Abstract
The mutation F508del is the commonest cause of the genetic disease cystic fibrosis (CF). CF disrupts the function of many organs in the body, most notably the lungs, by perturbing salt and water transport across epithelial surfaces. F508del causes harm in two principal ways. First, the mutation prevents delivery of the cystic fibrosis transmembrane conductance regulator (CFTR) to its correct cellular location, the apical (lumen-facing) membrane of epithelial cells. Second, F508del perturbs the Cl− channel function of CFTR by disrupting channel gating. Here, we discuss the development of rational new therapies for CF that target F508del-CFTR. We highlight how structural studies provide new insight into the role of F508 in the regulation of channel gating by cycles of ATP binding and hydrolysis. We emphasize the use of high-throughput screening to identify lead compounds for therapy development. These compounds include CFTR correctors that restore the expression of F508del-CFTR at the apical membrane of epithelial cells and CFTR potentiators that rescue the F508del-CFTR gating defect. Initial results from clinical trials of CFTR correctors and potentiators augur well for the development of small molecule therapies that target the root cause of CF: mutations in CFTR.
Keywords: ATP-binding cassette transporter, epithelial ion transport, cystic fibrosis, CFTR, chloride ion channel, F508del, CFTR corrector, CFTR potentiator
Introduction
Salty sweat is diagnostic of cystic fibrosis (CF), an autosomal recessive genetic disease common in Caucasians1, 2. The elevated concentration of salt in sweat is indicative of the underlying molecular defect in CF, the loss of chloride ion (Cl−) channel function in the apical (lumen-facing) membrane of epithelia lining ducts and tubes throughout the body1. The impermeability of the apical membrane to Cl− in CF disrupts fluid and electrolyte transport across epithelia and, hence, the function of a variety of organs. This leads to the wide-ranging manifestations of the disease, which include chronic lung disease, exocrine pancreatic insufficiency, meconium ileus (blockage of the terminal ileum), male infertility and salty sweat1, 2. The median survival of CF patients in North America and Western Europe is around 40 years2.
There are two principal causes of debilitation and death in CF patients1, 3, 4. First, chronic obstructive lung disease caused by thick tenacious mucus that prevents normal mucociliary clearance. Second, persistent bacterial infections, typically with Pseudomonas aeruginosa, which result in bronchiectasis, respiratory failure and eventually death. Current therapies for CF include physiotherapy, mucolytic drugs and antibiotics to treat lung disease, and pancreatic enzyme replacement and supplementary nutrition to overcome gastrointestinal dysfunction1, 2. These therapies treat the symptoms of CF; they do not target the root cause of the disease.
In 1989, the defective gene responsible for CF was identified and predicted to encode a protein with five domains: two membrane-spanning domains (MSDs), two nucleotide-binding domains (NBDs) and a unique regulatory domain (RD)5. Shortly thereafter, the protein product of this gene, the cystic fibrosis transmembrane conductance regulator (CFTR), was demonstrated to be a unique member of the ATP-binding cassette (ABC) transporter superfamily6. Instead of forming an ATP-driven pump like most family members, CFTR was demonstrated to function as an ATP-gated pathway for anion movement driven by the transmembrane electrochemical gradient7, 8, 9, 10. Subsequent research has aimed to understand the physiological role of CFTR, learn how CF mutations cause CFTR dysfunction and develop rational new therapies for CF patients. Here, we selectively review progress in the development of drug therapies for CF, focusing on small molecules that target the most common CF mutation, F508del, deletion of the three base pairs that result in the loss of the phenylalanine residue at position 508 of the CFTR protein sequence; 90% of CF patients carry at least one copy of the F508del mutation.
The F508del-CFTR mutant retains some residual channel function
When Rich et al11 found that expression of F508del-CFTR in CF airway epithelial cells failed to correct the defective Cl− permeability of these cells and Cheng et al12 subsequently demonstrated that the F508del mutation disrupts CFTR biosynthesis and membrane trafficking in COS-7 cells, it was widely assumed that F508del-CFTR had no Cl− channel function. Surprisingly however, Drumm et al13 demonstrated that F508del-CFTR generates a cAMP-activated Cl− conductance when expressed in Xenopus oocytes. Because F508del-CFTR Cl− currents had similar conduction and permeation properties, but reduced magnitude compared with those of wild-type CFTR, Drumm et al13 speculated that F508del-CFTR forms a channel with attenuated sensitivity to cAMP agonists, a conclusion that was to prove prescient. Concurrently, Dalemans et al14 used the patch-clamp technique to demonstrate that F508del-CFTR forms a Cl− channel regulated by cAMP-dependent phosphorylation in Vero cells. The authors demonstrated that F508del-CFTR had many properties in common with those of wild-type human CFTR14. However, there was one notable exception, the pattern of channel gating of F508del-CFTR differed dramatically from that of wild-type CFTR14.
Biophysical properties of the F508del-CFTR Cl– channel
Like other mutations that affect specific residues within the NBDs15, F508del has no discernable effect on the conduction and permeation properties of the CFTR Cl− channel. First, the F508del-CFTR Cl− channel has a small single-channel conductance, which does not differ from that of wild-type CFTR (6–10 pS; eg Li et al16). Second, like wild-type CFTR (but see17), the current-voltage (I–V) relationship of F508del-CFTR is linear (eg Dalemans et al14). Third, both wild-type and F508del-CFTR are highly selective for anions over cations (PNa/PCl=0.08; eg Li et al16). Fourth, wild-type and F508del-CFTR share the identical anion permeability sequence of Br−>Cl−>I− (eg Dalemans et al14). Finally, wild-type and F508del-CFTR both exhibit time- and voltage-independent gating behavior (eg Denning et al18). Consistent with these data, using excised membrane patches from gallbladder epithelial cells of wild-type and F508del-CFTR mice French et al19 demonstrated that the F508del mutation is without effect on the biophysical properties of murine CFTR. Thus, the data suggest that the F508del mutation does not affect the pore properties of CFTR.
The gating defect of the F508del-CFTR Cl– channel
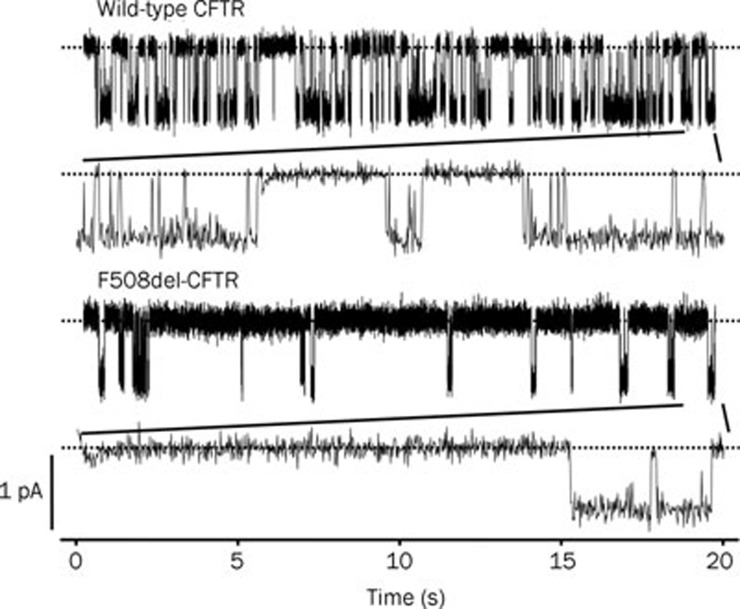
Figure 1 illustrates the gating pattern of wild-type and F508del-CFTR Cl− channels following phosphorylation by protein kinase A (PKA). The gating behavior of wild-type CFTR is characterized by frequent bursts of channel activity that are interrupted by brief flickery closures and separated by longer closures between bursts (Figure 1). By contrast, the gating pattern of F508del-CFTR is characterized by infrequent bursts of channel activity that are interrupted by brief flickery closures, but separated by long closures of prolonged duration (Figure 1). Work by a number of investigators using a variety of cells and experimental conditions demonstrate that the open probability (Po; a measure of the average fraction of time that a channel is open) of F508del-CFTR is about one third that of wild-type CFTR14, 18, 20, 21, 22, 23, although Miki et al24 argue that the Po of F508del-CFTR is likely to be substantially lower (∼fifteen-fold less than that of wild-type CFTR). Surprisingly, and in marked contrast to these data, other authors found that the Po of F508del-CFTR did not differ from that of wild-type CFTR16, 19. A likely explanation for these differences is that the rate of activation of F508del-CFTR is more than seven-fold slower than that of wild-type CFTR25.
Figure 1.
Single-channel activity of wild-type and F508del-CFTR. Representative recordings of wild-type and F508del-CFTR Cl− channels in excised inside-out membrane patches from C127 cells expressing recombinant CFTR. ATP (1 mmol/L) and PKA (75 nmol/L) were continuously present in the intracellular solution, voltage was clamped at -50 mV, and a large Cl− concentration gradient was imposed across the membrane patch ([Cl−]Ext=10 mmol/L; [Cl−]Int=147 mmol/L). Dashed lines indicate where the channels are closed and downward deflections correspond to channel openings. Beneath each of the prolonged 20 s recordings, the last 1 s of the record is shown on an expanded scale. Other details are as described in Cai and Sheppard23. Modified, with permission, from Cai and Sheppard23.
To understand how the F508del mutation disrupts CFTR channel gating, several investigators have examined the gating kinetics of the F508del-CFTR Cl− channel. Dalemans et al14 first demonstrated that in cell-attached membrane patches on Vero cells voltage-clamped at -60 mV, the F508del mutation was without effect on open times, but decreased mean closed times five-fold compared with those of wild-type CFTR. Haws et al20 and our group23, 26 examined the gating kinetics of F508del-CFTR in membrane patches from BHK and C127 cells. Both groups found that the F508del mutation was without effect on open and closed times within bursts. Instead, F508del caused a large decrease in Po by (i) markedly prolonging the closed time interval between bursts and (ii) reducing mean burst duration20, 23, 26. These data suggest that the F508del mutation disrupts CFTR channel gating in two ways: first, F508del dramatically slows the rate of entry into a burst of channel activity. Second, F508del accelerates the rate of channel closure.
F508del is located at a critical interface in the CFTR gating pathway
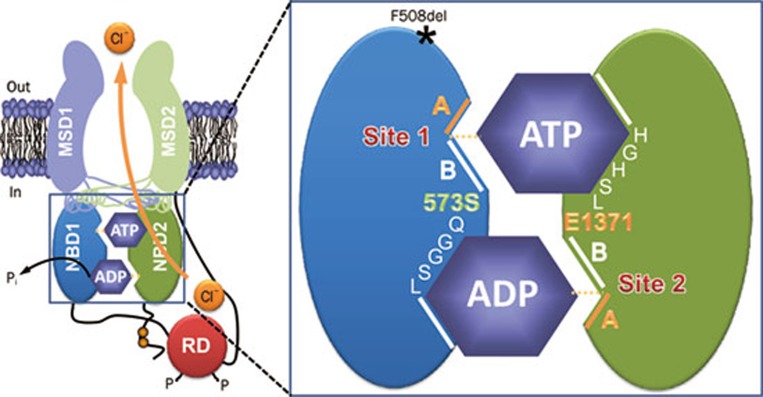
The F508del mutation profoundly disrupts CFTR channel gating by slowing dramatically the rate of channel opening and by accelerating the rate of channel closure. An explanation for the gating behavior of F508del-CFTR is provided by the ATP-driven NBD dimerization model of CFTR channel gating27, 28. This model integrates the results of functional studies of CFTR channel gating with biochemical and structural data. Structural studies of ABC transporters suggest that the NBDs are organized as a head-to-tail dimer with two ATP-binding sites located at the NBD1:NBD2 interface29, 30, 31, 32. The data suggest that one ATP-binding site is formed by the Walker A and B motifs of NBD1 and the LSGGQ motif of NBD2 (termed site 1), while the other is formed by the Walker A and B motifs of NBD2 and the LSGGQ motif of NBD1 (termed site 2) (Figure 2). However, photolabeling studies argue that the ATP-binding sites of CFTR are not equivalent in function; site 1 stably binds nucleotides, whereas site 2 rapidly hydrolyses them33, 34. Because CFTR Cl− channels transit between the closed and open configurations in seconds, Vergani et al27, 28 interpreted the photolabeling data to suggest that CFTR channel gating is controlled by ATP binding and hydrolysis at site 2, driving cycles of NBD dimer assembly and disassembly. To test this model, Vergani et al28 applied mutant cycle analysis to residues predicted to lie on opposite sides of the NBD1:NBD2 dimer interface. Of note, the authors demonstrated that R555 (NBD1) and T1246 (NBD2) are energetically coupled only in open channels, arguing convincingly that the NBDs undergo dynamic reorganization during channel gating28. For further discussion of how ATP gates the CFTR Cl− channel, see35, 36, 37.
Figure 2.
The organization of the ATP-binding sites in CFTR. The simplified model shows the molecular architecture of ATP-binding site 1 (site 1) and ATP-binding site 2 (site 2) in an open CFTR Cl− channel. Each ATP-binding site is formed by the Walker A and B motifs (labeled A and B, respectively) of one NBD and the LSGGQ motif of the other NBD. Site 2 contains a canonical LSGGQ motif, whereas site 1 contains a non-canonical LSGGQ motif (LSHGH). Site 2 also contains a catalytic base (E1371) at the distal end of the Walker B motif, but this residue is absent in site 1 (S573). The location of the CF mutation F508del on the surface of NBD1 opposite intracellular loop 4 (ICL4) is shown by an asterisk. Abbreviations: MSD, membrane-spanning domain; NBD, nucleotide-binding domain; P, phosphorylation of the RD; Pi, inorganic phosphate; RD, regulatory domain. In and out denote the intra- and extracellular sides of the membrane, respectively. See text for further information. Modified, with permission, from Hwang and Sheppard36.
Using the ATP-driven NBD dimerization model of CFTR channel gating27, 28, Roxo-Rosa et al26 speculated that the exceptionally slow rate of channel opening of F508del-CFTR might be explained by F508del inducing misfolding and/or structural instability of NBD1, which would hamper ATP binding. Moreover, the reduced open time of F508del-CFTR might reflect weakening of the binding energy for stable NBD1:NBD2 dimer formation by the mutation26. In support of this idea, Pissarra et al38 demonstrated that the solubilizing mutations used to promote crystallization of human NBD132 traffic F508del-CFTR to the surface and abrogate, albeit incompletely, the channel's gating defect. Thus, deletion of F508 might cause intrinsic misfolding and/or structural instability of NBD126.
However, two lines of evidence argue against the idea that deletion of F508 causes misfolding of NBD1. First, F508del perturbs the local topography of NBD1, without affecting domain folding32, but see Pissarra et al38. Second, F508 is located at the surface of NBD1, where it might interact with the MSDs31, 32. The residue is remote from the NBD1:NBD2 interface, the location of the ATP-binding sites (Figure 2).
Following the elucidation of the atomic structure of the ABC transporter Sav1866, the multidrug transporter of S aureus39, structural models of the entire CFTR protein have been developed40, 41, 42 and used to understand better CFTR function (eg Alexander et al43). Of note, these structural models have provided important new insight into how the F508del mutation disrupts CFTR channel gating. They also reveal the function of the four intracellular loops (ICLs), which connect transmembrane segments within the MSDs. Each ICL consists of two long α-helical extensions of transmembrane segments with an intervening short α-helix at its most cytoplasmic location orientated parallel to the plane of the membrane. Because this short α-helix interacts with the NBDs, it is termed the coupling helix40, 41.
For two reasons, the positions of the ICLs in structural models of CFTR are notable. First, the ICLs communicate both with the same and the opposite NBD (eg ICL1 (MSD1) with NBD1 and ICL2 (MSD1) with NBD240, 41). Prior to these structural models, communication between the NBDs and MSDs was presumed to be only vertical (eg NBD1:MSD1). However, the structural models of Serohijos et al40 and Mornon et al41 argue that communication between the NBDs and MSDs is both vertical and orthogonal (eg NBD1:MSD1 and NBD1:MSD2). Second, the coupling helix of ICL4 interacts with the surface of NBD1 in the region of F50840, 41. This observation provides an explanation for why the F508del mutation profoundly disrupts CFTR channel gating. The mutation affects a residue located at a critical interface in the CFTR gating pathway, the sequence of conformation changes initiated by ATP-driven NBD dimerization, which leads to opening of the CFTR pore and Cl− flow through the channel. Thus, understanding the interface between the F508 region of NBD1 and the coupling helix of ICL4 is central to the development of drug therapies that target the root cause of CF.
Rational new therapies for CF that target defects in F508del-CFTR
To target the root cause of CF, future therapies should (i) overcome the F508del-CFTR processing defect and traffic the mutant protein to the apical membrane12; (ii) extend the residence time of F508del-CFTR at the apical membrane44 and abrogate channel “rundown” (eg Schultz et al22) and (iii) rescue the defective channel gating of F508del-CFTR14. Thus, small molecules with two or possibly three types of activity are required to restore function to the F508del-CFTR Cl− channel.
Small molecules that overcome the processing defect of F508del-CFTR and traffic the mutant protein to the apical membrane are termed CFTR correctors because they rescue the cell surface expression of F508del-CFTR45, 46. CFTR correctors might interact with CFTR itself, by acting as either substrate mimics or active site inhibitors. Alternatively, they might target one or more of the many CFTR interacting proteins that orchestrate and control processing of CFTR, its delivery to, and expression at the apical membrane. This latter group of CFTR correctors is termed proteostasis regulators because they aim to treat CF by manipulating the concentration, conformation, quaternary structure and/or location of CFTR47.
Small molecules that repair the gating defect of the F508del-CFTR Cl− channel are termed CFTR potentiators because they do not open silent channels, but instead enhance ATP-dependent channel gating following the phosphorylation of F508del-CFTR by PKA45. Although some agents (eg bromotetramisole48) enhance CFTR gating by modulating activity of the protein kinases and phosphatases that control the phosphorylation status of CFTR, CFTR potentiators interact directly with CFTR to enhance channel gating. Interestingly, a small number of compounds have been identified which possess both CFTR corrector and potentiator activity49, 50. These small molecules are termed CFTR corrector-potentiators or dual-acting molecules.
Because there is insufficient information at the present time to design rationally CFTR correctors and potentiators, the strategy of choice to identify drug-like small molecule CFTR modulators is high-throughput screening (HTS)45. HTS exploits a reliable, sensitive, cost-effective assay to screen libraries of chemically diverse small molecules (eg approved drugs51, drug-like chemicals52 and natural products53) to identify lead compounds for medicinal chemistry optimization. For example, Alan Verkman (UCSF, San Francisco, USA) used Fischer rat thyroid cells, epithelial cells devoid of CFTR expression and cAMP-stimulated Cl− currents54 engineered to co-express recombinant human CFTR and a green fluorescent protein (GFP) with ultra high halide sensitivity in a halide flux assay (eg Yang et al52). By contrast, Vertex Pharmaceuticals (San Diego, USA) employed NIH-3T3 cells expressing recombinant human CFTR in a fluorescence resonance energy transfer (FRET)-based membrane voltage-sensing assay (eg Van Goor et al55). Both of these HTS assays monitor the change in CFTR-mediated anion flux elicited by CFTR modulators in real time.
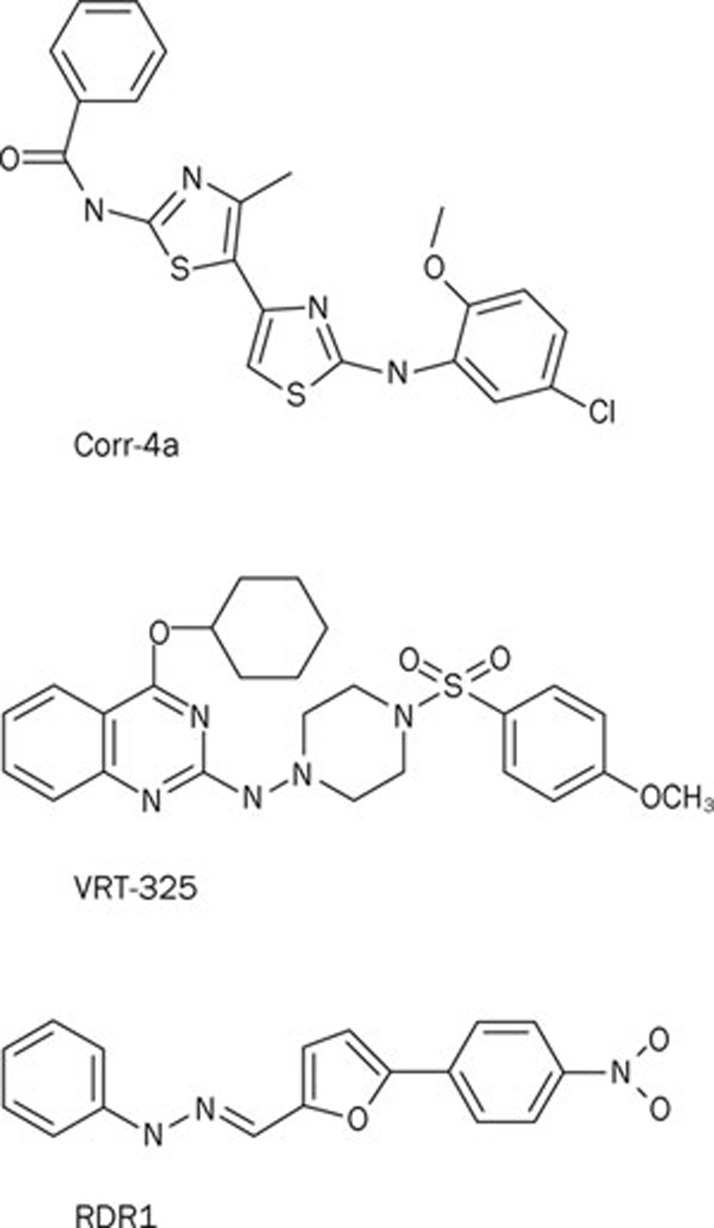
By screening 150 000 drug-like compounds using F508del-CFTR expressing FRT cells, Verkman's group were the first to identify CFTR correctors using HTS56. Among the chemical scaffolds with CFTR corrector activity, the bisaminomethylbithiazole corr-4a (Figure 3) deserves special attention. Pedemonte et al56 demonstrated that corr-4a is equipotent to low temperature correction at restoring function to F508del-CFTR-expressing human bronchial epithelia (CFBE), achieving levels of CFTR function approximately 8% of that of human bronchial epithelia expressing wild-type CFTR (HBE). Of special note, the aminoarylthiazole corr-2b identified by Pedemonte et al56 exhibits dual activity as both a CFTR corrector and a CFTR potentiator50. When compared with small molecules that act as CFTR correctors, corr-2b generated double the amount of forskolin-activated CFTR Cl− current (IFSK) relative to the total CFTR Cl− current measured in the presence of forskolin and the CFTR potentiator genistein (ITOT) (CFTR correctors (eg corr-4a): IFSK/ITOT ∼40%; corr-2b: IFSK/ITOT ∼80%; see Figure 1 of Pedemonte et al50). Moreover, the authors demonstrated that aminoarylthiazoles do not act as typical CFTR potentiators because they require protein synthesis to exert their effects (see below and50). Elucidation of the mechanism of action of corr-2b and related dual-acting small molecules is a priority for future research.
Figure 3.
Chemical structures of some CFTR correctors identified by HTS. Abbreviations: Corr-4a, N-[2-(5-Chloro-2-methoxy-phenylamino)-4′-methyl-[4,5′]bithiazolyl-2′-yl]-benzamide; VRT-325, 4-Cyclohexyloxy-2-{1-[4-(4-methoxy-benzensulfonyl)-piperazin-1-yl]-ethyl}-quinazoline; RDR1, 5-(4-nitrophenyl)-2-furaldehyde 2-phenylhydrazone.
In a ground-breaking program funded by the Cystic Fibrosis Foundation (Bethesda, USA), a nonprofit, donor-supported organization, Vertex Pharmaceuticals identified 13 distinct chemical scaffolds with CFTR corrector activity after screening ∼164 000 chemically diverse drug-like small molecules55. Following medicinal chemistry optimization, Vertex Pharmaceuticals identified the quinazoline VRT-325 (Figure 3) as a potent and efficacious CFTR corrector that enhances the maturation of native F508del-CFTR protein and augments CFTR-mediated transepithelial Cl− secretion in CFBE55. Biochemical studies of VRT-325 suggest that it acts at the endoplasmic reticulum to promote CFTR folding55. Because VRT-325 decreases the apparent ATP affinity of purified, reconstituted F508del-CFTR57, it might rescue the processing and trafficking of F508del-CFTR, at least in part, by interacting directly with the mutant protein.
To identify CFTR correctors that interact directly with F508del-CFTR, Sampson et al58 employed differential scanning fluorimetry, which identifies ligands of a target protein by monitoring their effects on the thermal unfolding of the protein. Among 224 hits identified in a previous HTS for CFTR correctors, just one chemical, the substituted phenylhydrazone RDR1 (Figure 3), was able to thermally stabilize murine F508del-CFTR58. As with previous studies of CFTR correctors by the Hanrahan and Thomas groups59, 60, the authors deployed a battery of biochemical and functional assays to investigate F508del-CFTR rescue by RDR1 in heterologous cells, polarized epithelia and genetically-modified mice. The authors' data demonstrate that RDR1 thermally stabilizes murine F508del-NBD1, increases the maturation of human F508del-CFTR protein and augments the function of human CFTR in vitro and murine CFTR in vivo58. Taken together, these data and the additive effect of RDR1 treatment and low temperature incubation on human F508del-CFTR maturation argue convincingly that RDR1 is a CFTR corrector that targets directly F508del-NBD1 to exert its effects. Identification of the RDR1-binding site on CFTR should be an important goal of future research.
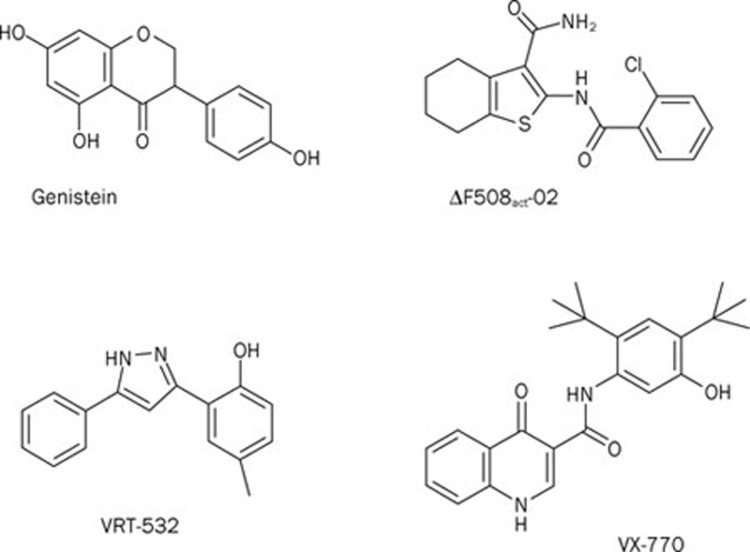
To identify CFTR potentiators that rescue the gating defect of F508del-CFTR, Yang et al52 studied FRT cells expressing low temperature-corrected F508del-CFTR. A screen of 100 000 compounds identified six novel classes of high-affinity F508del-CFTR potentiators52. However, by screening additional structural analogues, Yang et al52 discovered tetrahydrobenzothiophenes (eg ΔF508act-02; Figure 4), which potentiate F508del-CFTR with Kd<100 nmol/L. Subsequently, Pedemonte et al61 screened 50 000 compounds searching for further ligands that rescue the gating defect of F508del-CFTR. After secondary analyses, Pedemonte et al61 identified phenylglycines and sulfonamides that potentiate F508del-CFTR with nanomolar potency. Interestingly, by screening a library of 2000 compounds, including drugs approved for clinical use, Pedemonte et al51 demonstrated that the antihypertensive drugs 1,4-dihydropyridines (DHPs) act as F508del-CFTR potentiators by a mechanism independent of their effects on voltage-gated Ca2+ channels. To identify DHPs that potentiate F508del-CFTR without inhibiting voltage-gated Ca2+ channels, Pedemonte et al62 investigated structure-activity relationships using a panel of 333 analogues of felodipine, the most potent CFTR potentiator identified in their original screen. The authors' data demonstrate that substituents with hydrophobic groups enhance the potency of DHPs as CFTR potentiators62. They also reveal that some DHPs are excellent lead compounds for the development of therapeutically active CFTR potentiators.
Figure 4.
Chemical structures of some CFTR potentiators identified by HTS. Abbreviations: ΔF508act-02, 2-(2-chlorobenzamido)-4,5,6,7-tetrahydro-3H-indene-1-carboxamide; VRT-532, 4-methyl-2-(5-phenyl-1H-pyrazol-3-yl)phenol; VX-770, N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide. For comparison, the chemical structure of genistein is shown.
To identify therapeutically active potentiators of F508del-CFTR, Vertex Pharmaceuticals screened 122000 synthetic compounds from their compound collection using NIH-3T3 cells expressing temperature-corrected F508del-CFTR55. After careful scrutiny, Van Goor et al55 selected for further study 53 compounds consisting of 10 distinct chemical scaffolds. One compound, the pyrazole VRT-532 (Figure 4), rescued the gating defect of F508del-CFTR by accelerating the rate of channel opening and slowing the rate of channel closure55. Critically, VRT-532 augmented robustly CFTR-mediated transepithelial Cl− secretion in CFBE (EC50, 2.7±0.2 μmol/L55). Importantly, the effects of VRT-532 on CFBE were synergistic with the CFTR corrector VRT-325. CFBE incubated with VRT-325 and then treated with cAMP agonists and VRT-532 generated levels of CFTR-mediated transepithelial Cl− secretion in CFBE >20% of those observed in HBE55.
Subsequently, Vertex Pharmaceuticals screened 228 000 chemically diverse drug-like compounds to identify chemical scaffolds for development into therapeutically active CFTR potentiators. Following medicinal chemistry optimization, Vertex Pharmaceuticals identified VX-770 (Figure 4), a potent, selective and orally bioavailable CFTR potentiator63. Interestingly, by increasing the frequency and duration of channel openings, VX-770 (1 μmol/L) restored the channel activity (measured by Po) of F508del-CFTR to wild-type CFTR levels63. Moreover, treatment of CFBE (genotype F508del/G551D) with VX-770 (10 μmol/L) increased airway surface liquid volume and ciliary beat frequency to levels about half those of HBE63.
Based on its performance in preclinical studies, VX-770 became the first CFTR potentiator to be tested in the clinic. The drug was first tested in 39 adult CF patients carrying the CFTR mutation G551D, which has no effect on the processing and trafficking of CFTR, but profoundly disrupts channel gating64, 65. The CF patients in this study took VX-770 orally in a randomized, double-blind, placebo-controlled trial66. VX-770 was well tolerated by CF patients, and at high concentration (150 mg), VX-770 decreased the sweat Cl− concentration to a level approaching the normal range (<60 mmol/L) and improved lung function (measured by forced expiratory volume in one second, FEV1) by 9%66. Further clinical studies of VX-770 are ongoing. Of special note, initial results from the phase III clinical trial of VX-770 on 83 CF patients with the G551D mutation demonstrated a sustained improvement in lung function at 48 weeks with drug-treated CF patients 55% less likely to experience a pulmonary exacerbation (http://investors.vrtx.com/releasedetail.cfm?ReleaseID=551869). Vertex Pharmaceuticals indicate that they plan to apply for US and European drug approval later in 2011 (http://investors.vrtx.com/releasedetail.cfm?ReleaseID=551869).
Knowledge of how ATP gates the CFTR Cl− channel, particularly the ATP-driven NBD dimerization model27, 28, provides explanations for the mechanism(s) of action of CFTR potentiators. Ai et al67 first proposed that genistein and other CFTR potentiators might enhance CFTR channel gating by affecting NBD dimerization. The authors speculated first that the binding of genistein at the interface of the NBD dimer might lower the free energy of the transition state and, hence, accelerate channel opening67. Second, the authors proposed that genistein might slow the rate of channel closure by stabilizing the NBD dimer conformation67. Finally, the authors argued that the binding site for genistein might be located at the dimer interface67. Consistent with this idea, Moran et al68 used a molecular model of the NBD1:NBD2 dimer to show that genistein, apigenin and a series of novel CFTR potentiators identified by HTS bind to CFTR at the dimer interface. As predicted69 and verified70 by functional data, this drug-binding site is distinct from the two ATP-binding sites of CFTR. Moreover, sequences from both NBD1 (Walker A, Walker B and LSGGQ) and NBD2 (LSGGQ) contribute to the drug-binding site, with those from NBD1 forming a cavity in which CFTR potentiators dock68. Following the development of structural models of the entire CFTR protein40, 41, 42, in silico structure-based screening is likely to become a powerful tool to identify small molecules that interact directly with F508del-CFTR and other CF mutants. Of note, using this approach Kalid et al49 identified a ligand-binding site in the vicinity of F508 at the interface of the NBDs and MSDs. Finally, differences in the molecular pharmacology of CFTR homologs from different species (eg human and murine CFTR63, 71, 72) argue that chimeric CFTR proteins may be valuable tools to identify where CFTR potentiators dock with CFTR73.
Conclusions
Two decades after the identification of the defective gene responsible for CF, therapies based on a molecular understanding of the disease are beginning to be tested in the clinic. Early results from these trials are encouraging. They raise the prospect of personalized medicine, whereby specific therapies are designed to target precisely the genetic defects harbored by individuals afflicted by CF. However, the development of efficacious and safe drug therapies for CF patients will require much more work. For example, it is currently unknown how much CFTR function is required to rescue CF mutants, whether drug therapy for CF is mutation specific and if long-term treatment with CFTR correctors and potentiators causes adverse effects. Answers to these pressing questions will play an important role in shaping future therapeutic strategies for CF.
Author contribution
The authors researched the literature and wrote the review.
Acknowledgments
We thank former laboratory colleagues for stimulating discussions. Work in the authors' laboratory is supported by the Cystic Fibrosis Trust and the Engineering and Physical Sciences Research Council [grant no. EP/F03623X/1]. J Liu is supported by scholarships from the University of Bristol and the Overseas Research Students Awards Scheme of Universities UK.
References
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR.Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease New York: McGraw-Hill Inc; 2001. p5121–88. [Google Scholar]
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–82. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–70. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Holland IB, Cole SPC, Kuchler K, Higgins CF. London: Academic Press; 2003. ABC Proteins: from bacteria to man. [Google Scholar]
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–5. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Bear CE, Li CH, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, et al. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–18. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–84. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994;91:4698–702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358–63. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–34. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Drumm ML, Wilkinson DJ, Smit LS, Worrell RT, Strong TV, Frizzell RA, et al. Chloride conductance expressed by ΔF508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–9. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, et al. Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature. 1991;354:526–8. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79 (1 Suppl):S23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Reyes E, Jensen T, Chang X, Rommens JM, et al. The cystic fibrosis mutation (ΔF508) does not influence the chloride channel activity of CFTR. Nat Genet. 1993;3:311–6. doi: 10.1038/ng0493-311. [DOI] [PubMed] [Google Scholar]
- Cai Z, Scott-Ward TS, Sheppard DN. Voltage-dependent gating of the cystic fibrosis transmembrane conductance regulator Cl− channel. J Gen Physiol. 2003;122:605–20. doi: 10.1085/jgp.200308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–4. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- French PJ, van Doorninck JH, Peters RH, Verbeek E, Ameen NA, Marino CR, et al. A ΔF508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. J Clin Invest. 1996;98:1304–12. doi: 10.1172/JCI118917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, et al. ΔF508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am J Physiol. 1996;270:C1544–55. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Wang F, Yang IC, Reenstra WW. Genistein potentiates wild-type and ΔF508-CFTR channel activity. Am J Physiol. 1997;273:C988–98. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]
- Schultz BD, Frizzell RA, Bridges RJ. Rescue of dysfunctional ΔF508-CFTR chloride channel activity by IBMX. J Membr Biol. 1999;170:51–66. doi: 10.1007/s002329900537. [DOI] [PubMed] [Google Scholar]
- Cai Z, Sheppard DN. Phloxine B interacts with the cystic fibrosis transmembrane conductance regulator at multiple sites to modulate channel activity. J Biol Chem. 2002;277:19546–53. doi: 10.1074/jbc.M108023200. [DOI] [PubMed] [Google Scholar]
- Miki H, Zhou Z, Li M, Hwang TC, Bompadre SG. Potentiation of disease-associated cystic fibrosis transmembrane conductance regulator mutants by hydrolyzable ATP analogs. J Biol Chem. 2010;285:19967–75. doi: 10.1074/jbc.M109.092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zeltwanger S, Hu S, Hwang TC. Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels. J Physiol. 2000;524:637–48. doi: 10.1111/j.1469-7793.2000.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxo-Rosa M, Xu Z, Schmidt A, Neto M, Cai Z, Soares CM, et al. Revertant mutants G550E and 4RK rescue cystic fibrosis mutants in the first nucleotide-binding domain of CFTR by different mechanisms. Proc Natl Acad Sci U S A. 2006;103:17891–6. doi: 10.1073/pnas.0608312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Nairn AC, Gadsby DC. On the mechanism of MgATP-dependent gating of CFTR Cl− channels. J Gen Physiol. 2003;121:17–36. doi: 10.1085/jgp.20028673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–80. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher KP, Lee AT, Rees DC. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–8. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–49. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, et al. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–93. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HA, Zhao X, Wang C, Sauder JM, Rooney I, Noland BW, et al. Impact of the ΔF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–53. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J Biol Chem. 2002;277:15419–25. doi: 10.1074/jbc.M111713200. [DOI] [PubMed] [Google Scholar]
- Basso C, Vergani P, Nairn AC, Gadsby DC. Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating. J Gen Physiol. 2003;122:333–48. doi: 10.1085/jgp.200308798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–83. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TC, Sheppard DN. Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol. 2009;587:2151–61. doi: 10.1113/jphysiol.2009.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K, Wang W. A unified view of cystic fibrosis transmembrane conductance regulator (CFTR) gating: combining the allosterism of a ligand-gated channel with the enzymatic activity of an ATP-binding cassette (ABC) transporter. J Biol Chem. 2011;286:12813–9. doi: 10.1074/jbc.R111.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissarra LS, Farinha CM, Xu Z, Schmidt A, Thibodeau PH, Cai Z, et al. Solubilizing mutations used to crystallize one CFTR domain attenuate the trafficking and channel defects caused by the major cystic fibrosis mutation. Chem Biol. 2008;15:62–9. doi: 10.1016/j.chembiol.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature 2006. 14;443:180–5. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- Serohijos AW, Hegedus T, Aleksandrov AA, He L, Cui L, Dokholyan NV, et al. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci U S A. 2008;105:3256–61. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornon JP, Lehn P, Callebaut I. Atomic model of human cystic fibrosis transmembrane conductance regulator: membrane-spanning domains and coupling interfaces. Cell Mol Life Sci. 2008;65:2594–612. doi: 10.1007/s00018-008-8249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornon JP, Lehn P, Callebaut I. Molecular models of the open and closed states of the whole human CFTR protein. Cell Mol Life Sci. 2009;66:3469–86. doi: 10.1007/s00018-009-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Ivetac A, Liu X, Norimatsu Y, Serrano JR, Landstrom A, et al. Cystic fibrosis transmembrane conductance regulator: using differential reactivity toward channel-permeant and channel-impermeant thiol-reactive probes to test a molecular model for the pore. Biochemistry. 2009;48:10078–88. doi: 10.1021/bi901314c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, et al. The ΔF508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–8. [PubMed] [Google Scholar]
- Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–71. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD. Targeting CFTR: How to treat cystic fibrosis by CFTR-repairing therapies. Curr Drug Targets. 2011;12:683–93. doi: 10.2174/138945011795378586. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Becq F, Jensen TJ, Chang XB, Savoia A, Rommens JM, Tsui LC, et al. Phosphatase inhibitors activate normal and defective CFTR chloride channels. Proc Natl Acad Sci U S A. 1994;91:9160–4. doi: 10.1073/pnas.91.19.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalid O, Mense M, Fischman S, Shitrit A, Bihler H, Ben-Zeev E, et al. Small molecule correctors of F508del-CFTR discovered by structure-based virtual screening. J Comput Aided Mol Des. 2010;24:971–91. doi: 10.1007/s10822-010-9390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N, Tomati V, Sondo E, Caci E, Millo E, Armirotti A, et al. Dual activity of aminoarylthiazoles on the trafficking and gating defects of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel caused by cystic fibrosis mutations. J Biol Chem. 2011;286:15215–26. doi: 10.1074/jbc.M110.184267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N, Diena T, Caci E, Nieddu E, Mazzei M, Ravazzolo R, et al. Antihypertensive 1,4-dihydropyridines as correctors of the cystic fibrosis transmembrane conductance regulator channel gating defect caused by cystic fibrosis mutations. Mol Pharmacol. 2005;68:1736–46. doi: 10.1124/mol.105.015149. [DOI] [PubMed] [Google Scholar]
- Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, et al. Nanomolar affinity small molecule correctors of defective ΔF508-CFTR chloride channel gating. J Biol Chem. 2003;278:35079–85. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- Xu LN, Na WL, Liu X, Hou SG, Lin S, Yang H, et al. Identification of natural coumarin compounds that rescue defective ΔF508-CFTR chloride channel gating. Clin Exp Pharmacol Physiol. 2008;35:878–83. doi: 10.1111/j.1440-1681.2008.04943.x. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Carson MR, Ostedgaard LS, Denning GM, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am J Physiol. 1994;266:L405–13. doi: 10.1152/ajplung.1994.266.4.L405. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, et al. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–30. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, et al. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–71. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Chiaw P, Wellhauser L, Huan LJ, Ramjeesingh M, Bear CE. A chemical corrector modifies the channel function of F508del-CFTR. Mol Pharmacol. 2010;78:411–8. doi: 10.1124/mol.110.065862. [DOI] [PubMed] [Google Scholar]
- Sampson HM, Robert R, Liao J, Matthes E, Carlile GW, Hanrahan JW, et al. Identification of a NBD1-binding pharmacological chaperone that corrects the trafficking defect of F508del-CFTR. Chem Biol. 2011;18:231–42. doi: 10.1016/j.chembiol.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Robert R, Carlile GW, Pavel C, Liu N, Anjos SM, Liao J, et al. Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Mol Pharmacol. 2008;73:478–89. doi: 10.1124/mol.107.040725. [DOI] [PubMed] [Google Scholar]
- Robert R, Carlile GW, Liao J, Balghi H, Lesimple P, Liu N, et al. Correction of the ΔF508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol Pharmacol. 2010;77:922–30. doi: 10.1124/mol.109.062679. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, et al. Phenylglycine and sulfonamide correctors of defective ΔF508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Boido D, Moran O, Giampieri M, Mazzei M, Ravazzolo R, et al. Structure-activity relationship of 1,4-dihydropyridines as potentiators of the cystic fibrosis transmembrane conductance regulator chloride channel. Mol Pharmacol. 2007;72:197–207. doi: 10.1124/mol.107.034702. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–30. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Taddei A, Sheppard DN. Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel. J Biol Chem. 2006;281:1970–7. doi: 10.1074/jbc.M510576200. [DOI] [PubMed] [Google Scholar]
- Bompadre SG, Sohma Y, Li M, Hwang TC. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol. 2007;129:285–98. doi: 10.1085/jgp.200609667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai T, Bompadre SG, Wang X, Hu S, Li M, Hwang TC. Capsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride-channel currents. Mol Pharmacol. 2004;65:1415–26. doi: 10.1124/mol.65.6.1415. [DOI] [PubMed] [Google Scholar]
- Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–60. doi: 10.1007/s00018-004-4422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zeltwanger S, Yang IC, Nairn AC, Hwang TC. Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating. Evidence for two binding sites with opposite effects. J Gen Physiol. 1998;111:477–90. doi: 10.1085/jgp.111.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarra-Moran O, Monteverde M, Galietta LJ, Moran O. Functional analysis of mutations in the putative binding site for cystic fibrosis transmembrane conductance regulator potentiators. Interaction between activation and inhibition. J Biol Chem. 2007;282:9098–104. doi: 10.1074/jbc.M611411200. [DOI] [PubMed] [Google Scholar]
- Lansdell KA, Delaney SJ, Lunn DP, Thomson SA, Sheppard DN, Wainwright BJ. Comparison of the gating behaviour of human and murine cystic fibrosis transmembrane conductance regulator Cl− channels expressed in mammalian cells. J Physiol. 1998;508:379–92. doi: 10.1111/j.1469-7793.1998.379bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge H, Wilke M, Bot A, Sheppard DN. Responsiveness of mouse versus human CFTR-ΔF508 to small molecule correctors and potentiators. Pediatr Pulmonol Suppl. 2009;32:291–92. [Google Scholar]
- Scott-Ward TS, Cai Z, Dawson ES, Doherty A, Da Paula AC, Davidson H, et al. Chimeric constructs endow the human CFTR Cl− channel with the gating behavior of murine CFTR. Proc Natl Acad Sci U S A. 2007;104:16365–70. doi: 10.1073/pnas.0701562104. [DOI] [PMC free article] [PubMed] [Google Scholar]