Abstract
Aim:
To evaluate the effects of the fibrinolytic enzyme FIIa from Agkistrodon acutus venom on acute pulmonary thromboembolism (APT) in animal models.
Methods:
Both rabbit and dog APT models were used. For the rabbit APT model, the thrombi weight before and after administration was measured. Central venous pressure (CVP) and mean arterial pressure (MAP) were measured before and 15, 30, 60, and 120 min after the injection of the blood clot. Partial thromboplastin time (APTT), prothrombin time (PT), platelet count, and fibrinogen concentration were measured using auto analyzers. Plasminogen activity was measured based on chromogenic substrates. In the dog APT model, pulmonary blood flow was recorded using pulmonary angiography.
Results:
Intravenous administration of FIIa (0.1–5.0 mg/kg) improved the APT-induced hemodynamic derangements and reduced thrombi weight. The angiography evidence also showed that the pulmonary emboli had almost disappeared after FIIa infusion. FIIa (0.1, 0.5, or 1.0 mg/kg) did not impair the coagulation pathways, although very high doses of FIIa (5.0 mg/kg) could stimulate the production of plasminogen and result in impairment of the pathways.
Conclusion:
FIIa could effectively protect against APT via degradation of thrombi with less activation of plasminogen, and may provide a novel fibrinolytic enzyme for targeting the main pathological processes of the disease.
Keywords: snake venom, fibrinolytic enzyme, pulmonary thromboembolism, angiography, plasminogen, central venous pressure, mean, arterial pressure, urokinase, plasminogen
Introduction
Acute pulmonary thromboembolism (APT) is defined as an embolic occlusion of a pulmonary artery, which results in a sudden increase in pulmonary resistance, right ventricular afterload, and O2 consumption with a reduction in right coronary artery perfusion1. Despite advances in diagnosis and therapy, the mortality associated with APT remains high2, 3. Various approaches are available for the treatment of APT, including anticoagulant drugs and thrombolytic agents. Anticoagulant therapy, such as heparin, is thought to enhance the effect on thrombolysis by preventing formation of new thrombi4, 5. However, anticoagulants have minimal effects on previously existing thrombi. Enhanced fibrinolysis prevents disturbances in the circulation to organs by dissolving thrombi. It may be reasonable to assume that fibrinolytic therapy is effective against APT6, 7, 8, 9, 10. Current thrombolytic agents, such as tissue plasminogen activator (t-PA) and urokinase, which act as plasminogen activators, are effective at dissolving intravascular thrombi. These agents act indirectly on the thrombi by converting plasminogen, both circulating and fibrin-bound, into plasmin, which is the primary enzyme responsible for the removal of emboli. These conditions may “overflow” the systemic circulation and lead to systemic fibrinolysis and degradation of other clotting proteins11, which may increase the bleeding tendency or may result in local activation of coagulation.
FIIa is a novel fibrinolytic enzyme that is purified from Anhui Agkistrodon acutus venom and is a type of snake venom metalloproteinase. Based on its crystal structure, binding to ZnP2+ is known to be essential for it hydrolytic activity12, 13. We have demonstrated that FIIa has the ability to directly degrade fibrin in vitro and effectively dissolve thrombi in vivo without activating plasminogen or influencing the activities of t-PA and plasminogen activator inhibitor-1 (PAI-1)14, 15. These findings show that FIIa has a different mechanism of action from t-PA and urokinase. Additionally, upon examination of the tissue sections from kidney, liver, heart, and lung16, the thrombolytic activities of FIIa did not lead to hemorrhage. Although many of the fibrinolytic properties of FIIa are known, there are few studies investigating its fibrinolytic effects on APT. In the present study, we mimicked clinical APT models and found that FIIa could ameliorate the hemodynamic derangements by degrading fibrin clots.
Materials and methods
Preparation of the enzyme FIIa
FIIa, the fibrinolytic enzyme that was isolated from Agkistrodon acutus venom, was prepared according to the method previously described by Liang et al 14.
Reagents
Urokinase was purchased from Sigma (St Louis, USA). The reagent pack for the plasminogen activity assay was obtained from Sun Biotechnology Company (Shanghai, China). All other reagents were of analytical grade and obtained from commercial sources.
Animals
All animal experiments were conducted in accordance with the National Guide for the Care and Use of Laboratory Animals and were approved by Sun Yat-sen University Animal Care and Use Committee (Guangzhou, China). Adult male New Zealand white rabbits (weight 2-3 kg, grade II) were supplied by the Experimental Animal Center of Zhongshan Medical College, Sun Yat-san University (China). Adult male Beagle dogs (weight 15-20 kg, grade II) were supplied by the Experimental Animal Center of Guangdong Province, China.
Experimental model and hemodynamic measurements in rabbits
The APT experimental model in the rabbit was performed according to the method described by Todd 17. An autologous thrombus was produced in a 10-mL tube (ID=0.2 cm) by incubation at 37 °C for 2 h with constant agitation using a clinical rotor (160 r/min). Thrombus fragments (2 cm) were cut and weighed according to an established protocol. The extracted blood clots were injected into the lungs through the right ventricular catheter, which then embolized the pulmonary arteries. Mean artery pressure (MAP) and central venous pressure (CVP) were measured before and 15, 30, 60, and 120 min after the injection of the blood clot.
Treatment was started simultaneously with the injection of the blood clots through the left marginal ear vein. Seven different groups were established, each containing 10 animals as follows: treatment groups were injected with 0.1 mg/kg, 0.5 mg/kg, 1.0 mg/kg or 5.0 mg/kg FIIa in 20 mL of saline solution over a period of 2 h (at a rate of 10 mL/h); the embo-control group was infused with saline solution (at a rate of 10 mL/h) instead of FIIa; the urokinase-control group was infused with 100 000 IU/kg over a period of 2 h (10 mL/h); and the remaining rabbits, which were neither injected with blood clots nor FIIa, were regarded as the sham-operated group and were infused with saline solution through the left marginal ear vein.
Two hours after the start of the experiments, the animals were sacrificed with an intravenous injection of 60 mg/kg pentobarbital sodium. The blood clots were carefully removed from the lungs and weighed.
Pulmonary angiography in dogs
This animal model was performed according to the methods described in Dias-Junior et al 18. Under sterile dissection, a polyethylene catheter was placed into the right pulmonary artery through the right femoral vein. An autologous thrombus was produced in a 20-mL tube (ID=0.2 cm) by incubation at 37 °C for 2 h with constant agitation using a clinical rotor (160 r/min). Then, APT was induced by infusing the prepared clot into the lung through the polythene catheter. A pigtail catheter was repositioned in the pulmonary trunk. The extent of pulmonary embolism and the reperfusion rate were documented by the method of intra-arterial digital subtraction angiography (DFP-2000A, Toshiba) at 0, 30, and 60 min following the infusion of FIIa.
FIIa infusions were performed 2 h after the injection of the blood clots. Two different groups were established, each containing 6 animals as follows: (1) the dogs in the FIIa group were embolized animals that received FIIa infusions (1 mg/kg) over 1 h (at a rate of 10 mL/h); and (2) the dogs in the embo-control group were embolized animals that were infused with saline solution instead of FIIa.
Sample collection and handling
Blood samples from rabbits were obtained immediately before and 2 h after blood clot injection through a catheter that was inserted into a femoral artery. Blood samples were collected in 3.8% sodium citrate (1:10 v/v citrate/blood) and centrifuged at 2000×g for 15 min at 4 °C. All samples were then stored at -70 °C until assayed.
Laboratory methods
An auto analyzer (Sysmex CA1500, Japan) was used to determine the partial thromboplastin time (APTT), prothrombin time (PT), and fibrinogen concentration. An automatic blood cell analyzer (Sysmex SE-9500, Japan) was used to determine platelet counts. Plasminogen activity was measured according to the reagent pack instructions based on chromogenic substrates.
Data analysis
Data at 2 h were converted to percentages, with a value of 100% corresponding to basal data, and were expressed as mean±SEM. One-way ANOVA, followed by Tukey's B test was used for multiple comparisons. A non-parametric test (Kruskal–Wallis H test) was used to determine the changes in the hemodynamic and biochemical parameters as well as to compare the embo-control, FIIa and urokinase-control groups. Differences with P values of less than 0.05 were considered to be statistically significant.
Results
Effect of FIIa on coagulation in APT in rabbits
To elucidate the effect of FIIa on APT, we systematically investigated its coagulation effects using a rabbit model. Table 1 summarizes the APTT, PT, platelet counts, fibrinogen concentration and plasminogen activity in normal rabbits, embo-control rabbits, FIIa -treated rabbits and urokinase-treated rabbits. APTT, PT, and plasminogen values in the urokinase-control group were all significantly higher than those in the sham-operated group (P<0.05). However, the values for plasma levels of both fibrinogen concentration and platelet count were significantly lower than those of the normal rabbits (P<0.05). No changes were detected in the hemodynamic parameters that were measured 2 h post-FIIa infusion (0.1, 0.5, or 1.0 mg/kg) compared with the sham-operated group. Although a significant decrease in fibrinogen and platelet count was observed, APTT, PT, and plasminogen activity increased in the animals that were treated with the highest dose of FIIa (P<0.05, compared with sham-operated group). No blood loss was observed at the site of infusion in any treatment group.
Table 1. Thrombolytic characteristics after infusion of urokinase and FIIa in a rabbit APT model. Data were expressed as percentage of the basal value. n=10. Mean±SEM. bP<0.05 compared with the sham-operated group. APTT: partial thromboplastin time. PT: prothrombin time.
| Fibrinogen (%) | Plasminogen (%) | APTT (%) | PT (%) | Platelet counts (%) | |
|---|---|---|---|---|---|
| Sham-operated | 101.28±14.27 | 97.68±20.74 | 101.36±6.85 | 99.29±17.84 | 101.40±10.19 |
| Embo-control | 105.37±13.39 | 103.83±18.65 | 99.54±16.16 | 98.48±12.22 | 100.83±12.61 |
| Urokinase | 69.14±26.09b | 180.58±30.96b | 283.12±40.37b | 242.87±37.63b | 59.82±19.07b |
| FIIa(0.1 mg/kg) | 107.51±20.07 | 104.48±24.57 | 97.38±10.31 | 101.69±11.06 | 99.52±8.52 |
| FIIa(0.5 mg/kg) | 93.41±15.56 | 109.62±26.74 | 100.59±9.63 | 104.19±6.72 | 93.84±11.93 |
| FIIa(1.0 mg/kg) | 89.37±18.92 | 115.35±24.90 | 104.62±11.49 | 103.94±14.27 | 90.81±13.29 |
| FIIa(5.0 mg/kg) | 75.31±24.15b | 143.37±31.42b | 173.80±30.62b | 143.64±25.33b | 70.35±18.98b |
Effect of FIIa on APT-induced hemodynamic derangements in rabbits
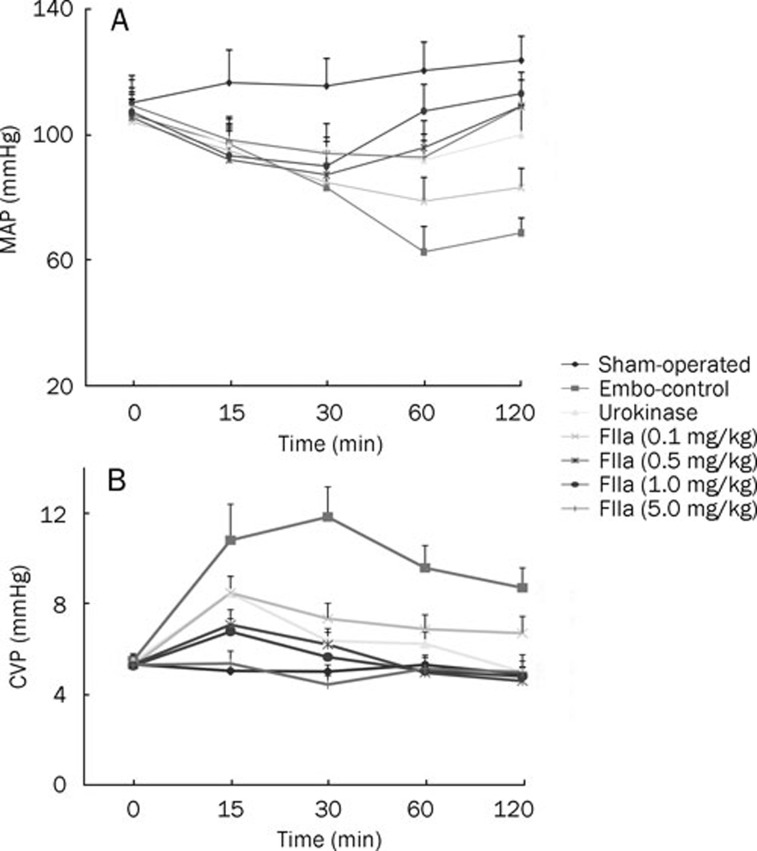
Baseline hemodynamic parameters were similar in all experimental groups and showed no significant changes in the sham-operated animals throughout the study period (Figure 1). APT caused a sustained reduction in MAP and an increase in CVP in embolized animals, compared with the sham-operated group (P<0.05, Figure 1).
Figure 1.
Effects of embolization and thrombolytic agents on mean arterial pressure (MAP, A) and central venous pressure (CVP, B). The data demonstrate that (1) embolization leads to an increase in CVP and a decrease in MAP and (2) FIIa and urokinase consistently ameliorate the hemodynamic derangements.
The animals treated with urokinase showed a decrease in CVP throughout the study period. An increase in MAP was observed 30 min following infusion of urokinase (P<0.05, compared with sham-operated group).
Two-hour measurements taken at all doses of infused FIIa showed the following: a decrease in CVP throughout the study period (P<0.05, compared with the embo-control group, Figure 1); an increase in MAP for 60 min following the infusion of FIIa in the 0.1 mg/kg and 0.5 mg/kg dose groups; and an increase in MAP for 30 min following the infusion of FIIa in the 1.0 mg/kg and 5.0 mg/kg dose groups (P<0.05, compared with the embo-control group, Figure 1).
Thrombolysis effect of FIIain vivo
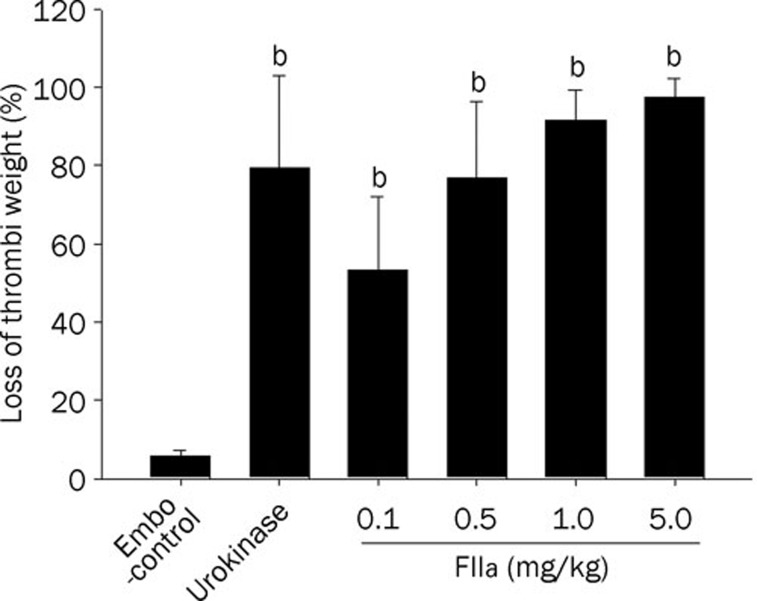
The in vivo thrombolysis effect of FIIa was studied in the rabbit APT model. No change in the thrombi weight was observed in the emboli-control group. The blood clots showed a weight loss of 79.38%±23.72% at 2 h post-infusion time point in the urokinase-treated group (Figure 2). FIIa dose-dependently reduced thrombi weight, which ranged from 53.37%±18.67% for a 0.1 mg/kg dose to 91.36%±7.83% for a 1.0 mg/kg dose (Figure 2). No blood loss was observed in any of the animals treated with FIIa.
Figure 2.
Effect of FIIa on thrombi weight in rabbits (n=10 in each group). Dose-dependent reduction in thrombus weight by FIIa infusion. Data were expressed as mean±SEM. bP<0.05, compared with embo-control.
Pulmonary angiography in the Beagle APT models
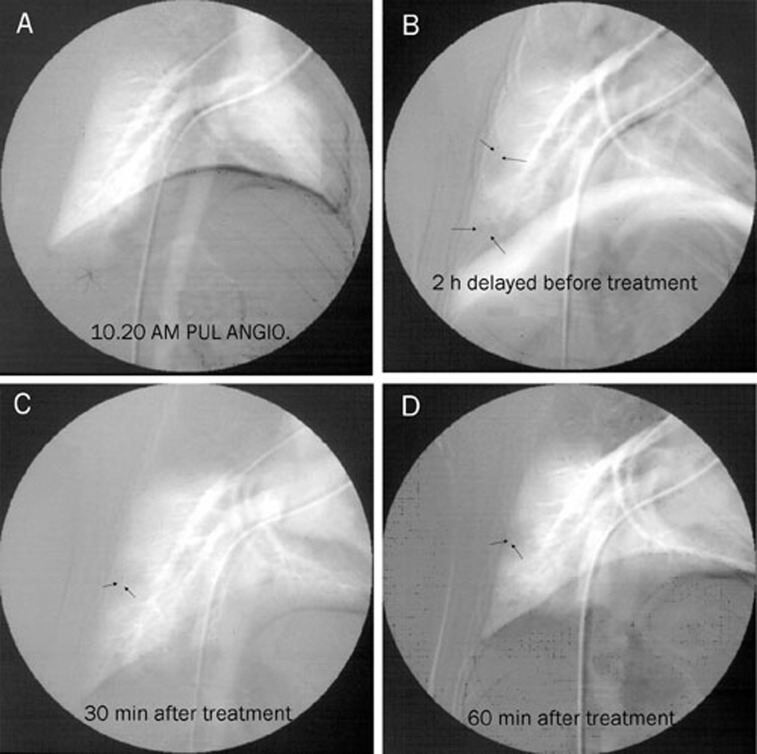
Most pulmonary emboli occurred in the lower lobes of the right lung. Serious obstructions occurred in the branches of the right lower lobar pulmonary artery in the embo-control group, following a significant decrease in the perfusion of the right lower lobes of the lung (the reperfusion rate was 0.6%±0.07%, Figure 3). Administration of 1 mg/kg of FIIa resulted in the gradual disappearance of the emboli. Thirty minutes after the infusion of FIIa, a lower level of obstruction was detected in the branches of the lower lobar pulmonary artery, and the reperfusion rate was 70.41%±7.62% (Figure 3). Sixty minutes after the infusion of FIIa, the pulmonary emboli had almost completely disappeared, and the reperfusion rate of the upper to lower lobe was 85.34%±8.42% (Figure 3).
Figure 3.
Digital subtraction angiography in APT. Arrows indicate thrombi in the right pulmonary artery. (A) Before pulmonary thromboembolism; (B) Two hours after pulmonary thromboembolism. The right pulmonary artery shows a complete obstruction. (C) FIIa treatment for 30 min. A reduction in obstruction was detected in the lower lobar pulmonary artery branches. (D) FIIa treatment for 60 min. The pulmonary emboli almost completely disappeared.
Discussion
In the present study, we reported that FIIa from Agkistrodon acutus venom demonstrated significantly protective properties in APT induced by the injection of preformed blood clots. An improvement in the APT-induced hemodynamic derangements and a reduction in thrombi weight were observed. The angiography evidence in the dog APT model also showed that the pulmonary emboli almost completely disappeared with the infusion of FIIa. In addition, FIIa did not impair the coagulation pathways.
APT can produce severe cardiopulmonary dysfunction that is characterized by pulmonary arterial hypertension, right ventricular failure, and hypoxemia, which is the principal cause of APT-related death19, 20, 21, 22. In addition, the major adverse effect is attributed to the direct mechanical obstruction of the pulmonary arteries. This evidence suggests that promoting fibrinolysis might be a promising target for therapeutic strategies for APT 23.
In this study, we found that lung embolization resulted in typical changes associated with APT that were characterized by a significant decrease in MAP and a dramatic increase in CVP, which were consistent with the results previously described by Todd et al 17. The angiography evidence in the dog APT model also showed that the pulmonary blood flow was almost completely occluded 2 h after pulmonary embolization.
Using the rabbit model of APT, we found that all doses of FIIa not only significantly attenuated the increase in CVP and the decrease in MAP but also effectively reduced the thrombi weight in the pulmonary artery. Furthermore, the angiography evidence in the dog APT model also showed that the pulmonary emboli almost completely disappeared after a 60-min FIIa infusion, and pulmonary blood flow was almost fully returned to baseline levels. In our previous study 14, 15, we found that fibrin clots were hydrolyzed more efficiently by FIIa than by urokinase in vitro. Because FIIa is a snake venom fibrinolytic enzyme, its proteolytic action should involve the mechanism of directly degrading fibrin. Furthermore, FIIa lysed fibrin by direct proteolysis without activating intrinsic plasminogen24, 25, which resulted in a greater effectiveness than urokinase. These findings suggest that FIIa could be beneficial in improving APT-induced hemodynamic derangements by its direct fibrinolytic effect on thrombi.
Because of the coagulation abnormalities, bleeding is a major complication of thrombolytic therapy. The risk of hemorrhage usually occurs at a site of previous surgery or puncture 26, 27. As a measure of ongoing coagulation during the infusion of the agents, we determined the fibrinogen concentration, platelet counts, plasminogen activity, PT and APTT.
In this study, the significant changes in these coagulation parameters in the urokinase-treated group indicated that infusion of urokinase impaired the intravascular coagulation in the animals. After a 2-h FIIa infusion (0.1, 0.5, or 1.0 mg/kg), no significant changes in the parameters of coagulation were detected, and no blood loss was observed at the site of infusion. These results suggest that intravascular administration of FIIa did not impair the coagulation system and produced no hemorrhages. FIIa effectively decreased the thrombi weight in a rabbit APT model. Because FIIa does not activate plasminogen, many complex secondary effects, such as platelet activation and fibrinogen degradation, are avoided. The advantages of the plasminogen-independent fibrinolysis induced by FIIa was further demonstrated by the findings of no bleeding in any of the rabbits treated with FIIa in our previous studies 13, 14 and a much wider therapeutic window compared with urokinase.
However, it should be emphasized that FIIa, at its extremely high dose, may result in internal hemorrhaging in some animals. In this study, the values of plasminogen activity, fibrinogen concentration, platelet count, APTT, and PT showed significant changes compared to the respective baseline values in the group with the highest dose of FIIa. Our previous study showed that FIIa influenced blood coagulation by inhibiting the platelet aggregation that was induced by adenosine phosphate and degradation of prothrombin and factor X 13. Plasminogen provides an important control of fibrinolysis by its degradation of blood coagulation proteins28. In addition to its fibrinolytic activity, FIIa is also involved in the degradation of gelatin and collagen, which are the main proteins that compose the vascular basement membrane. Plasminogen also activates collagenases and disrupts the basement membrane barriers 29. This evidence suggests that extremely high dose of FIIa might stimulate the production of plasminogen in vivo, which may be the reason for the impairment of the coagulation pathways. These results suggest that if FIIa is employed in the treatment of APT, it should be utilized in a manner that does not cause systemic activation of the fibrinolytic system. Additional work is needed to further characterize this issue.
In conclusion, FIIa may have protective effects on TAPT by it direct degradation of fibrin clots. These therapeutic effects support the hypothesis that FIIa could be used for reducing thrombi, restoring blood flow in the lung, and improving cardiovascular function. However, we emphasize that the true utility of FIIa in combating APT will require further direct testing in clinical trials.
Author contribution
Jia-shu CHEN and Guang-mei YAN designed the study; Xi LIN, Xiu-xia LIANG and Peng-xin QIU performed the research; Jian-jun TANG contributed new analytical reagents and tools; and Xi LIN analyzed the data and wrote the paper.
Abbreviations
APT, acute pulmonary thromboembolism; MAP, mean arterial pressure; CVP, central venous pressure; APTT, partial thromboplastin time; PT, prothrombin time; PAI-1, plasminogen activator inhibitor-1.
Acknowledgments
Project was supported by the National Natural Science Foundation of China (No 81000209), the key Project of Chinese Ministry of Education (No 210255), and the Fundamental Research for the Central Universities (No 21609304).
The authors thank Dr Jun XIE and the staff at the Molecular Imaging Lab in the Department of Radiology at the 3rd Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, for their technical assistance.
References
- Keymel S, Rassaf T, Kelm M. Nitrite in action: a commentary on “Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboemolism. Free Radic Biol Med. 2006;41:1750–2. doi: 10.1016/j.freeradbiomed.2006.10.046. [DOI] [PubMed] [Google Scholar]
- Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- Barritt DW, Jordon SC. Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet. 1960;1:1309–12. doi: 10.1016/s0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- Hull R, Delmore T, Centon E, Hirsh J, Gent M, Sackett D, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301:855–8. doi: 10.1056/NEJM197910183011602. [DOI] [PubMed] [Google Scholar]
- Vassalli G, Dichek DA. Gene therapy for arterial thrombosis. Cardiovas Res. 1997;35:459–69. doi: 10.1016/s0008-6363(97)00153-3. [DOI] [PubMed] [Google Scholar]
- Goldhaber SZ. Thrombolytic therapy in venous thromboembolism: Clinical trials and current indications. Clin Chest Med. 1995;16:307–20. [PubMed] [Google Scholar]
- Sherry S, Bell WR, Duckert FH, Fletcher AP, Gurewich V, Long DM, et al. Thrombolytic therapy in thrombosis: a National Institutes of Health consensus development conference. Ann Intern Med. 1980;93:141–4. doi: 10.7326/0003-4819-93-1-141. [DOI] [PubMed] [Google Scholar]
- Marder VJ, Sherry S. Thrombolytic therapy: current status (1) N Engl J Med. 1988;318:1512–20. doi: 10.1056/NEJM198806093182306. [DOI] [PubMed] [Google Scholar]
- Marder VJ, Sherry S. Thrombolytic therapy: current status (2) N Engl J Med. 1988;318:1585–95. doi: 10.1056/NEJM198806163182406. [DOI] [PubMed] [Google Scholar]
- Ouriel K. Comparison of safety and efficacy of the various thrombolytic agents. Rev Cardiovasc Med. 2002;3:S17–24. [PubMed] [Google Scholar]
- Lou Z, Hou J, Liang X, Chen J, Qiu P, Liu Y, et al. Crystal structure of a non-hemorrhagic fibrin (ogen)olytic metalloproteinase complexed with a novel natural tri-peptide inhibitor from venom of Agkistrodon acutus. J Struct Biol. 2005;152:195–203. doi: 10.1016/j.jsb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Liang XX, Zhou YN, Chen JS, Qiu PX, Chen HZ, Sun HH, et al. Enzymological characterization of FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom. Acta Pharmacol Sin. 2005;26:1474–8. doi: 10.1111/j.1745-7254.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- Liang XX, Chen JS, Zhou YN, Qiu PX, Yan GM. Purification and biochemical characterization of FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom. Toxicon. 2001;39:1133–9. doi: 10.1016/s0041-0101(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Lin X, Liang XX, Chen JS, Chen Q, Qiu PX, Yan GM. The effect of fibrinolytic enzyme FIIa from Agkistrodon acutus venom on disseminated intravascular coagulation in rabbits. Transl Res. 2007;150:295–302. doi: 10.1016/j.trsl.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chen JS, Liang XX, Qiu PX, Yan GM. Thrombolysis effect with FIIa from Agkistrodon acutus venom in different thrombosis model. Acta Pharmacol Sin. 2001;22:420–2. [PubMed] [Google Scholar]
- Todd MH, Forrest JB, Cragg DB. The effects of aspirin and methysergide, singly and in combination, on systemic haemodynamic responses to pulmonary embolism. Can Anaesth Soc J. 1981;28:373–80. doi: 10.1007/BF03007806. [DOI] [PubMed] [Google Scholar]
- Dias-Junior CA, Gladwin MT, Tanus-Santos JE. Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboembolism. Free Radic Biol Med. 2006;41:1764–70. doi: 10.1016/j.freeradbiomed.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Gurewich V, Cohen ML, Thomas DP. Humoral factors in massive pulmonary embolism: an experimental study. Am Heart J. 1968;76:784–94. doi: 10.1016/0002-8703(68)90264-0. [DOI] [PubMed] [Google Scholar]
- Daily PO, Griffin A, Blackstone E, Moulder PV. The hemodynamic effects of localized pulmonary embolism. Surg Gynecol Obstet. 1966;123:765–73. [PubMed] [Google Scholar]
- Hyamn AL, Myers WD, Meyer A. The effect of acute pulmonary embolus upon cardiopulmonary hemodynamics. Am Heart J. 1964;67:313–23. doi: 10.1016/0002-8703(64)90005-5. [DOI] [PubMed] [Google Scholar]
- Halmagyi DF, Starzecki B, Horner GJ. Humoral transmission of cardiorespiratory changes in experimental lung embolism. Circ Res. 1964;14:546–54. doi: 10.1161/01.res.14.6.546. [DOI] [PubMed] [Google Scholar]
- Ibrahim SA, Stone RA, Obrosky DS, Geng M, Fine MJ, Aujesky D. Thrombolytic therapy and mortality in patients with acute pulmonary embolism. Arch Intern Med. 2008;168:2183–90. doi: 10.1001/archinte.168.20.2183. [DOI] [PubMed] [Google Scholar]
- Ueshima S, Matsuo O. Development of new fibrinolytic agents. Curr Pharm Des. 2006;12:849–57. doi: 10.2174/138161206776056065. [DOI] [PubMed] [Google Scholar]
- Dobrovolsky AB, Titaeva EV. The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochemistry (Mosc) 2002;67:99–108. doi: 10.1023/a:1013960416302. [DOI] [PubMed] [Google Scholar]
- Stein PD, Matta F. Acute pulmonary embolism. Curr Probl Cardiol. 2010;35:314–76. doi: 10.1016/j.cpcardiol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Werf FV, Barron HV, Armstrong PW, Granger CB, Berioli S, Barbash G, et al. Incidence and predictors of bleeding events after fibrinolytic therapy with fibrin-specific agents. A comparison of TNK-tPA and rt-PA. Eur Heart J. 2001;22:2253–61. doi: 10.1053/euhj.2001.2686. [DOI] [PubMed] [Google Scholar]
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–45. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- Niedbala MJ, Picarella MS. Tumor necrosis factor induction of endothelial cell urokinase-type plasminogen activator mediated proteolysis of extracellular matrix and its antagonism by gamma-interferon. Blood. 1992;79:678–87. [PubMed] [Google Scholar]