reduced both by an NMDA receptor antagonist (MK-801, 300 μmol/L) and by a non-NMDA receptor antagonist (CNQX, 30 μmol/L). Application of exogenous glutamate (200 μmol/L) or NMDA (25 μmol/L) resulted in increased neuronal discharges for ARC neurons, which was enhanced to a greater extent in inflamed rats than in control rats.
Conclusion:
Glutamate receptor activation in the hypothalamic ARC plays a crucial role in central sensitization associated with peripheral inflammation.
Keywords: arcuate nucleus (ARC), NMDA receptor, NR1 phosphorylation, inflammation, central sensitization, MK-801, CNQX
Introduction
Glutamate is a major excitatory neurotransmitter in the central nervous system (CNS). The receptors for glutamate comprise two large families: the ionotropic glutamate receptors (iGluRs) and the metabotropic glutamate receptors (mGluRs). The iGluRs are classified into the following receptor subtypes: N-methyl-D-aspartate (NMDA), kainate (KA) and alpha-amino 3-hydroxy-5-methyl-4-isoxazole propionate (AMPA). KA and AMPA receptor subtypes are collectively classified as non-NMDA receptors. Molecular studies have indicated that functional NMDA receptors contain a heteromeric combination of NR1 subunits (essential for channel-formation) and one or more NR2A to D subunits1, 2, 3.
NMDA receptors have been shown to play an important role in several physiological processes, such as long-term potentiation, learning and memory, as well as in some pathological conditions, including neurodegenerative diseases, ischemia and persistent nociception. The NMDA receptor has also been shown to be involved in the development of central sensitization, which is believed to underlie hyperalgesia during inflammatory pain and neuropathic pain2, 3. The majority of the data describing central sensitization were derived from studies in the dorsal horn of spinal cord, which is the first station to receive and integrate nociceptive information in the CNS3, 4, 5, 6, 7, 8, 9. However, studies investigating the molecular mechanisms of central sensitization in the supraspinal centers of nociceptive regulation remain elusive10, 11.
Previous studies have demonstrated that the arcuate nucleus (ARC) of mediobasal hypothalamus, which exhibits large clusters of β-endorphinergic neurons, is one of the critical structures in the modulation of nociception. Electrical or chemical stimulation of ARC can elicit antinociceptive effects12, 13, 14, while electrolytic or chemical lesioning of ARC attenuates the morphine-induced analgesia, acupuncture-induced analgesia and stress-induced analgesia12, 15, 16. Furthermore, peripheral noxious stimulation modulates the spontaneous discharges of neurons in ARC, indicating that peripheral nociceptive information is sent to the hypothalamic nucleus and can result in neuronal activation in ARC17. In addition, glutamate and glutamate receptors, such as NMDA receptors, are highly expressed in the nuclei of the medial hypothalamus, including ARC18, 19, 20, 21, 22, 23, 24.
Based on these observations, we hypothesized that hypothalamic ARC, a supraspinal center in pain modulation, might be a locus for central sensitization induced by injuries in peripheral tissues. We investigated the function of the NR1 subunit of the NMDA receptor and neuronal activation in ARC in mediobasal hypothalamic slices from rats suffering from peripheral inflammatory injury. We found that peripheral inflammation enhanced NR1 phosphorylation and increased neuronal activation in ARC. These findings provide new insights into how noxious signals are centrally processed during persistent nociception, which may be important in the development of novel analgesic strategies.
Materials and methods
Animals
Adult male Wistar rats (180 to 220 g) were housed in a light- (lights on 06.00–18.00 h) and temperature- (22±1 °C) controlled room and were fed rat chow and tap water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee at the Medical College, Soochow University and were in accordance with the ethical standards of the Helsinki Declaration and the guidelines of the International Association for the study of Pain for pain research in animals.
Drugs and reagents
In this study L-glutamic acid (glutamate), NMDA, MK-801 (a non-competitive NMDA receptor antagonist), CNQX (a non-NMDA receptor antagonist), rabbit anti-mouse NR1 antibody and rabbit anti-serine-897-phosphorylated-NR1 (pNR1) antibody were purchased from Sigma Chemical Co. The avidin-biotin complex and the biotinylated goat anti-rabbit IgG secondary antibody were purchased from Vector Laboratories Inc. All other chemical reagents were purchased from Sinopharm Chemical Reagent Co, Ltd.
Inflammatory pain model
Inflammatory pain was induced by injecting complete Freund's adjuvant (CFA, 50% in saline, with 5 mg/mL heat-killed Mycobacterium tuberculosis, 0.1 mL) into the plantar surface of the left hind paw. Western blotting and electrophysiological experiments were conducted one week after CFA injection, when symptoms of inflammatory pain were evident, such as redness, swelling of the ankle joint, hyperalgesia and impairment in motor activity. Normal rats injected with an equal volume of saline were prepared as a control.
Western blot analysis
One week after CFA injection, rats (including control rats) were deeply anesthetized and decapitated to quickly isolate the ARC region. Three (anterior, middle and posterior) ARC slices (500 μm thick) were isolated from each animal and transferred into the ice-cold artificial cerebrospinal fluid (ACSF). The ARC slices were then homogenized in the presence of protease and phosphatase inhibitors. The homogenate was centrifuged twice at 13 000×g for 10 min at 4 °C. The supernatant was used for Western blot analyses. The concentration of protein in the homogenate was measured using a bicinchoninic acid (BCA) kit and was used to calculate volume for equal protein loading in the gel. Proteins were separated and were transferred onto PVDF membranes (Invitrogen) by SDS-PAGE using Criterion XT Precast 6% Bis-Tris gels (Bio-Rad, Hercules, CA) in standard transfer buffer (25 mmol/L Tris, 192 mmol/L glycine, 10% v/v methanol, pH 8.3) for 1.5 h at room temperature. After being blocked with 5% milk in Tris-buffered saline and 0.1% Tween 20 (TBS-T) for 1 h, the membranes were incubated in specific antibodies against NR1 (1:2000, rabbit affinity-purified polyclonal antibody, Sigma), pNR1-Ser897 (1:500, rabbit affinity-purified polyclonal antibody, Upstate) and β-actin (1:20 000, mouse monoclonal, Sigma) overnight at 4 °C. After extensive washing in TBST, the membranes were incubated in goat anti-rabbit horseradish peroxidase (HRP) secondary antibody (1:5000, Jackson Immunoresearch Co) for 1 h at room temperature. After extensive washing, signals were detected by Western Lightning ECL and were quantified relative to a β-actin control by densitometry on Image-Pro Plus 6.0.
Mediobasal hypothalamus slice preparation
Hypothalamic slices (400 μm) were prepared as described previously25, 26. In brief, hypothalamus slices containing ARC in a recording chamber were continuously perfused with ACSF (3 mL/min) saturated with 5% CO2 and 95 % O2 at 33±1 °C. The ACSF media (pH 7.35–7.40) contained the following reagents: NaCl (124 mmol/L), NaHCO3 (26 mmol/L), KCl (5 mmol/L), CaCl2 (2.4 mmol/L), MgSO4 (1.3 mmol/L), NaH2PO4 (1.24 mmol/L), and D-glucose (10 mmol/L). After 1 h of ACSF perfusion, extracellular single unit recordings were collected.
Electrophysiological recording
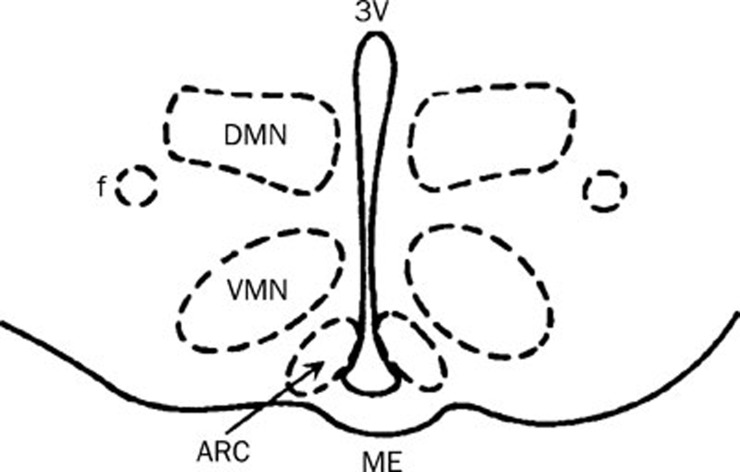
The spontaneous unit discharges from the ARC were recorded extracellularly with glass microelectrodes containing 0.5 mol/L sodium acetate and 2% pontamine sky blue (10–20 MΩ). Using a stereomicroscope and a microelectrode manipulator, the glass microelectrode was lowered into the ARC, which was located anatomically with respect to the third ventricle (3V) and medium eminence (Figure 1). The electrical signals were amplified (microelectrode amplifier, MEZ-8201, Nihon Kohden, Japan) and sent to a dual-beam oscilloscope (VC-10, Nihon Kohden, Japan). Neuronal unit discharges were continuously recorded online. The firing rate and interspike interval of neuronal discharges were analyzed with Powerlab/4SP (AD Instruments, Australia).
Figure 1.
Schematic drawing of a mediobasal hypothalamic slice that contains the ARC. Dashed lines indicate the boundaries of the nucleus, as indicated. DMN: dorsomedial nucleus; VMN: ventromedial nucleus; ME: medium eminence; 3V: third ventricle; f: fornix.
Drug application
All the drugs used in our experiments were freshly prepared, dissolved in ACSF saturated with 5% CO2 and 95% O2, and perfused hypothalamic slices via a three-way stopcock near the recording chamber. The baseline activity of neuronal discharges was recorded for 5 to 10 min as control. After establishing a good baseline, glutamate receptor agonists (glutamate 200 μmol/L, NMDA 25 μmol/L) or antagonists (MK-801 300 μmol/L, CNQX 30 μmol/L) were applied. The drug effects were observed continuously for 8 min after application.
Statistical analysis
The ARC neuron responses to glutamate or NMDA were determined according to the critical ratio criterion (CR), using the following formula: CR=(E–S)/(S+E)1 (where E is discharge frequency after drug application and S is discharge frequency before drug application). The response was considered excitatory, if the ratio exceeded 1.9627. All data are presented as mean±SD. Statistical comparisons were performed using one-way ANOVA or student's t-test. P<0.05 was considered statistically significant.
Results
Expression of NMDA receptor in ARC
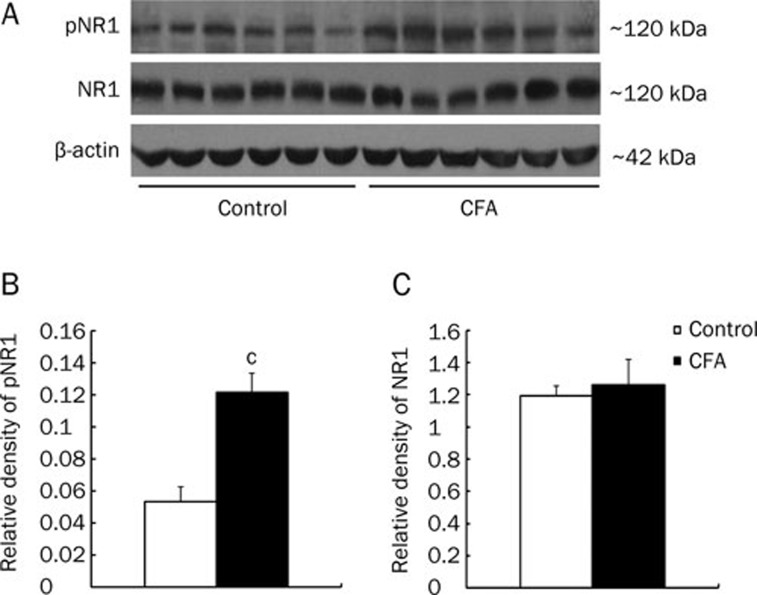
NMDA receptors are composed of NR1 subunits, which are essential, and one of the NR2 subunits (NR2A, NR2B, NR2C, or NR2D). In the present study, we used Western blotting to measure the relative amounts of NR1 and pNR1 in ARC isolated from control and inflamed animals. Figure 2 illustrates that the relative amount of pNR1 in CFA-induced inflamed rats was significantly increased as compared with control rats (n=6, P<0.01, Figure 2A and 2B). Meanwhile the relative amounts of NR1 in inflamed and control rats were not statistically different (n=6, P>0.05, Figure 2A, 2C). These data suggest that peripheral inflammation increased NR1 phosphorylation in ARC, but it had no effect on NR1 subunit upregulation.
Figure 2.
Enhanced pNR1 but not NR1 expression in the ARC from CFA-inflamed rats. (A) Immunoblots of phosphorylated NR1 (pNR1) and NR1 in the ARC of control and CFA-inflamed rats. β-actin was as control. (B) The relative density of pNR1 protein was significantly increased in the ARC of CFA-inflamed rats (n=6) compared to control rats (n=6; cP<0.01, unpaired t-test). (C) There was no significant difference in NR1 protein expression between control (n=6) and CFA-inflamed (n=6) rats.
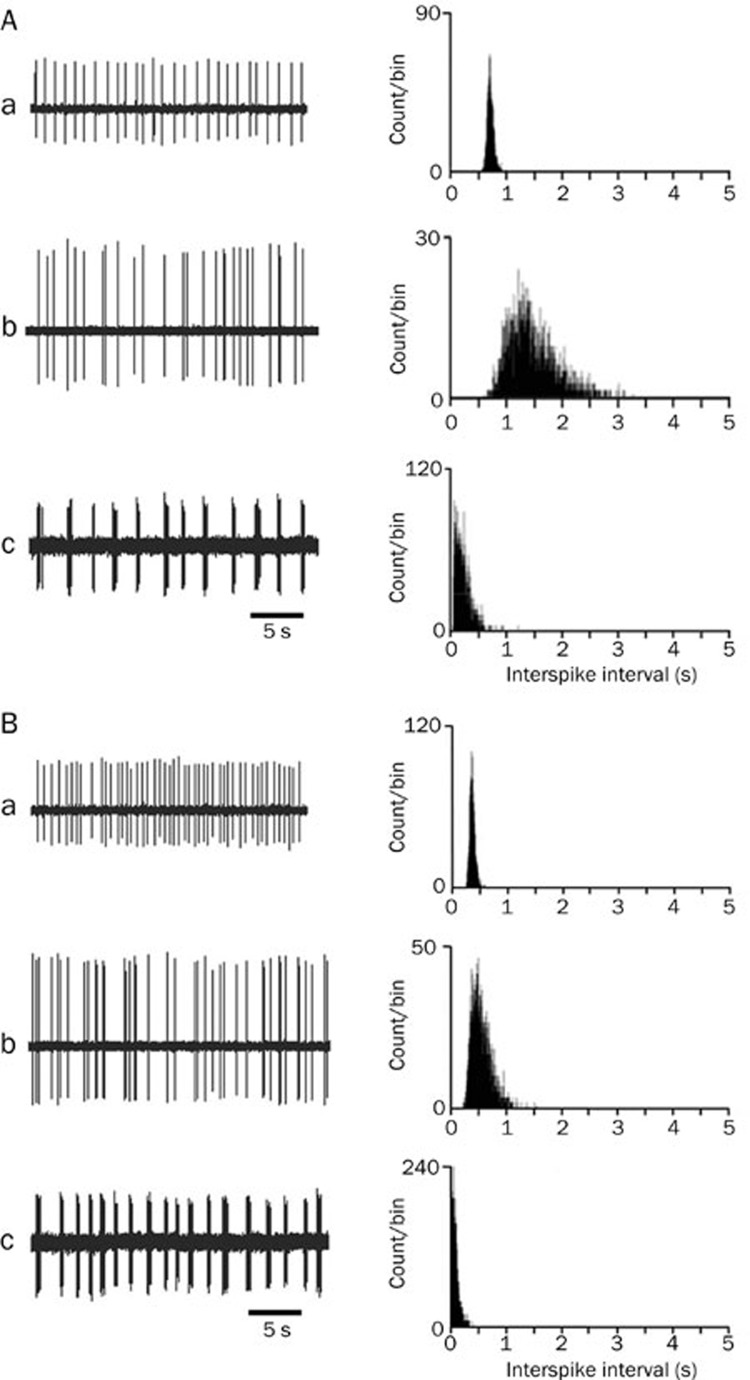
The discharge patterns of ARC neurons
We also measured the neuronal discharge activity in ARC. We found that the spontaneous discharges of ARC neurons in the slices could be divided into three firing patterns: regular, irregular and burst firing (Figure 3). Irregular discharges were observed in the majority of recorded neurons. As indicated in Table 1, the number of neurons exhibiting regular, irregular and burst firing was similar across control and inflamed rats (Table 1).
Figure 3.
The discharge patterns of arcuate neurons. Examples of electrical signals (left) and their inter-spike interval histograms (right) recorded from arcuate neurons in control and inflamed rats are shown in A and B, respectively. a, b, and c show examples of regular, irregular and burst firing, respectively.
Table 1. Frequency of spontaneous discharges of ARC neurons from control and inflamed rats. bP<0.05, cP<0.01 vs control. The number of neurons in each firing pattern is indicated in parenthesis.
| Regular firing (Hz) | Irregular firing (Hz) | Burst firing (Hz) | Mean (Hz) | |
|---|---|---|---|---|
| Control | 1.59±0.34 (21) | 1.07±0.13 (86) | 1.54±0.29 (16) | 1.22±0.24 (123) |
| CFA-inflamed | 2.36±0.38b (14) | 1.52±0.11c (95) | 1.94±0.36b (33) | 1.70±0.27b (142) |
Discharge frequency in ARC neurons
The frequency of spontaneous discharge in ARC neurons was calculated for each discharge pattern, as well as for the mean of the three discharge patterns. The mean firing rate and the firing rate of each discharge pattern in ARC neurons from inflamed rats were significantly elevated as compared with the normal group (P<0.05, Table 1). This significant difference was also observed for the inter-spike interval histograms. As shown in Figure 3, as the firing rate increased, the inter-spike intervals shifted to the left in the inflamed animals.
Effect of glutamate on the electrophysiological activities of ARC neurons
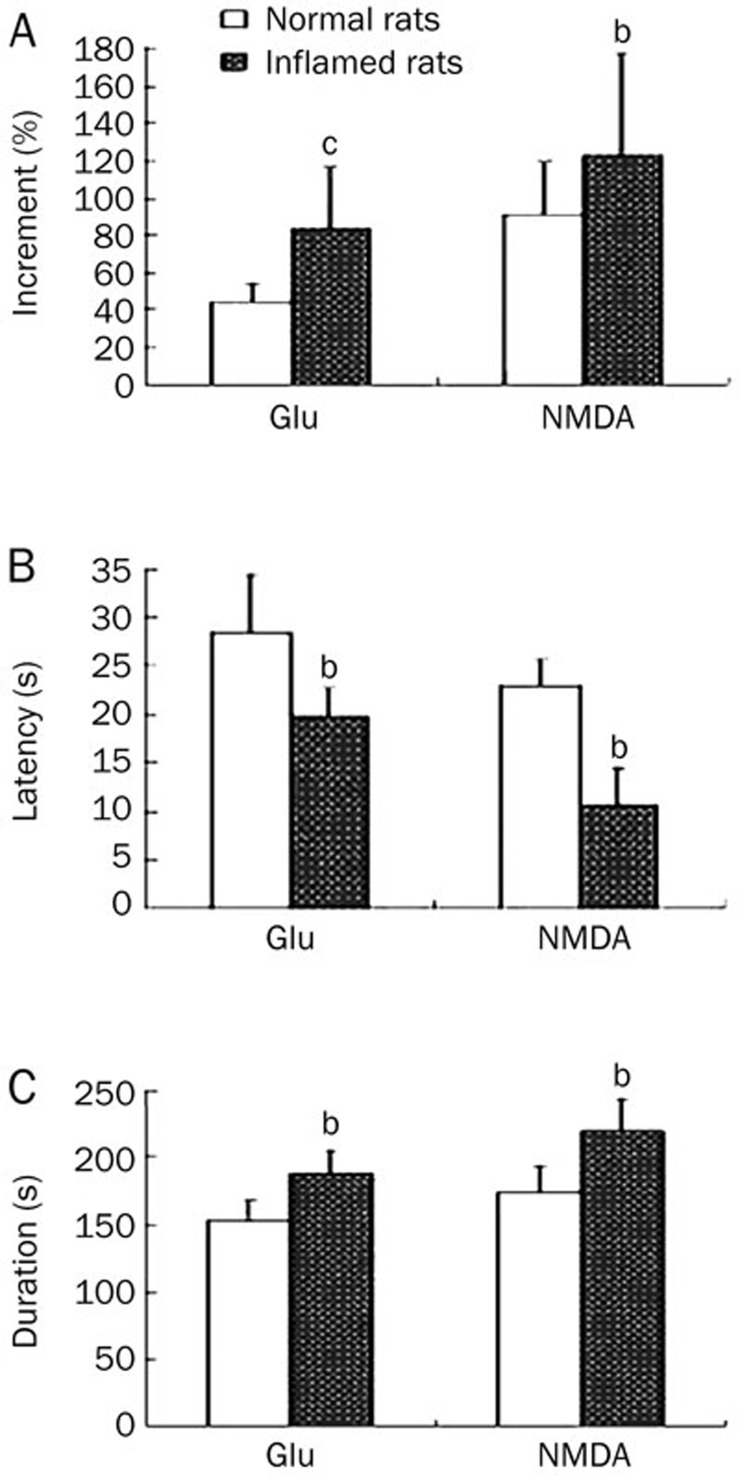
We investigated the response of ARC neurons to application of exogenous glutamate (200 μmol/L) to monitor their electric activities. In control animals, neuronal discharges increased significantly following glutamate application with a latency of 28.5±5.9 s (n=26). The firing rate increased by 44.5%±9.9%, and this increase persisted for up to (152.5±15.3) s. In inflamed rats, the glutamate-induced excitatory response was more rapid, with a latency of (19.8±2.8) s (n=23), and was more long lasting (188.5 s±20.1 s). The firing rate increased by 84.0%±32.7%. These results indicate that the glutamate-induced excitatory response in ARC neurons from inflamed rats exhibited a shorter latency (P<0.05), longer duration (P<0.05) and an enhanced firing rate (P<0.01) as compared with control rats (Figure 4).
Figure 4.
Effects of exogenous glutamate and NMDA on neuronal discharges in the ARC. Relative increases in neuronal discharge frequency following glutamate (200 μmol/L, n=26 in control rats, n=23 in inflamed rats) or NMDA (25 μmol/L, n=29 in control rats, n=24 in inflamed rats) application are shown in A. The baseline discharge frequency before glutamate application in control and inflamed rats were 1.26±0.31 Hz and 1.58±0.22 Hz, respectively; before NMDA application they were 1.27±0.22 Hz and 1.69±0.34 Hz, respectively. The latency and duration of glutamate or NMDA-induced increases in neuronal discharges are shown in B and C, respectively. bP<0.05, cP<0.01 as compared with control rats.
Effect of NMDA on the electric activities of ARC neurons
Similar to application of exogenous glutamate, application of an NMDA receptor agonist, NMDA (25 μmol/L), also induced an excitatory response in ARC neurons in both the control and inflamed rats. In inflamed rats (n=24), the excitatory response exhibited a shorter latency, larger increment and longer duration, all of which were significantly different (P<0.05) from ARC neurons in control animals (n=29, Figure 4). These results indicate that both NMDA and glutamate could induce a stronger excitatory response in inflamed rats than in control rats.
Effects of MK-801 and CNQX on the spontaneous discharge of ARC neurons
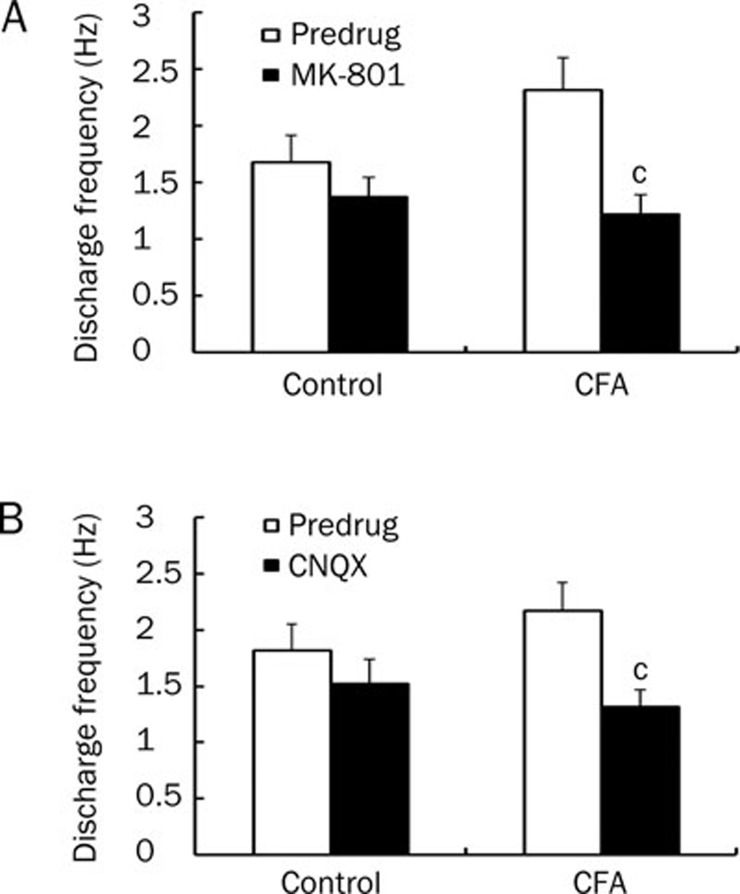
It is well known that the increased excitability of nociceptive neurons in the spinal cord during persistent inflammation is mediated by glutamate receptors, including NMDA and non-NMDA receptor subtypes. To examine the role of glutamate receptors in the neuronal activation of ARC neurons during peripheral inflammation, we tested the effects of MK-801 (NMDA receptor antagonist) and CNQX (non-NMDA receptor antagonist) on the increased spontaneous discharges of ARC neurons from inflamed rats. Application of either MK-801 (300 μmol/L) or CNQX (30 μmol/L) resulted in a significant reduction in the discharge frequency of ARC neurons from inflamed rats. After MK-801 application, the discharge frequency was reduced from (2.32±0.29) Hz to (1.23±0.17) Hz (n=11, P<0.01, Figure 5A). After CNQX application, the discharge frequency decreased from (2.17±0.25) Hz to (1.32±0.15) Hz (n=11, P<0.01, Figure 5B). In control rats neither antagonist induced a significant reduction in the discharge frequency of ARC neurons (Figure 5A, 5B). These results suggest that the increase in spontaneous discharges of ARC neurons from inflamed rats was mediated by NMDA and non-NMDA receptors.
Figure 5.
MK-801 (NMDA receptor antagonist) and CNQX (non-NMDA receptor antagonist) inhibit spontaneous discharge of ARC neurons in inflamed rats. (A) A significant decrease in neuronal discharge frequency was observed following MK-801 application (300 μmol/L) in inflamed rats (n=11; P<0.01, unpaired t-test) but not in control rats (n=12). (B) A significant decrease in neuronal discharge frequency was observed following CNQX application (30 μmol/L) in inflamed rats (n=11; cP<0.01, unpaired t-test), but not in control rats (n=10).
Discussion
The results of this study were threefold: 1) CFA-inflamed rats exhibited elevated levels of phosphorylated NR1 but not unphosphorylated NR1; 2) ARC neurons from CFA-inflamed rats exhibited a higher frequency of neuronal discharges, which was both NMDA and non-NMDA receptor dependent; and 3) ARC neurons from CFA-inflamed rats exhibited enhanced responses to exogenous glutamate and NMDA.
NMDA receptors in spinal dorsal horn have been shown to play a critical role in nociceptive transmission2, 3. Peripheral tissue injury dramatically enhances the function of spinal NMDA receptor that is involved in the initiation and maintenance of central sensitization, a persistent increase in the excitability of nociceptive neurons2, 3, 28, 29. The hyperfunction of the NMDA receptor could result from phosphorylation, upregulation, or a combination of both30, 31. Our western blot analysis indicated that the NMDA receptor NR1 subunit was phosphorylated but not upregulated in the ARC, and this effect was enhanced in inflamed rats. These results are consistent with previous studies. Yang et al found that CFA treatment in mice did not affect total NR1 levels, but it did result in a marked increase in NR1 phosphorylation32. Bird et al demonstrated that mono-arthritis induced by an injection of a kaolin suspension with carrageenan into the knee joint in rats resulted in increased NR1 phosphorylation rather than receptor upregulation in the amygdala33. Similarly, Maneepak et al reported that dural stimulation by topical application of an inflammatory soup enhanced phosphorylation but not expression of NR1 in the trigeminal nucleus caudalis of the spinal cord in rats34.
Phosphorylation of NR1 has been shown to induce NMDA receptor trafficking from storage sites in the endoplasmic reticulum to the synaptic plasma membrane, leading to a hyperactivation state of NMDA receptor in nociceptive transmission35, 36. Furthermore, NR1 phosphorylation has been correlated with hyperalgesia and allodynia, which are characteristic behavioral manifestations of central sensitization31, 37. Previous investigations have demonstrated that the blockage of NR1 phosphorylation could reverse allodynia37. These data indicate that NR1 phosphorylation plays an important role in the initiation and maintenance of central sensitization. In the present study, NR1 phosphorylation was enhanced in ARC neurons from inflamed rats, thereby suggesting that central sensitization during peripheral inflammation might also occur in this supraspinal center.
Many studies have demonstrated that increased excitability of nociceptive neurons in the spinal dorsal horn is an important manifestation of central sensitization38. In addition, central sensitization has been shown to result from glutamate-induced activation of multiple signaling pathways in dorsal horn neurons, which involve both ionotropic (NMDA and non-NMDA) receptors and metabotropic receptors38, 39. In present study we also observed increased excitability of ARC neurons from CFA-inflamed rats, as evidenced by the increased frequency of spontaneous discharges that was NMDA and non-NMDA receptor dependent. The enhanced responsiveness of ARC neurons from inflamed rats by glutamate and NMDA application indicates that the excitability of ARC neurons increased following peripheral inflammation.
In the present study, hypothalamic ARC neurons from CFA-inflamed rats exhibited enhanced NR1 phosphorylation, increased excitability and increased responsiveness. These data suggest that the hypothalamic ARC may be important in the development of central sensitization associated with inflammatory injuries.
Author contribution
Xing-hong JIANG, Xian-min YU, and Gen-cheng WU designed the experiments; Long-sheng XU, Jian-ming PENG, Qi ZHU, and Shan GONG performed all experiments; Shi-yu GUO and Jin TAO analyzed data; Xing-hong JIANG and Jin TAO wrote the manuscript.
Acknowledgments
This work was supported by Funding from State Key Laboratory of Medical Neurobiology (09-09, Fudan University, Shanghai, China), the National Natural Science Foundation of China (No 30900437), Natural Science Funding for Colleges and Universities in Jiangsu Province (No 09KJB180008), Natural Science Funding of Jiangsu Province (No BK2009118), and Doctoral Funding of Ministry of Education of China (No 20093201110018).
References
- Dingledine K, Borges K, Bowie D, Traynelis S. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fundytus ME. Glutamate receptors and nociception: implications for the drug treatment of pain. CNS Drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–16. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- Rygh LJ, Svendsen F, Hole K, Tjolsen A. Natural noxious stimulation can induce long-term increase of spinal nociceptive responses. Pain. 1999;82:305–10. doi: 10.1016/S0304-3959(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapse by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–80. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and spinal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann NY Acad Sci. 2001;933:142–56. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Salter MW. Cellular neuroplasticity mechanism mediating pain persistence. J Orofac Pain. 2004;18:318–24. [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–71. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Guo SY, Yin WP, Zhang HQ, Yin QZ. Role of hypothalamic arcuate region in lip-acupuncture analgesia in rats. Acta Physiol Sin. 1982;34:71–7. [Google Scholar]
- Zhang N, Yin QZ. Involvement of rat locus coeruleus in the analgesic effect induced by monosodium glutamate injection into the hypothalamic arcuate nucleus area. Acta Physiol Sin. 1988;40:529–38. [PubMed] [Google Scholar]
- Wang Q, Mao LM, Han JS. Characterization of inhibition of spinal nociceptive reflex by stimulation of the arcuate nucleus of the hypothalamus in the pentobarbital-anesthetized rat. Pain. 1990;41:101–8. doi: 10.1016/0304-3959(90)91114-X. [DOI] [PubMed] [Google Scholar]
- Guo SY, Yin WP, Yin QZ. Effects of neonatal administration of monosodium glutamate on morphine-, acupuncture- and stress-analgesia in adult rats. Acta Pharmacol Sin. 1983;4:14–6. [PubMed] [Google Scholar]
- Zhu MY, Wang XY, Zhang DX, Wan XX. Effects of lesion of hypothalamic arcuate nucleus region on brain β-endorphin, 5-hydroxytryptamine and norepinephrine content and acupuncture analgesia in rats. Acta Physiol Sin. 1984;36:42–8. [Google Scholar]
- Yu XM, Yin QZ. Changes in unit discharge of hypothalamic arcuate nucleus area during noxious stimulation or acupuncture in rats. Acta Physiol Sin. 1984;36:33–41. [Google Scholar]
- Palkovits M, Láng T, Patthy A, Elekes I. Distribution and stress-induced increase of glutamate and aspartate levels in discrete brain nuclei of rats. Brain Res. 1986;373:252–7. doi: 10.1016/0006-8993(86)90339-2. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaba Z, Csáki A, Halász B. Glutamatergic innervation of growth hormone-releasing hormone-containing neurons in the hypothalamic arcuate nucleus and somatostatin-containing neurons in the anterior periventricular nucleus of the rat. Brain Res Bull. 2006;70:278–88. doi: 10.1016/j.brainresbull.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Deli L, Turi GF, Kalló I, Liposits Z. Glutamatergic innervation of the hypothalamic median eminence and posterior pituitary of the rat. Neuroscience. 2007;144:1383–92. doi: 10.1016/j.neuroscience.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaba Z, Csáki A, Halász B. Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur J Neurosci. 2005;21:2111–9. doi: 10.1111/j.1460-9568.2005.04012.x. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Robertson HA, Wilkinson M. Expression of c-fos protein by N-methyl-D-aspartic acid in hypothalamus of immature female rats: blockade by MK-801 or neonatal treatment with monosodium glutamate. Brain Res Dev Brain Res. 1990;56:294–7. doi: 10.1016/0165-3806(90)90096-h. [DOI] [PubMed] [Google Scholar]
- Hu L, Fernstrom JD, Goldsmith PC. Exogenous glutamate enhances glutamate receptor subunit expression during selective neuronal injury in the ventral arcuate nucleus of postnatal mice. Neuroendocrinology. 1998;68:77–88. doi: 10.1159/000054353. [DOI] [PubMed] [Google Scholar]
- Khan AM, Stanley BG, Bozzetti L, Chin C, Stivers C. Currás-Collazo MC. N-methyl-D-aspartate receptor subunit NR2B is widely expressed throughout the rat diencephalon: an immunohistochemical study. J Comp Neurol. 2000;428:428–49. [PubMed] [Google Scholar]
- Liu YL, Yu GD, Jiang XH, Yin QZ. Effects of serotonin on circadian rhythm of electric activity of suprachiasmatic neurons in rat hypo-thalamic slice. Acta Acad Med Suzhou. 1999;18:897–900. [Google Scholar]
- Zhou XJ, Jiang XH, Yu GD, Yin QZ. Modulation of circadian rhythm of discharge of suprachiasmatic nucleus neurons in rat hypothalamic slices by melatonin. Acta Physiol Sin. 2000;52:215–9. [PubMed] [Google Scholar]
- Feldman S, Dafny N. Effects of cortisol on unit activity in the hypothalamus of the rats. Exp Neurol. 1970;27:375–87. doi: 10.1016/0014-4886(70)90101-9. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–44. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–70. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25:11107–16. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–97. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang HB, Xie QJ, Liu XH, Hu XD. Peripheral inflammation increased the synaptic expression of NMDA receptors in spinal dorsal horn. Pain. 2009;144:162–9. doi: 10.1016/j.pain.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou XJ, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol. 2005;564:907–21. doi: 10.1113/jphysiol.2005.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneepak M, le Grand S, Srikiathachorn A. Serotonin depletion increases nociception-evoked trigeminal NMDA receptor phosphorylation. Headache. 2009;49:375–82. doi: 10.1111/j.1526-4610.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implication for synaptyic transmission and plasticity. Trends Neurosci. 2002;25:571–7. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–67. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhanncement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms. Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang HB, van der Meer C, et al. Ionotropic and metaboteropic receptors, protein kinase A, protein kinase C, and Src contribuite to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–21. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]