Abstract
Searching for effective pharmacological agents for stroke treatment has largely been unsuccessful. Despite initial excitement, antagonists for glutamate receptors, the most studied receptor channels in ischemic stroke, have shown insufficient neuroprotective effects in clinical trials. Outside the traditional glutamate-mediated excitotoxicity, recent evidence suggests few non-glutamate mechanisms, which may also cause ionic imbalance and cell death in cerebral ischemia. Transient receptor potential melastatin 7 (TRPM7) is a Ca2+ permeable, non-selective cation channel that has recently gained attention as a potential cation influx pathway involved in ischemic events. Compelling new evidence from an in vivo study demonstrated that suppression of TRPM7 channels in adult rat brain in vivo using virally mediated gene silencing approach reduced delayed neuronal cell death and preserved neuronal functions in global cerebral ischemia. In this review, we will discuss the current understanding of the role of TRPM7 channels in physiology and pathophysiology as well as its therapeutic potential in stroke.
Keywords: ion channels, TRP, TRPM7, cerebral ischemia, stroke, in vivo test, siRNA, neuroprotection
Introduction
Stroke is one of the leading causes of death and disability in the world1, 2. The disease itself and associated morbidity have caused significant social and economic impacts on society and individuals worldwide. The prevalence of stroke is expected to increase and our aging population is especially vulnerable to stroke insults. The clinical trials of anti-excitotoxic therapies (AET) have failed to benefit stroke patients3, thus diminishing the initial excitement of translating research from bench to bedside and using glutamate receptor blockers in treating stroke patients. Even though the mechanisms underlying cerebral ischemia are beginning to be better understood, there is still no clinical or experimental treatment that has shown improved outcome for stroke patients. To ease personal and societal burden of stroke, continuous efforts have been directed towards searching for new therapeutic targets in stroke. This review provides a current view on one of the non-glutamate mechanisms of stroke that mediates through TRPM7 channels from a recent in vivo study 4.
A major event during cerebral ischemia is a concomitant massive release of the excitatory neurotransmitter glutamate, which results in intracellular calcium overload and eventual cell death5. The excitotoxicity in ischemia has been in the centre of stroke research for a long period of time. Triggered release of excessive glutamate causes cell death following ischemia, which is associated with an increase of the intracellular calcium (Ca2+) concentration6, 7, 8. Thus, identifying the source of the excessive Ca2+ influx and/or release from the intracellular Ca2+ stores during ischemia has been a research focus. Traditionally, Ca2+-permeable NMDA (N-methyl-D-aspartic acid), AMPA (DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors8 and L-type voltage-dependent Ca2+ channels9 were considered as the major calcium entry paths and the causes of Ca2+ overload during ischemia. This Ca2+ overload, or a broad spectrum of ion imbalance, during ischemia is considered to initiate a wide range of sequential events that lead to irreversible damage to protein synthesis, mitochondria, cytoskeleton and plasma membrane, and to eventual cell death. In an ideal scenario, the interruption of this Ca2+ overload in ischemia is thought to be clinically beneficial for stroke patients. Blocking these receptor channels prevents the intracellular Ca2+ overload and provides significant neuroprotection in the laboratory. Some of the findings from the bench have been translated into many clinical trials in stroke treatment. However, the results of clinical trials testing AET, which include NMDA and AMPA receptor blockers, turned out to be ineffective and even with unwanted side effects10, 11, 12, 13, 14. This may be due to multiple factors and it will not be the focus of this review15. Because of the limitation of the glutamate mechanism and the unfavourable outcomes of AET trials, stroke researchers have been seeking for alternative, non-glutamate related therapeutic targets that cause ionic imbalance and cell death. Some of these channels include: acid-sensing ion channels16, 17, transient receptor potential (TRP) channels4, 7, 18, 19, 20, 21, and hemichannels22, 23, 24, volume-regulated anion channels25, sodium-calcium exchangers26, 27 and non-selective cation channels28.
Based on the recommendations from the Stroke Therapy Academic Industry Roundtable (STAIR) committee, it is important to validate the preclinical development in proof of concept starting with in vivo rodent models as experimental animal stroke models29. Recent in vivo studies aimed at identifying the non-glutamate mechanisms for stroke have demonstrated the involvement of acid-sensing ion channels16, 17 first, and then the TRPM7 (transient receptor potential melastatin 7) channel4, 7, 18, 19, 20, 21. In this review, we will mainly focus on the current understanding of the molecular, biophysical, and pharmacological properties of TRPM7 as well as its physiological and pathophysiological roles and its therapeutic potential in stroke.
Classification, structures and distributions
Classification
The TRP superfamily is comprised of a group of non-selective cation channels30, 31, 32, 33. Its nomenclature was originated from the first found member of this superfamily, which was identified in a Drosophila phototransduction mutant showing transient receptor potential to a continuous light34. Currently, about 30 mammalian TRP channels have been discovered and named according to their sequence homologous structures. They are classified into six subfamilies: 1) TRPC (canonical), 2) TRPM (melastatin), 3) TRPV (vanilloid), 4) TRPA (ankyrin), 5) TRPML (mucolipin) and 6) TRPP (polycystin). Different TRP channels are activated by different physical and chemical stimuli. The diverse gating mechanisms of TRP channels make them good cellular signal integrators critical for physiological and pathological functions30, 31, 32, 33.
TRPM7 belongs to the melastatin-related subfamily of TRP channels, which is comprised of eight members (eg, TRPM1-8). It was suggested that TRPM7 may also form heteromers with TRPM2 as application of TRPM7 siRNA also down-regulated TRPM2 channel mRNA in an in vitro study21. This is important as TRPM2 has also shown to play a role in oxidative stress-mediated cell death, which is a cellular condition shown in stroke.
Gene and protein structures
In human, TRPM7 gene is located on chromosome 15 in the q21.2 region, and encoded by 39 exons that spans about 127 kb of DNA sequence. The mouse TRPM7 gene is 95% identical to human gene35. It is located on chromosome 2 on cytoband F2 and it is also encoded by 39 exons that spans about 85 kb of DNA sequence.
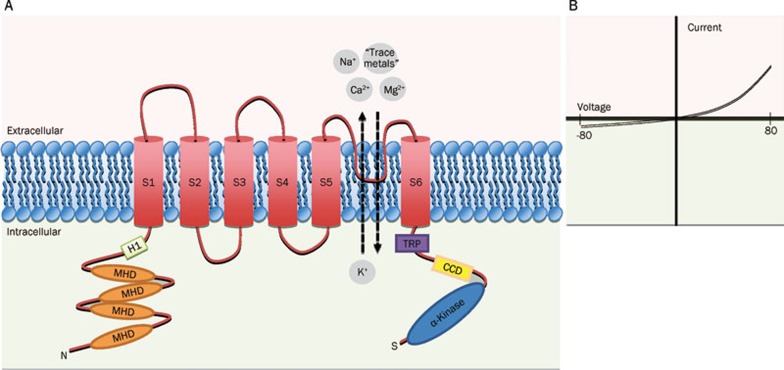
TRPM7 is a large protein (1864 amino acids in human; 1863 amino acids in mouse) with a predicted molecular weight of approximately 212 kDa. Each subunit has six transmembrane (TM) spanning domains (S1–S6) with a re-entrant pore-forming loop (known as P-loop) between the fifth (S5) and sixth (S6) segments32, 33 (Figure 1). The N-terminus has another hydrophobic region (H1) and four regions of TRPM subfamily homology domain (MHD), but their biological significance is largely undefined. The C-terminus contains a TRP box of ∼25 highly conserved residues, which may interact with phosphatidylinositol 4,5-bisphosphate (PIP2), a positive regulator of some TRP channel36. A coiled-coil domain close to the C-terminus may mediate subunit-subunit interactions and tetrameric assembly of TRPM737. The most unique structural feature of the channel is the enzymatic domain located at the end of C-terminus. In TRPM7, the distal C-terminus has an atypical serine/threonine protein kinase domain that is homologous to a family of α-kinases38. Although this kinase domain does not seem to affect channel activity directly33, 39, 40, it may be important for the regulation of channel function by Mg2+ nucleotides41.
Figure 1.
Schematic diagram showing proposed transmembrane topology of TRPM7. (A) The putative membrane topology of a single subunit of TRPM7 is shown. Each subunit has six transmembrane (TM) spanning domains (S1–S6) with a re-entrant pore-forming loop between the fifth (S5) and sixth (S6) segments. The intracellularly located N-terminus has another hydrophobic region (H1) and four regions of TRPM subfamily homology domain (MHD). The intracellularly located C-terminus contains a TRP box of ∼25 highly conserved residues (TRP) and a coiled-coil domain (CCD). The distal C-terminus has an atypical serine/threonine protein kinase domain. As indicated in the figure, TRPM7 is a non-selective cation channel that conducts both monovalent ions (eg, Na+ and K+) and divalent ions (eg, Ca2+, Mg2+ and other trace metal ions). (B) Representative current-voltage (I-V) relationship of TRPM7.
Tissue and cellular distribution
TRPM7 channel mRNA is ubiquitously expressed in almost all tissues33, 42, 43. Recently, real-time quantitative RT-PCR analyses with either Taqman or SYBR Green were used to create comparative distribution profiles of TRPM channels in selected human tissues, including brain, pituitary, heart, lung, liver, fetal liver, skeletal muscle, stomach, intestine, spleen, peripheral blood mononuclear cells, macrophages, pancreas, prostate, placenta, cartilage, bone and bone marrow42. TRPM7 mRNA has the highest expression in heart, pituitary, bone, and adipose tissue42. Similar distribution patterns of TRPM7 were also observed in mouse tissue samples44. Compared to other TRP members, TRPM7 mRNA expression levels were significantly higher in most tissues.
The TRPM7 protein shown by immunofluorescent labeling is strongly expressed at the plasma membrane in N1E-115 neuroblastoma cells45, and in vascular smooth muscle cells46. In N1E-115 neuroblastoma cells, HA-tagged TRPM7 antibodies were localized in membrane ruffles45. Similarly, protein expression of TRPM7 is also shown in cell bodies and processes of hippocampal neurons with immunostaining4, 47, 48. In superior cervical ganglion neurons, TRPM7 is exclusively localized within cholinergic vesicles49.
Biophysical properties, regulatory mechanisms, pharmacology
Biophysical properties
TRPM7 is a non-selective cation channel that displays several biophysical features that make this channel distinguishable from other TRP members. TRPM7 channel has a reversal potential of approximately 0 mV, and a prominent outward rectification30, 33, 43, 50 (Figure 1B). At negative membrane potentials, TRPM7 conducts a small inward current by transporting divalent cations (eg calcium and magnesium) down their concentration gradients35. TRPM7 current density is usually under 20 pA/pF35, 50. At positive membrane potential, TRPM7 conducts a strong outward current as intracellular cations experience strong driving force to exit the cell. This outwardly rectifying property is entirely due to a voltage-dependent block of monovalent cation influx by extracellular divalents. For instance, in the absence of divalent cations, TRPM7 conducts inward monovalent cations35. Consequently, its I-V relation becomes quasi-linear suggesting the lack of voltage-dependent gating and channel selectivity (Figure 1B).
Unlike many other Ca2+ permeating channels, TRPM7 is characteristically more permeable to a series of trace metal ions. Using equimolar divalent ion substitution approaches, Monteilh-Zoller and colleagues reported a permeation profile for TRPM7 in a sequence of: Zn2+≈Ni2+>>Ba2+>Co2+>Mg2+≥ Mn2+≥Sr2+≥Cd2+≥Ca2+ 51. TRPM7 allows entry of these divalent ions even with physiological levels of extracellular Ca2+ and Mg2+. TRPM7 is constitutively active and this feature makes TRPM7 a good candidate for both sensing the extracellular concentration of divalents and maintaining intracellular Mg2+ homeostasis during ischemic episodes that lead to intense neuronal activity47.
TRPM7 channel activity is regulated by extracellular pH. A decrease in extracellular pH (acidic) strongly potentiated current activity of the recombinant TRPM7 channel expressed in HEK-293 cells (∼10-fold increase at pH 4.0, and 1–2 fold increase at pH 6.0)52 and in CHOK1 cells (∼12-fold increase at pH 4.0)53. However, the TRPM7-like current in the FaDu cell line was insensitive to the acidic condition (pH 5.0)54, while the TRPM7-like inward current in human cervical epithelial HeLa cells was increased at pH 4.055. Protons likely compete with Ca2+ and Mg2+ for their binding sites, thus increase the inward current by releasing the divalent cation block52. Point mutation of Glu1047 (E1047Q) of TRPM7 channels eliminated the proton-enhanced inward current activity, indicating the residue may be involved in the pH sensitivity of the channels53. Although the effects of protons on endogenous TRPM7 remains controversial, TRPM7 can be regulated in acidic pathophysiological conditions, including ischemic stroke56.
TRPM7 is not a mechanosensitive channel, however, shear stress in vascular smooth muscle cells doubled the number of TRPM7 channels near the plasma membrane57. Further studies are required to deduce the mechanism of stress-induced regulation of TRPM7 channels.
Regulatory mechanisms
The heterologously expressed foreign and native TRPM7 channels are constitutively active, and their activities can be modulated by several extracellular and intracellular factors43, 50. TRPM7 activities can be tonically inhibited by intracellular Mg2+, and Mg-complexed nucleotides, MgATP, MgGTP, and such tonic inhibition is usually less than 10% of maximal conductance35, 41, 50. Whole-cell patch-clamp recordings have shown that either adding Mg2+ chelators (eg HEDTA or Na-ATP) intracellularly or omitting Mg2+ and Mg2+-complexed nucleotides in intracellular solutions increased activation of TRPM740, 41, 43. These inhibitory effects may be mediated by binding to C-terminal kinase domain39, 43, which in itself is not essential for the activation of TRPM733, 39, 40. Compared to the wild-type, phosphotransferase-deficient mutant channels (K1648R and G1799D) demonstrated a reduced sensitivity to inhibition by Mg2+ at intermediate concentrations close to the IC5039. Moreover, Demeuse and colleagues41 reported differential sensitivity to Mg2+ and Mg2+-nucleotides inhibition in the wild-type, phosphotransferase deficient point mutant (K1648R), and the Δ-kinase truncation mutant. These findings lead to a hypothetical model: only Mg2+-nucleotides bind to the kinase domain but this domain interacts with the Mg2+ binding site, which is responsible for regulating the channel activity.
Activation of TRPM7 can be regulated via PIP2, which is a substrate of phospholipase C (PLC)58. The C2 domain of PLC is directly associated with the kinase domain of TRPM7. When carbachol, an agonist for Gαq-linked muscarinic type 1 (M1) receptors, was used, PLC-beta was activated. This activation of PLC-beta led to the hydrolysis of localized PIP2, which caused a rapid decreased ITRPM7.
TRPM7 may also be regulated by phosphorylation. A variant of TRPM7 with a missense mutation (T1482I) is found in a subset of patients with Guamanian amyotrophic lateral sclerosis (ALS-G) and Parkinsonism-dementia (PD-G)59. When recombinant TRPM7s with T1482I mutation were heterologously expressed in HEK-293 cells, these channels were functional but showed increased sensitivity to Mg2+ inhibition and reduced phosphorylation compared to wild-type59. Based on the computer analysis of the secondary structure, both Thr-1482 in fish, amphibian, avian, and primate species, and Ser-1482 in murine species are the potential substrates for autophosphorylation by the C-terminus serine/threonine α-kinase domain in TRPM7. Ile-1482 mutation found in these patients, however, cannot be phosphorylated. TRPM6, the closest member to TRPM7, also regulates TRPM7 via cross-phosphorylation, and alters Mg2+ homeostasis regulation60.
Pharmacological properties
There are currently no selective pharmacological tools (both agonists and antagonists) that can specifically modulate TRPM7 channels61. Gene deletion in global TRPM7 knockout animal is confirmed to be embryonically lethal62, 63. Thus, inability to modulate the TRPM7 channels pharmacologically creates a huge obstacle for investigating the physiological and pathophysiological roles of TRPM7 in stroke. There are some successful studies of gene silencing using small interfereing RNA (siRNA) to knockdown the TRPM7 in either mRNA and/or protein expression in central neurons4, 21, 47, peripheral neurons49, vascular endothelial cells64, vascular smooth muscle cells65, gastrointestinal tract interstitial pacemaker cells66 and human epithelial cells67. TRPM7 can be blocked non-specifically by trivalent ions, such as Gd3+ ((IC50 ∼1.4–2.5 μmol/L) and La3+ (IC50 ∼17 μmol/L)21 and 2-Aminoethoxydiphenyl borate (2-APB) (IC50 ∼50 μmol/L), which is a well known non-specific blocker of many TRP channels68. Recently, it has been reported that inhibitors of 5-lipoxygenase (5-LOX), NDGA (nordihydroguaiaretic acid, IC50 ∼6.3 μmol/L), AA861(IC50 ∼6.0 μmol/L), and MK886 (IC50 ∼8.6 μmol/L), can suppress the TRPM7 current in HEK-293 cells69. Application of these molecules also prevented some of the phenomena (eg cell death) associated with TRPM7 when exposed to low extracellular divalent cations and other apoptotic stimuli. These effects seem to be independent of their actions on 5-LOX, and the expression level and cellular concentrations of TRPM7 at the plasma membrane were not affected. Previously, studies have reported less tissue damage during cerebral ischemia and myocardial ischemia-reperfusion injury with 5-LOX inhibition70, 71. Although drawing a connection between cellular protective effects during ischemic injury with 5-LOX and blockade of TRPM7 with 5-LOX would be premature, these findings emphasize the importance of future follow-up studies. Thus, there is a pressing need of specific pharmacological agents for studying the physiological and pharmacological roles of the TRPM7 channels in vivo and potential therapeutic uses.
Physiological functions
Our current knowledge of the physiological functions of TRPM7 channels has recently been improved, even with limited molecular and specific pharmacological tools. Under physiological conditions, several lines of evidence suggest the role of TRPM7 in cell survival and proliferation39, 62, 72. The early embryonic lethality in global TRPM7 knockout mice hints at the requirement of TRPM7 in cell survival and proliferation as embryonic development involves extensive cell proliferation62. In the same study, TRPM7 gene was selectively deleted in developing thymocytes. These T-cells did not differ in its ability of uptake Mg2+ or maintaining global cellular Mg2+, but showed defective thymopoiesis. A more recent study showed that knocking out of TRPM7 kinase domain homozygously resulted in embryonic lethality63, while heterozygous knockout mice were viable, but exhibited abnormal homeostasis63. TRPM7 knockout in chicken DT40 B cells caused growth arrest and eventual cell death in culture39, which may be linked to a regulation of Mg2+ homeostasis72. Supplementing TRPM7 knockout cells with a high Mg2+ containing medium, but not Ca2+ or Zn2+, could restore normal cell growth and survival in culture. Knockdown of TRPM7 with RNA interference reduced Ca2+ and Mg2+ influxes, and decreased cell proliferation in human osteoblast-like cells72, and retinoblastoma cells73. TRPM7 dependence for proliferation and differentiation was also shown in zebrafish mutants as they displayed severe growth retardation and general alterations in skeleton development74.
Several studies have shown the importance of TRPM7 in cell adhesion. Over-expression of TRPM7 in HEK-293 cells lead to cell rounding, loss of adhesion and cell death35. Consistent with these findings, knockdown of TRPM7 in HEK-293 cells increased cell adhesion75. Over-expression of TRPM7 may produce cell rounding by stimulating the activity of the Ca2+-dependent protease m-calpain. TRPM7 has also been implicated in cell motility76. Knockdown of TRPM7 by RNA interference reduced the number of high Ca2+ micro-domains induced by platelet-deprived growth factor (PDGF) and disrupted the turning of migrating WI-38 fibroblasts.
It has also been suggested that TRPM7 is involved in the neurotransmitter release by mediating Ca2+ influx49. In primary rat superior cervical ganglion neurons, TRPM7 is localized in the synaptic vesicles and interacts with synaptic vesicular snapin, synapsin 1 and synaptotagmin 1. Furthermore, there were some correlations between TRPM7 expression levels and quantal sizes, amplitudes and decay times of the excitatory postsynaptic potential (EPSPs). When TRPM7 specific siRNA was used to suppress endogenous TRPM7 in PC12 cells, acetylcholine-secreted-synaptic-like vesicle fusion was inhibited.
Pathophysiological relevance in cerebral ischemia and stroke
Unregulated monovalent or divalent cation influx is implicated in several different cellular mechanisms (eg, excitotoxicity, apoptosis, and oxidative stress) underlying neural cell death during ischemic periods of stroke61. Since cation channels are the main pathways for cation influx from extracellular space, they are closely involved in neuronal cell death. Conventionally, Ca2+ permeable NMDA and AMPA receptor channels are widely accepted as the main pathways of Ca2+ entrance during ischemia as well as the promising therapeutic targets7, 8, 77. Numerous clinical trials testing AETs in stroke patients, however, yielded disappointing outcomes. The shortcomings of AET led researchers to consider other non-glutamate dependent mechanisms10, 11, 12, 13, 14, such as non-specific cation channels including acid-sensing ion channels16, 17, TRP channels4, 7, 18, 19, 20, 21, hemichannels22, 23, 24, volume-regulated anion channels25, sodium-calcium exchangers26, 27 and non-selective cation channels28.
Previous studies demonstrated pathophysiological involvement of TRPM7 in stroke from in vitro data. When primary cultured cortical neurons were subjected to oxygen-glucose deprivation (OGD) for a prolonged period, there was an increase in ROS production, a Ca2+ influx mediated by TRPM7 and cell deaths21. When the primary mouse cortical neurons were transfected with siRNA vector directed against TRPM7, the TRPM7 mRNA expression was suppressed, ROS-mediated activation was inhibited and subsequent cell death under anoxia was reduced. Such effects were consistently shown with cocktail of blockers for glutamate NMDA and AMPA receptor and L-type calcium channels (MK-801, CNQX, and nimodipine), indicating the independent role of TRPM7 in mediating intracellular Ca2+ elevation and subsequent cell death during the prolonged anoxia. In another study, the contribution of TRPM7 channels in cell membrane depolarization, intracellular Ca2+ accumulation and cell swelling during the initial period of brain ischemia is also observed in native CA1 neurons of brain slices78.
TRPM7 in vivo studies have been scarce for a period of time because both the knockout model and selective pharmacological agents are not available. Eventually, a report demonstrated in vivo changes in TRPM7 channels during focal ischemia. Jiang and colleagues48 studied the interaction of nerve growth factor (NGF) with TRPM7 channels using both in vivo cerebral ischemia-reperfusion and in vitro OGD models. NGF, a neurotrophic factor, showed neuroprotective effects during ischemia. In their in vivo model, middle cerebral artery occlusion (MCAO) was performed on rats for 1 h and it was followed by reperfusion that lasted for 5, 10, 20, and 30 h. Both mRNA and protein levels of TRPM7 were up-regulated compared with pre-ischemia, peaking at 20 h after the reperfusion with about 2–3 fold increase. Given that there are increases in both mRNA and protein levels of TRPM7, up-regulation of the channels may be another mechanism that increases TRPM7-like current. Interestingly, these expression levels of TRPM7 were close to the normal level when 500 ng of NGF was applied 30 min before ischemia. The effects of NGF on TRPM7, however, disappeared when NGF was introduced after K252a, which is an inhibitor for the NGF-activated TrkA pathway. When wortmannin, which is an inhibitor for phosphatidylinositol-3 kinase (PI-3K) signal pathway, was applied, NGF effects were also abolished. These findings indicate that TRPM7 may be involved in neuronal cell damage in vivo during ischemia.
Recently, Sun and colleagues4 demonstrated that suppression of TRPM7 channels in vivo reduced neuronal cell death and preserved functions after global cerebral ischemia. The study used virally mediated gene silencing with shRNA to knockdown TRPM7 channels in hippocampal CA1 pyramidal neurons of adult rat brains. The viral vectors were delivered in vivo using stereotaxic microinjection to CA1 area. First, the authors showed that infecting adult hippocampal CA1 neurons in vivo was feasible by using the adeno-associated viral vectors (AAV serotype-1). Secondly, suppression of TRPM7 channels was convincingly demonstrated by measuring: 1) mRNA level in conjunction with the Laser Capture Microdissection for infected hippocampal CA1 cells; 2) protein level with both Western Blot and immunohistochemistry in conjunction with Laser Confocal microscope; and 3) functional level with electrophysiology. Thirdly, the injected viral vectors and transient suppression of TRPM7 channels in the adult rat brains in vivo showed no ill effects on cell survival, neuronal and dendritic morphology, neuronal excitability, or synaptic plasticity. Finally, they showed that following fifteen minutes of global cerebral ischemia induced by occluding both common carotid and vertebral arteries, TRPM7 suppression reduced hippocampal CA1 neuronal death in vivo and preserved functional outcomes after stroke. The survived neurons preserved their morphological integrity and fine structures, and even maintained their electrophysiological properties (LTP) and hippocampal-dependent behaviours, such as fear-associated and spatial-navigation memory tasks. This is the first in vivo evidence showing the important role of TRPM7 channel in mediating ischemic neuronal cell death in stroke.
Working model of TRPM7 activation during cerebral ischemia
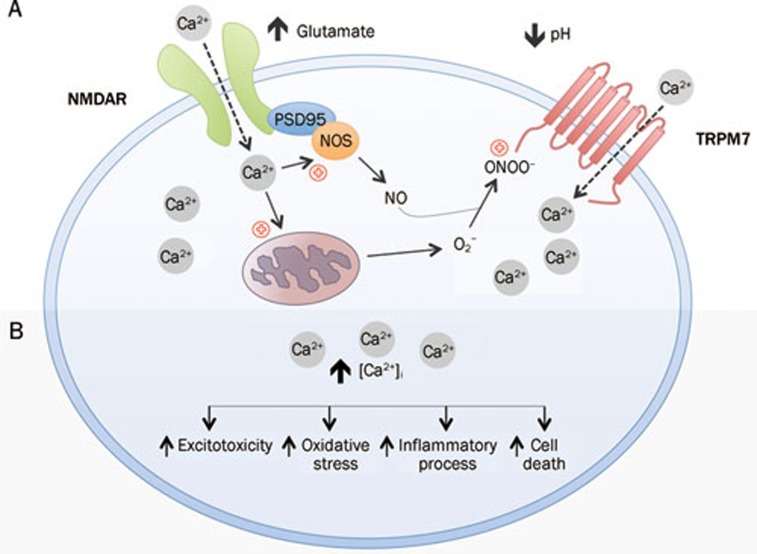
During the initial phase of an ischemic attack, a strong NMDA receptor activation leads to a large influx of Ca2+, and the resulting Ca2+ directly stimulates (i) production of nitric oxide (NO) by neuronal nitric oxide synthase (NOS) and (ii) production of superoxide (O2−) from mitochondria17, 18, 19. When NO and O2− are combined, highly reactive species peroxynitrite (ONOO−) form. Along with other factors, such as decreases in pH and extracellular divalents, that are associated with ischemic episodes, ONOO− enhances TRPM7 activation. This completes the lethal positive feedback loop of free radical production. In this model, the failure of AET could be partly explained: AET could delay the process but insufficient to ultimately prevent lethal TRPM7 activation (Figure 2).
Figure 2.
Working model of TRPM7 activation during cerebral ischemia. (A) During the early phase of an ischemic attack, an increase in the extracellular glutamate activates NMDA receptors. Ca2+ influx due to activated NMDA receptors stimulates: (i) production of nitric oxide (NO) by nitric oxide synthase (NOS) and (ii) production of superoxide (O2−) from mitochondria. NO and O2− combine to produce highly reactive species peroxynitrite (ONOO−). Along with factors, such as decrease in pH, that are associated with ischemia, these free radicals promote sustained activation of TRPM7, which leads to further Ca2+ build-up in the intracellular space. (B) Consequences of unchecked Ca2+ influx. Increased intracellular Ca2+ concentration may lead to excitotoxicity, oxidative stress, inflammatory processes and eventual cell death.
TRPM7 channels may also be involved in the ischemic lethal process by conducting metal ions other than Ca2+. For instance, Zn2+, which is the most permeable trace ion through TRPM7, is highly toxic to cells if its concentration exceeds the physiological level79, 80 and has been implicated in cerebral ischemia. After the brief global ischemic insults, a delayed increase in intracellular Zn2+ is observed before cell death in some selective hippocampal CA1 neurons81. Increases in intracellular Zn2+ and neuronal cell death were prevented with the application of the membrane-impermeable zinc-chelator calcium-EDTA (calcium-ethylenediaminetetraacetic acid) before the ischemia. Recently, it has been shown that Zn2+-induced neurotoxicity may be mediated by TRPM782. Both Zn2+-mediated neurotoxicity and neuronal injury associated with oxygen-glucose deprivation (OGD) were reduced by non-specific blockers (Gd3+ and 2-APB) and knockdown of TRPM7 by siRNA. Overexpression of TRPM7 in HEK-293 cells led to increase in intracellular Zn2+ accumulation and Zn2+-mediated cell deaths.
Clinical potentials and therapeutic perspectives
To date, therapeutic intervention for stroke is very scarce. The only approved treatment of acute ischemic stroke by the US Food and Drug Administration (FDA) is the tissue plasminogen activator (tPA), which relieves vascular occlusion by dissolving clots83. Although tPA is a potent treatment for stroke, the usage and effectiveness of tPA are still limited by its short therapeutic window, and intrinsic toxicity. With disappointing preliminary clinical results from drugs targeting glutamate-induced excitotoxicity, considerable efforts have been put into searching for alternative targets.
Several lines of evidence support our hypothesis that TRPM7 is involved in ischemic stroke77. Even though findings from cellular and animal studies are compelling, TRP channels should be studied in their native cellular environment, as the specific cellular environment and expression levels seem to be important for the normal physiological functions. It would be necessary to validate their diverse physiological and pathophysiological functions using in vivo animal models. For in vivo studies, developing tissue-specific or inducible TRPM7 knockout models will be useful as the conventional TRPM7 knockout mouse is not viable62, 63.
Without the development of specific pharmacological modulators of TRPM7, we do not expect to see any preliminary clinical trials in the near future. At the initial stage of most drug development, potential therapeutic targets are first identified, and experimental high-throughput screening (HTS) is used to narrow down the drug candidates that bind to the targets and changes their activities84. Hence, in order to design specific and potent inhibitors, it is important to understand their molecular or structural properties in detail84, 85. This is especially true for TRPM7, which seems to have conflicting roles in cell death and cellular survival4, 21, 62. It may be the case that TRPM7 function would have to be regulated separately, either enhanced or depressed, in different tissues for the prevention of cerebral ischemia and stroke. For instance, Touyz and colleagues86 have shown that reduced Mg2+ influx in cultured vascular smooth muscle cells (VSMCs) of the spontaneously hypertensive rat (SHR) is associated with down-regulation of TRPM7. Furthermore in the normotensive Wistar-Kyoto rat, TRPM7 expression and activity in VSMCs of the SHR were attenuated by angiotensin II. Since hypertension is a well-known risk factor for cerebral ischemia and stroke, it suggests that TRPM7 channels may enhance cerebral ischemia and stroke by regulation of Mg2+ homeostasis87. However, either supplement or depletion of Mg2+ showed no effect on hypertension, thus questioning the role of TRPM7 in vasculature regulation87. In contrast, reduction of TRPM7 expression prevented cerebral ischemia and stroke4 indicating TRPM7 may play a differential role in vascular smooth muscle cells and neurons. Further investigation is required to evaluate pathophysiological roles of TRPM7 channels in different cell types. Therefore, designing activity-dependent antagonists that preferentially target TRPM7 during stroke is critical in the future study. Understanding its temporal, spatial expressions and interactions with other proteins may aid the development of selective drugs for modulating TRPM7 activity.
When specific TRPM7 channel modulators are developed, these might be added in combination therapy for cerebral ischemic stroke in future. Now it is suspected that, although the recruitment of NMDARs is the key event in the early phase of cell death cascades in cerebral ischemia, there is also progressive recruitment of other non-selective cation channels, such as TRPM7, in the later stages. Based on this hypothesis, combination therapy, which includes drugs that are applied at empirically determined time points for each target, will be more effective in providing neuroprotection and potentially facilitating the recovery of function.
TRPM7 genetic variants may be related to various human diseases. For instance, heterologously expressed T1482I TRPM7 variant was found in a subset of ALS-G (Guamanian amyotrophic lateral sclerosis) and PD-G (Parkinsonism dementia) patients59. The mutation resulted in an increase in sensitivity of the channels to intracellular Mg2+-mediated inhibition, thus the patients were more vulnerable to the diseases59. No test has been performed related to the risk assessment of ischemic stroke. Romero and colleagues88, recently conducted a prospective, nested case-control investigation to evaluate the associations of TRPM7 gene variations with the risk of ischemic stroke, and showed that 16 tag-single-nucleotide TRPM7 polymorphisms from 259 Caucasian men had no direct association with the risk assessment of ischemic stroke. However, no test has been done to suggest whether these TRPM7 mutants have neither dysfunction nor abnormality of the expression level of the channels. Thus, these human studies lead to no conclusion between TRPM7 activation and cerebral stroke. Further study is needed to fully understand the biophysical properties of the TRPM7 polymorphisms. Such information will dramatically contribute to our current understanding of the pathophysiological role of TRPM7 in cerebral ischemia and stroke.
Conclusions
With the failure of using NMDA and AMPA antagonists in clinical trials for stroke treatment, other non-glutamate mechanisms to ischemic cell death have been rigorously investigated. Such disappointing clinical outcomes may originate from the insufficient understanding of non-glutamate mechanisms and their molecular cascades involved in stroke, problems in drug development, delivery of drugs, side effects of drug, or limited time windows for treatment. While the compelling findings from both in vitro and in vivo studies indicate the involvement of TRPM7 channels in ischemic neuronal injury, further extensive preclinical testing is required to assess the therapeutic potential of the TRPM7 blockade in stroke.
References
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–46. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, et al. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke. 2000;31:347–54. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–7. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Macdonald J, Xiong Z, Jackson M. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 2006;29:75–81. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Horn J, Limburg M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst Rev. 2000;(2):CD001928. doi: 10.1002/14651858.CD001928. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Secondary Ca2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res. 1993;607:319–23. doi: 10.1016/0006-8993(93)91523-u. [DOI] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–12. [PubMed] [Google Scholar]
- Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992;12:1882–95. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies — the need for new approaches. Cerebrovasc Dis. 2004;17:153–66. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42:S24–7. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006;209:59–68. doi: 10.1007/s00232-005-0840-x. [DOI] [PubMed] [Google Scholar]
- McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Arch. 2005;451:235–42. doi: 10.1007/s00424-005-1440-4. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Arch. 2005;451:243–9. doi: 10.1007/s00424-005-1439-x. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Tymianski M. TRPM7 and ischemic CNS injury. Neuroscientist. 2005;11:116–23. doi: 10.1177/1073858404272966. [DOI] [PubMed] [Google Scholar]
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- de Pina-Benabou MH, Szostak V, Kyrozis A, Rempe D, Uziel D, Urban-Maldonado M, et al. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–7. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- Oguro K, Jover T, Tanaka H, Lin Y, Kojima T, Oguro N, et al. Global ischemia-induced increases in the gap junctional proteins connexin 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J Neurosci. 2001;21:7534–42. doi: 10.1523/JNEUROSCI.21-19-07534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–57. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, et al. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–56. [PubMed] [Google Scholar]
- Pignataro G, Tortiglione A, Scorziello A, Giaccio L, Secondo A, Severino B, et al. Evidence for a protective role played by the Na+/Ca2+ exchanger in cerebral ischemia induced by middle cerebral artery occlusion in male rats. Neuropharmacology. 2004;46:439–48. doi: 10.1016/j.neuropharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Moran M, Xu H, Clapham D. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–69. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Pedersen S, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–52. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An Introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–23. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–5. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Mei ZZ, Xia R, Beech DJ, Jiang LH. Intracellular coiled-coil domain engaged in subunit interaction and assembly of melastatin-related transient receptor potential channel 2. J Biol Chem. 2006;281:38748–56. doi: 10.1074/jbc.M607591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell. 2001;7:1047–57. doi: 10.1016/s1097-2765(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Kozak JA, Cahalan MD. MIC channels are inhibited by internal divalent cations but not ATP. Biophys J. 2003;84:922–7. doi: 10.1016/S0006-3495(03)74909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006;127:421–34. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26:159–78. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogi A, Callera GE, Tostes R, Touyz RM. Bradykinin regulates calpain and proinflammatory signaling through TRPM7-sensitive pathways in vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2009;296:R201–7. doi: 10.1152/ajpregu.90602.2008. [DOI] [PubMed] [Google Scholar]
- Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, et al. TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci U S A. 2007;104:16323–8. doi: 10.1073/pnas.0701149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Tian SL, Zeng Y, Li LL, Shi J. TrkA pathway(s) is involved in regulation of TRPM7 expression in hippocampal neurons subjected to ischemic-reperfusion and oxygen-glucose deprivation. Brain Res Bull. 2008;76:124–30. doi: 10.1016/j.brainresbull.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–96. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Penner R, Fleig A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb Exp Pharmacol. 2007;(179):313–28. doi: 10.1007/978-3-540-34891-7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–50. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, et al. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–30. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation [Research Support, NIH, Extramural] Cancer Res. 2007;67:10929–38. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata T, Okada Y. Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem. 2008;283:15097–103. doi: 10.1074/jbc.M709261200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehncrona S. Brain acidosis. Ann Emerg Med. 1985;14:770–6. doi: 10.1016/s0196-0644(85)80055-x. [DOI] [PubMed] [Google Scholar]
- Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–53. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol. 2002;4:329–36. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, et al. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A. 2005;102:11510–5. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–71. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta. 2007;1772:947–57. doi: 10.1016/j.bbadis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–60. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, et al. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Xiong ZG. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc Res. 2009;83:547–57. doi: 10.1093/cvr/cvp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–15. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, et al. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–17. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol. 2007;292:C460–7. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–37. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS ONE. 2010;5:e11161. doi: 10.1371/journal.pone.0011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatana M, Giri S, Ansari MA, Elango C, Singh AK, Singh I, et al. Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia. J Neuroinflammation. 2006;3:12. doi: 10.1186/1742-2094-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamek A, Jung S, Dienesch C, Laser M, Ertl G, Bauersachs J, et al. Role of 5-lipoxygenase in myocardial ischemia-reperfusion injury in mice. Eur J Pharmacol. 2007;571:51–4. doi: 10.1016/j.ejphar.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007;40:849–65. doi: 10.1111/j.1365-2184.2007.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, et al. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–19. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, et al. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for TRPM7. Curr Biol. 2005;15:667–71. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, et al. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–70. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Wang X, Chen M, Ou-Yang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–5. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–75. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Lipski J, Park TI, Li D, Lee SC, Trevarton AJ, Chung KK, et al. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res. 2006;1077:187–99. doi: 10.1016/j.brainres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–31. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–8. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–6. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–9. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambauer KZ, Johnston SC, Bambauer DE, Zivin JA. Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol. 2006;63:661–4. doi: 10.1001/archneur.63.5.661. [DOI] [PubMed] [Google Scholar]
- Jorgensen WL. The many roles of computation in drug discovery. Science. 2004;303:1813–8. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- Zheng C, Han L, Yap CW, Xie B, Chen Y. Progress and problems in the exploration of therapeutic targets. Drug Discov Today. 2006;11:412–20. doi: 10.1016/j.drudis.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, et al. Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R73–8. doi: 10.1152/ajpregu.00515.2005. [DOI] [PubMed] [Google Scholar]
- Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J. 2011;75:237–45. doi: 10.1253/circj.cj-10-1021. [DOI] [PubMed] [Google Scholar]
- Romero JR, Ridker PM, Zee RY. Gene variation of the transient receptor potential cation channel, subfamily M, member 7 (TRPM7), and risk of incident ischemic stroke: prospective, nested, case-control study. Stroke. 2009;40:2965–8. doi: 10.1161/STROKEAHA.109.558346. [DOI] [PMC free article] [PubMed] [Google Scholar]