Abstract
BACKGROUND
Immune deficient male mice bearing human prostate cancer xenografts are used to evaluate therapeutic response to novel androgen ablation approaches and the results compared to surgical castration based upon assumption that testosterone microenvironment in intact and castrated adult male mice mimics eugonadal and castrated aging adult human males.
METHODS
To test these assumptions, serum total testosterone (TT) and free testosterone (FT) were determined longitudinally in groups (n > 20) of intact versus castrated adult male nude, NOG, and immune competent C57BL/6 mice.
RESULTS
In adult male mice, TT and FT varies by 30- to 100-fold within the same animal providing a microenvironment that is only equivalent to hypogonadal, not eugonadal, adult human males (TT is 1.7 ± 1.2 ng/ml [5.8 ± 4.1 nM] in nude and 2.5 ± 1.3 ng/ml [8.7 ± 4.4 nM] in NOG mice versus >4.2 ng/ml [14.7 nM] in eugonadal humans). This was confirmed based upon enhanced growth of androgen dependent human prostate cancer xenografts inoculated into mice supplemented with exogenous testosterone to elevate and chronically maintain serum TT at a level (5 ng/ml [18 nM]) equivalent to a 50-year-old eugonadal human male. In castrated mice, TT and FT range from 2 to 20 pg/ml (7–70 pM) and <0.8 pg/ml (<2.6 pM), respectively, which is equivalent to castrate resistant prostate cancer (CRPC) patients treated with abiraterone. This was confirmed based upon the inability of another CYP17A1 inhibitor, ketoconazole, to inhibit the growth of CRPC xenografts in castrated mice.
CONCLUSIONS
Adult male mice supplemented with testosterone mimic eugonadal human males, while unsupplemented animals mimic standard androgen ablation and castrated animals mimic abiraterone treated patients. These studies confirm what is claimed in Robert Burns’ poem “To a Mouse” that “The best laid schemes of mice and men/often go awry.
Keywords: serum total testosterone, free testosterone, eugonadal levels, hypogonadal levels, mice versus humans
INTRODUCTION
Human prostate cancers growing as xenografts in immune deficient male mice are a well-established preclinical model system. Such xenografts are often used to evaluate the therapeutic response of the cancers to novel androgen ablation target compounds. To do this, intact male adult immune deficient mice bearing human prostate cancer xenografts are treated with such test compounds, and the tumor growth inhibitory response compared to that produced by surgical castration. The validation for such efficacy assays assumes that the testosterone microenvironment in intact and castrated adult male mice mimics those of eugonadal and castrated aging adult human males, respectively. This raises the issue of whether this assumption is warranted.
This point is especially relevant because it has been well established for more than 40 years that the circulating blood level of total testosterone (TT) varies by more than 150-fold from less than 0.3 ng/ml (1 nM) to >45 ng/ml (155 nM) in intact adult males of both outbred and inbred immune competent as well as immune deficient (e.g., nude) strains of mice with an overall mean TT of less than 2 ng/ml (7 nM) with a SEM of 1–2 ng/ml (3–7 nM) [1–7]. This extreme variation in serum TT occurs even if all of the animals are cross-sectionally sampled at the same time of day, or if the same animal is longitudinally sampled at multiple times throughout the day [1,2]. These observations are not a methodological artifact, because these extreme daily variations in serum TT are detectable using either radioimmunoassay or ELISA [1–7] or liquid chromatography-electrospray ionization-tandem mass spectrometry [7].
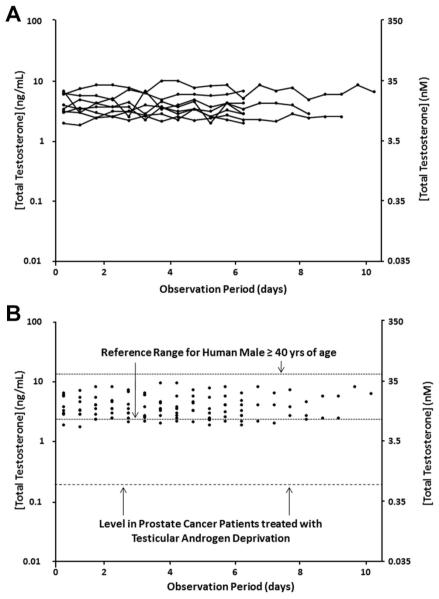
While serum TT decreases in an age-dependent manner in adult human males, the reference range among cross-sectional groups of eugonadal adult males varies from age 40 to 80 by less than sixfold (i.e., 1.6 ng/ml [5.4 nM]–9.22 ng/ml [32 nM]) with the mean value decreasing between the 5th and 8th decade of life by less than 25% (i.e., 5.4 ng/ml [18.7 nM]) vs. 4.2 ng/ml [14.7 nM]) [8] (Table I). Not only is the age-dependent inter-individual (i.e., cross-sectional) variation in serum TT much smaller in humans compared to mice, but also the circadian intra-individual (i.e., longitudinal) variation of serum TT is much smaller as well [9]. For example, Diver [10] evaluated the daily variation of serum TT longitudinally in normal men aged 25–60 followed over an 8- to 10-day observation period (Fig. 1). For this group of men, the mean TT is 4.5 ± 0.5 ng/ml (15.5 ± 1.7 nM) with a range of 1.9–10 ng/ml (6.6–35 nM). These combined cross-sectional and longitudinal data [8–10] document that eugonadal aging human males not only have a much smaller inter- and intra-individual variation than mice (i.e., 6-fold for humans compared to >130-fold for mice), but also a >2-fold higher serum TT concentration. This latter point is significant because aging human males are defined as “hypogonadal” if their serum TT is lower than 2.8 ng/ml (10 nM) [11] (Table I). These results raise the issue of whether intact adult male mice are “hypogonadal” relative to human males.
TABLEI.
Comparison of Serum Total and Free Testosterone Levels in Various Androgen States of Aging (>40 Years Old) Adult Human Males Versus Immune Deficient Adult (>120 Days Old) Male Mice
| Host type | Total testosterone | Free testosterone |
|---|---|---|
| Human | ||
| Eugonadal adult (>40 years old) human male | ||
| Range | 1.6–9.2 ng/ml (5.5–32 nM) | 52–263 pg/ml (183–912 pM) |
| Range of means from 40 to 80 years (8) | 5.4–4.2 ng/ml (19–15 nM) | 143–73 pg/ml (496–262 pM) |
| Hypogonadal adult (>40 years old) human male | ||
| Reference value (11) | <2.8 ng/ml (10 nM) | <74 pg/ml (<225 pM) |
| Castrated adult (>40 years old) human male | ||
| Reference value (23) | <200 pg/ml (700 pM) | <4 pg/ml (<14 pM) |
| Abiraterone treated castrated adult (>40 years old) human male | ||
| Reference value (25) | 3 pg/ml (10 pM) | <0.05 pg/ml (0.17 pM) |
| Mouse | ||
| Intact adult immune deficient male mice supplemented with exogenous testosterone | ||
| Reference value | 5.3 ± 0.5 ng/ml (18.3 ± 1.7 nM) | 145 ± 20 pg/ml (500 ± 69 pM) |
| Intact adult immune deficient nude male mice | ||
| Range | 0.1–45 ng/ml (0.3–138 nM) | 6–220 pg/ml (21–761 pM) |
| Mean | 1.7 ± 1.2 ng/ml [5.8 ± 4.1 nM] | 65 ± 28 pg/ml [224 ± 97 pM] |
| Median | 0.25 ng/ml (0.88 nM) | 10 pg/ml (35 pM) |
| Intact adult immune deficient NOG male mice | ||
| Range | 0.3–45 ng/ml (1–138 nM) | 5–210 pg/ml (42–727 pM) |
| Mean | 2.5 ± 1.3 ng/ml (8.7 ± 4.4 nM) | 78 ± 19 pg/ml (270 ± 65 pM) |
| Median | 0.43 ng/ml (1.48 nM) | 22 pg/ml (76 pM) |
| Castrated adult immune deficient male mouse | ||
| Range | 2–20 pg/ml (7–70 pM) | <0.8 pg/ml (>2.6 pM) |
Fig. 1.
Variation in serum total testosterone (expressed as ng/ml on left-axis and as nM on right-axis) followed longitudinally over a 10-day observation period for eight individual men ages 25–60. A: Each line is an individual followed longitudinally. B: Same data presented cross-sectionally and compared to the 95% reference range for human males age 40–80 derived from Mohr et al. [8] and for prostate cancer patients treated with testicular androgen deprivation derived from Morote et al. [23]. Data re-plotted from original publication of Diver [10].
In human males, approximately 50% of TT is loosely bound (i.e., Kd = 0.4 mM [12]) to serum albumin (SA), 44% is tightly bound (i.e., Kd = 0.6 nM [12]) to sex hormone-binding globulin (SHBG), 4% is bound to other proteins, and only 2% is non-protein bound, which is denoted as free T [FT] [10]. Only FT is able to diffuse from the circulation into the extracellular fluid of tissues; therefore, it is the level of FT that determines intracellular concentrations of T and thus, the biological dose–response [13]. Interestingly, while all mammals contain the SHBG gene, rats and mice do not express and secrete SHBG by their adult liver cells [14].Thus, there is no circulating level of SHBG in adult male mice [14]. Because SHBG tightly binds nearly half of the circulating TT preventing its diffusion into tissue in humans and because mice do not have SHBG in their serum, this raises the possibility that even though mice express less than half the level of serum TT, the biologically active serum FT might be similar in intact adult male mice and humans. If this is true, then even though the serum TT is lower in intact adult male mice, the FT level might be equivalent to eugonadal aging adult human males when used as xenograft hosts for human prostate cancers. Therefore, TT and FT in intact adult male mice were determined and compared to the values in eugonadal adult human males.
MATERIALS AND METHODS
Animals
Adult intact male immune competent C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Adult intact male T-cell immune deficient nude mice were obtained from Taconic Farms (Germantown, NY). Adult intact male T-, B-, and NK-cell immune deficient NOD/SCID/gamma-null (i.e., NOG) mice [15] were obtained from an in-house colony at Hopkins. Using a Johns Hopkins Animal Care and Use Committee approved protocol, blood was harvested between 9 and 10 AM from intact or castrated adult (i.e., >120 day old) nude and NOG male mice. The blood was allowed to clot, and the resulting serum used for testosterone measurements. Where indicated, castrated adult male nude male mice were given ketoconazole 5 days a week via oral gavage at a dose of 25 mg/kg. Ketoconazole was obtained from LKT Laboratories, Inc. (St. Paul, MN).
ExogenousTestosterone Supplementation
Where indicated, mice were implanted subcutaneously (SC) in the flank with two 1 cm long capsules made from silastic tubing (cat# EW96115-16 from Cole-Parmer, Vernon Hills, IL) with an inner diameter of 0.078 in. and outer diameter of 0.125 in., which was filled with testosterone (Steraloids, Newport, RI) and sealed on both ends with silastic sealer (PolyOne, Ayer, MA) as described previously [16].
Xenograft Studies
The history and characteristics of the human prostate cancer xenograft lines used (i.e., PC-82, LNCaP, LNCaP/A, LAPC-4, MDA-PCA-2b, VCaP, CWR-22RH) have been published previously [17]. Using a Johns Hopkins Animal Care and Use Committee approved protocol, approximately 120 days old intact male immune deficient nude or NOG mice where indicated, were inoculated SC in the flank with human prostate cancer cells in 200 μl of Matrigel (BD Biosciences, San Jose, CA) as described previously [16]. Tumor volumes were determined using microcaliper measurements as described previously [16].
Testosterone Measurements
For the unsupplemented and testosterone-supplemented intact animals, the total serum testosterone (TT) [i.e., protein bound plus non-protein bound testosterone (i.e., the latter being free T)] was determined using either a direct testosterone-ELISA kit (cat# BC-1115), which does not require serum extraction, purchased from BioCheck, Inc. (Foster City, CA). For the castrated animals, serum TT was determined using the testosterone high sensitivity-ELISA kit (cat# ADI-900-176) from Enzo Life Sciences (Farmingdale, NY), which involves assaying di-ethyl ether (5:1) extracted serum. To determine the non-protein bound (i.e., free T [FT]), serum was centrifuged for 5 min at 800g at 37°C in an Amicon Ultra-4 centrifugal filter unit from Millipore (cat# 801024) with a cut-off of 10 kDa to produce a protein-free ultra-filtrate containing FT. This ultra-filtrate was extracted (5:1) with diethyl ether, and the organic extract taken to dryness. The residue was resuspended in aqueous buffer and then assayed to determine FT using the testosterone high sensitivity-ELISA kit (cat# ADI-900–176) from Enzo Life Sciences.
Where indicated, FT was calculated using the calculation tool provide by the website of the International Society for the Study of the Ageing Male (www.ISSAM.ch) based upon the Sodergard equation assuming a fixed SA and SHBG concentration of 46.5 g/L and 30.3 nM/L, respectively, based upon reference values reported by Ho et al. [18].
PSA Determinations
Levels of PSA in mouse plasma were determined by the Clinical Chemistry laboratory at Johns Hopkins using the Hybritech assays on the Beckman Access Immunoassay System (Beckman Coulter, Inc., Brea, CA).
Statistics
All of the values reported are presented as means ± SEM. Statistical analysis was conducted by a 1-way ANOVA with the Newman–Keuls test for multiple comparisons. P < 0.05 was defined as statistically significant.
RESULTS
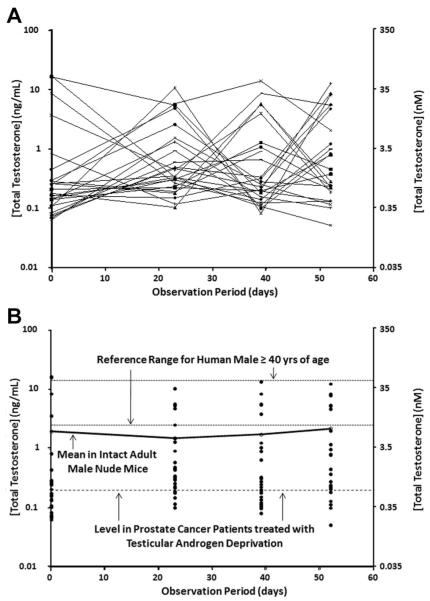
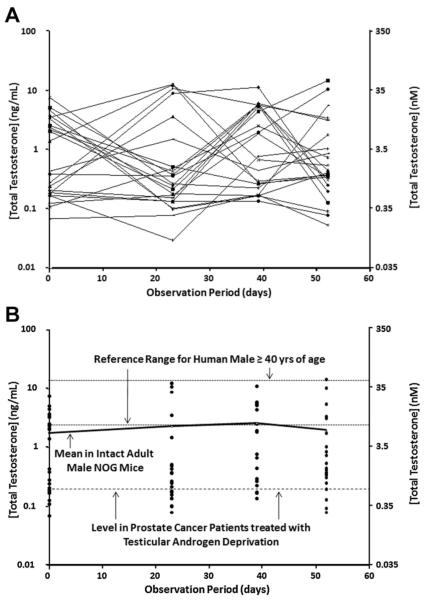
Groups of immune deficient, intact and adult (i.e., approximately 120 day old) male nude (n = 23) or NOG (n = 21) mice had their blood drawn between 9 and 10 AM on four separate occasions over an 8-week period and the serum TT determined. These results documented that for both types of immune deficient intact adult male mice, there is a >100-fold variation in TT even if measured in the same mouse longitudinally over the 8-week period (Figs. 2A and 3A). Only approximately a quarter of these intact adult nude or NOG male mice had TT values that reached the 95% reference range for eugonadal 40- to 80-year-old human males at any time during the 8-week observation period, while a quarter of the mice had TT values at or below the reference level in prostate cancer patients treated with testicular androgen deprivation (Figs. 2B and 3B). The fact that serum TT is both much more variable and much lower in intact adult male mice (i.e., mean ± SEM and median for nude vs. NOG mice are 1.7 ± 1.2 ng/ml [5.8 ± 4.1 nM] and 0.25 ng/ml [0.88 nM] vs. 2.5 ± 1.3 ng/ml [8.7 ± 4.4 nM] and 0.43 ng/ml [1.48 nM], respectively, Table I) than in eugonadal adult human males (i.e., >4.2 ng/ml [14.7 nM]) is not because these are immune deficient mice. A similar variable and low serum TT is observed in a group of 120-day-old immune competent C57BL/6 intact male mice in which two-thirds have TT values below the reference ranges for eugonadal human males and nearly half are below the reference level in prostate cancer patients treated with testicular androgen deprivation (Fig. 4).
Fig. 2.
Variation in serum total testosterone (expressed as ng/ml on left-axis and as nM on right-axis) over a 54-day observation period for 23 immune deficient intact adult male nude mice. A: Each line is an individual nude mouse followed longitudinally. B: Same nude mouse data presented cross-sectionally and compared to the 95% reference range for human males age 40–80 derived from Mohr et al. [8] and for prostate cancer patients treated with testicular androgen deprivation derived from Morote et al. [23]. Solid line is the mean for the group at the indicated time point.
Fig. 3.
Variation in serum total testosterone (expressed as ng/ml on left-axis and as nM on right-axis) over a 54-day observation period for 21 immune deficient intact adult male NOG mice. A: Each line is an individual NOG mouse followed longitudinally. B: Same NOG mouse data presented cross-sectionally and compared to the 95% reference range for human males age 40–80 derived from Mohr et al. [8] and for prostate cancer patients treated with testicular androgen deprivation derived from Morote et al. [23]. Solid line is the mean for the group at the indicated time point.
Fig. 4.
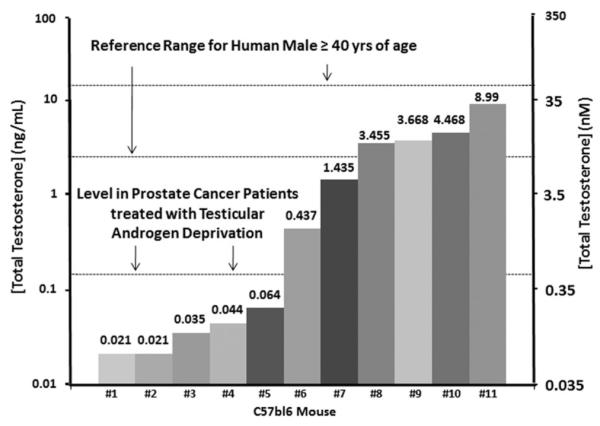
Variation in serum total testosterone (expressed as ng/ml on left-axis and as nM on right-axis) in immune competition intact adult male C57BL/6 mice compared to the 95% reference range for human males age 40–80 derived from Mohr et al. [8] and for prostate cancer patients treated with testicular androgen deprivation derived from Morote et al. [23].
To resolve whether the low serum TT in intact adult male mice results in a correspondingly low level of biologically active testosterone, the mean free testosterone (FT) ± SEM in a group of intact adult male nude mice was determined. The results (i.e., 65 ± 28 pg/ml [225 ± 97 pM] for nude and 78 ± 19 pg/ml [270 ± 65 pM] for NOG mice, Table I) document that 70% of these mice had FT values that are below the International Society for the Study of the Aging Male (ISSAM) value of 74 pg/ml (255 pM) used as criterion for the diagnosis of hypogonadism in human males [11] (Fig. 5). This criterion for determining hypogonadism in aging human males has recently been confirmed [19] (Table I). Based upon these combined serum results, the testosterone microenvironment in intact adult male mice is equivalent to hypogonadal, not eugonadal, aging human males.
Fig. 5.
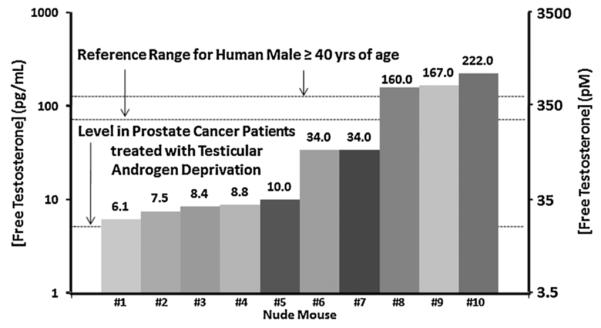
Variation in serum free testosterone (expressed as pg/ml on left-axis and as pM on right-axis) in ten immune deficient intact adult male nude mice compared to the 95% reference range for FT in 40- to 80-year-old human males derived from Mohr et al. [8] and for prostate cancer patients treated with testicular androgen deprivation derived from Morote et al. [23]. Values above the bar are the FTvalue in pg/ml.
Functionally to confirm this conclusion, the in vivo growth of the androgen-dependent PC-82 human prostate cancer xenograft line was compared when inoculated into either intact adult male nude mice versus animals supplemented with exogenous testosterone to elevate and chronically maintain TT at a serum level comparable to that in a eugonadal 50-year-old adult human male (i.e., approximately 5 ng/ml [18 pM]) [9]. To do this, intact adult male nude mice were implanted in the flank with silastic capsules filled with testosterone. Groups of intact adult nude male mice (n = 10) were implanted with two 1 cm-sized capsules and after 1 week, serum was analyzed for TT. These results documented that such testosterone implants elevate and maintain serum TT from ~1.5 ng/ml (5 nM) in the unsupplemented animals, which is equivalent to a hypogonadal adult human male, to 5.3 ± 0.5 ng/ml (18.3 ± 1.73 nM) in implanted animals, which is equivalent to a eugonadal adult human male. Raising serum TT to that equivalent of an intact adult eugonadal human male significantly enhanced (P < 0.05) the wet weight of the prostate complex (i.e., ventral + dorsal + lateral lobes) by nearly 50%, going from 84 ± 15 mg in the unsupplemented mice versus 122 ± 11 mg in the exogenously supplemented animals. In addition, raising the serum TT to that of an intact adult eugonadal human male significantly enhanced (P < 0.05) PC-82 cancer growth (Fig. 6).
Fig. 6.
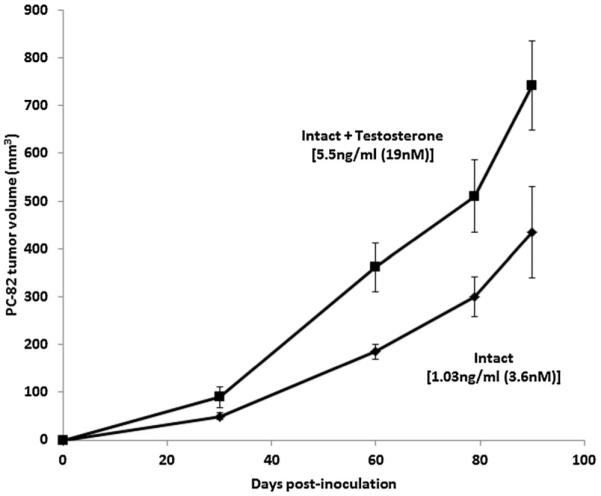
Growth response of androgen-dependent PC-82 human prostate cancer xenografts inoculated into immune deficient intact adult male nude mice (n = 10 per group), which were either un treated or supplemented with testosterone implants. Values are the mean ± SEM of the serum TT for the group. PC-82 was originally derived from a primary cancer from a hormonally năve patient with localized disease.
These results confirm that serum TT in intact adult male mice is equivalent to a hypogonadal adult human male. These results are important because they provide an explanation to a consistently observed paradox about the androgen responsiveness for the in vivo growth of human prostate cancer cells when xenografted into immune deficient mice. Nearly all of the presently available in vitro androgen receptor (AR)-expressing human prostate cancer cell lines (e.g., LNCaP, LAPC-4, MDA-PCA-2B, VCaP, etc.) were derived from castration resistant prostate cancer (CRPC) patients and yet, their in vivo xenograft growth is much faster in intact versus castrated immune deficient adult male mice (Fig. 7).
Fig. 7.
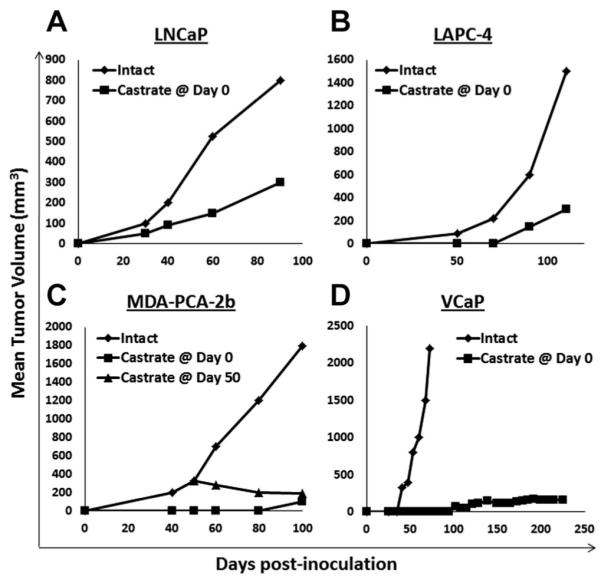
Growth response of human prostate cancer cell lines derived from castrate resistant prostate cancer patients when xenografted into immune deficient intact (n = 10) versus castrated (n = 10) adult male mice. A: LNCaP, (B) LAPC-4, and (C) MDA-PCA-2b were inoculated into nude mice and (D) VCaP was inoculated in NOG mice.
This paradoxical response is consistent with two facts. First, standard tissue culture media supplemented with 10% fetal bovine serum (FBS) used for the propagation of these human cells in vitro only provides a level of testosterone present in castrated human males (i.e., [50–100 pM]) [20]. Second, prostate cancer cells adaptively increase their AR protein expression to optimize their growth when systemic androgen is decreased (i.e., AR protein expression is enhanced 30-fold for LNCaP, 56-fold for LAPC-4, 27-fold for MDA-PCA-2b, and 90-fold for VCaP when propagated in standard media containing a castrate level of testosterone provided by 10% FBS [17]). Thus, when prostate cancer cells are harvested from CRPC patients, these cells have already auto-upregulated their AR protein to a level that allows them to survive and proliferate when propagated in vitro in media containing a castrate level of testosterone provided by 10% FBS. Thus, when such in vitro propagated cells are xenografted into immune deficient intact adult males, the cancer cells optimally grow because this hypogonadal in vivo environment replicates the low androgen microenvironment present in the original CRPC patient.
In contrast to the wide variability in intact immune deficient mice, serum TT in castrated nude or NOG mice is consistently low, ranging from 2 to 20 pg/ml (7–70 pM) (Table I). This is consistent with the lack of significant production and secretion of adrenal androgens by mice due to low adrenal expression of cytochrome P450 17A1 (CYP17A1) [21,22]. Importantly, the TT in these castrated mice is >10-fold lower than the reference value of 200 pg/ml (700 pM) for clinically “castrated” human males [23] (Table I). This reference value is validated by the fact that the median serum TT in a group of patients with advanced prostate cancer who underwent bilateral orchiectomy was 150 pg/ml (519 pM) (95% CI between 120 and 170 pg/ml [415–588 pM]) with minimum and maximum values of 100 and 300 pg/ml (346–1,038 pM), respectively [24]. Using a reference value for TT of <200 pg/ml, the FT for “castrated” human males is calculated to be <3.6 pg/ml (<12.5 pM) (Table I). Based upon the lowest level of assay sensitivity, the serum FT of castrated immune deficient nude and NOG adult male mice is nearly fourfold lower at <0.8 pg/ml (2.6 pM) (Table I), than in humans. When castrate resistant prostate cancer (CRPC) patients are treated with abiraterone to suppress CYP17A1, serum TT decreases from 70–200 pg/ml (242–700 pM) to 3 pg/ml (10 pM) [25]. Using a reference value for TT of 3 pg/ml, the FT for such abiraterone treated CRPC patients was calculated to be <0.05 pg/ml (<0.17 pM) (Table I).
These results document that immune deficient castrated adult male mice are equivalent to an abiraterone treated CRPC patients. Functionally to confirm this conclusion, castrated male nude mice were xenografted with the CWR-22RH human prostate cancer and the animals randomized into groups (n = 10) which were untreated as controls or given daily oral treatment with ketoconazole at a daily dose which inhibits Cyp-17A1 (i.e., 25 mg/kg/day) [26]. The CWR-22RH xenograft is castrate resistant as documented by its equal growth where inoculated into intact versus castrated adult male nude mice [17]. While the growth of the CWR-22RH is castrate resistant, serum PSA is nearly twofold higher (<0.05) in intact versus castrated hosts (i.e., 456 ± 109 vs. 225 ± 58 ng/ml/g tumor, respectively). These results document that AR is still actively signaling in these CRPCs even when grown in castrated hosts. When such castrated animals are simultaneously treated with ketoconazole, there is no inhibitory effect upon the in vivo growth of the CWR-22RH cancers (Fig. 8). These results confirm that castrated adult male mice are equivalent to abiraterone treated CRPC patients.
Fig. 8.
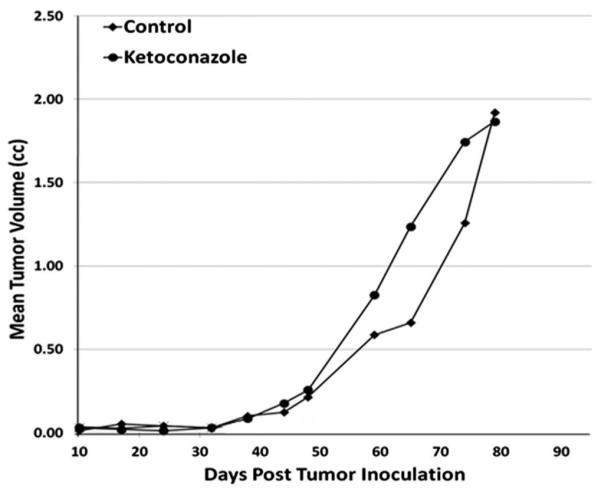
Response of castrate resistant human CWR-22RH xenograft inoculated into castrated adult male nude mice to the CYP17A1 inhibitor, ketoconazole (N = 10 per group). Ketoconazole was given 5 days per week via oral gavage at a dose of 25 mg/kg.
DISCUSSION
During mammalian evolution, breeding became episodically timed for optimal survival of both parents and offspring [27]. Thus, the male accessory sex glands, as well as sexual libido and sperm maturation, are atrophic during the seasonal period when breeding is not optimal [28]. When environmental conditions (i.e., food/temperature/light, etc.) are appropriate, male mammals come into “breeding season.” Such seasonal breeding has great reproductive advantage and was selected during evolution to restrict the energy requirements for maintaining male accessory sex glands, sperm maturation, and sexual libido; thus, limiting the dangers associated with the maniacal focus upon procreation at the expense of self-survival (i.e., suppression of normal flight instinct in the presence of predators) to the minimal time frame of the breeding season. Since breeding season for many mammals is short, evolutionary pressure drove development of a neuroendocrine (i.e., pineal gland–hypothalamic–pituitary) axis to restrict high production and secretion of testosterone into the circulation by the testes to only the breeding season [28].
Co-incident with the evolution of seasonal breeding, there was also selective pressure to develop a mechanism for the rapid rise of testosterone to rapidly induce sexual libido, sperm maturation and full growth of the atrophic male accessory sex tissues needed for reproduction during the short breeding season [27,28]. Thus, during evolutionary selection of testosterone as the master regulator of seasonal male reproduction, there was co-selection of enzymes needed to regulate intracellular ligand and the AR needed to generate intracellular signaling induced by its ligand binding [29]. In contrast with the other mammals, human males eventually evolved away from strictly seasonal breeding and acquired the ability to sustain serum testosterone at a sufficiently high chronic level to maintain the male accessory sex glands, spermatogenesis, and libido in a fully developed adult state so that fertility is constant. Acquiring such constant fertility is a definitive advantage for the highly mobile human species. This is because it enables reproduction to occur despite environmental restrictions allowing man to populate all of the biological niches throughout the world as opposed to “locking” the species into specific indigenous breeding ranges, like other mammals. Apparently, the price of such reproductive “freedom,” however, is the acquisition of BPH and prostate cancer by the human male.
In a eugonadal aging human male, serum TT varies longitudinally by <10-fold (Fig. 1) resulting in a serum FT of >500 pM (Table I). Within the prostate, testosterone is metabolized, particularly via 5-α reductase conversion to dihydrotestosterone (DHT). The concentrations of T and DHT in non-malignant human prostate tissue from eugonadal men are reported to be 0.04 ng/ml (0.14 nM) and 1.92 ng/ml (6.6 nM) (95% CI: 1.63–2.21 ng/ml [5.6–7.6 nM], respectively) [30]. Thus, a serum FT of 500 pM is sufficiently high to maintain a concentration gradient to drive diffusion of FT from the blood into prostate tissue. The equilibrium dissociation constant (Kd) for T and DHT binding to the human AR is 300 pM [31]. This means that at steady-state, the combined concentration of cellular T and DHT (i.e., >6 nM) is 20-fold higher than the binding Kd for AR. Based upon mass action kinetics, this means that there is sufficient androgen for 100% occupancy (i.e., saturation) of nuclear AR in prostate epithelial cells in a eugonadal adult male. This is consistent with the clinical observation that if such human males are given exogenous T, which substantially increases the blood level of T and thus FT, there is no increase in the level of serum prostate-specific antigen (i.e., a marker of androgen action) [32].
Unlike the situation in eugonadal aging adult human males where TT and FT fluctuate modestly (<6-fold), in adult male immune deficient as well as immune competent male mice, serum TT and FT are not only >2-fold lower, but vary by >150-fold for TT and >35-fold for FT (Figs. 2–4). These results are consistent with multiple major differences between humans and mice. The first difference is that mice do not express and secrete SHBG in their liver cells, and thus there is no serum level of SHBG [14]. Thus, there are no high affinity T binding proteins in mouse serum, and therefore, no pool of tightly bound T to buffer rapid changes in TT. The second is that mice do not make adrenal androgen precursors [21]. The third difference is that mice are seasonal breeders driven by seasonal changes in the hypothalamic–pituitary–testes axis, which results in much higher serum TT in breeding versus non-breeding males [33,34]. Due to these differences in the intact adult male mouse, FT does not fully saturate the prostatic AR (i.e., Kd for rodent AR is 400 pM ([35] while FT is <300 pM, Table I; therefore, AR is only <25% occupied, not saturated). This is documented in the present study by the fact that if immune deficient adult male mice are supplemented with exogenous T to raise their serum FT from <300 to >500 pM, so that now >65% of the nuclear AR is occupied, prostates increase by nearly 50% within 1 month of such treatment.
In localized prostate cancer from hormonally naïve patients, the concentrations of T and DHT have been reported to be 0.23 ng/ml (0.8 nM) (95% CI: 0.03–0.44 ng/ml [0.1–1.5 nM]) and 2.75 ng/ml (9.5 nM) (95% CI: 2.45–3.04 ng/ml [8–10 nM]), respectively [30]. This means that under steady-state conditions in a eugonadal patient, the concentration of T within prostate cancer is slightly higher than the ~500 pM serum concentration of FT. This is consistent with intracrine production of T within these localized prostate cancers from hormonally naïve patients as reported previously [30].This also means that based upon mass action kinetics, the steady-state combined concentration of cellular T and DHT (i.e., >10 nM) is >30-fold higher than the AR’s Kd, which is sufficient for 100% occupancy (i.e., saturation) of nuclear AR in prostate cancer cells in a eugonadal adult male. In contrast, when such human prostate cancer cells are xenografted into unsupplemented intact adult immune deficient male mice, the <250 pM serum FT does not produce a sufficient cellular T concentration within the cancer cells to fully saturate their nuclear AR as document by their sub-optimal growth (Fig. 7). These results document that unlike the situation in aging human males, there are insufficient adrenal precursors in the circulation of intact mice to allow the cancer cell’s intracrine metabolism to produce enough ligand for full occupancy of their nuclear AR. These results indicate that primary prostate cancer specimens from such hormonally naive patients should be xenografted into mice supplemented with T to achieve optimal tumor take and subsequent growth.
In conclusion, comparative serum total and FT levels for various androgen states in aging adult (>40 years old) human males versus immune deficient adult (>120 days old) male mice are summarized in Table I. These serum results when combined with the functional tumor growth assays document that when used as hosts for human prostate cancer xenografts, unsupplemented immune deficient intact adult male mice provide a circulating testosterone environment that is only equivalent to hypogonadal human males, while immune deficient castrated adult male mice are equivalent to an abiraterone treated CRPC patient. A corollary to these results is that if one wishes to mimic the testosterone environment of an intact aging adult human male, immune deficient intact male mice must be supplemented with exogenous testosterone to raise and maintain serum TT at approximately 5 ng/ml (18 pM).
These results illuminate the significant limitations of current assays utilizing human prostate cancer xenografts in immune deficient mice as the sole model system for the development of new hormone ablation therapies.
ACKNOWLEDGMENTS
We would like to thank Dr. Cindy Zahnow, Sherrie Hawkes, and Elen Romero of the immuno-deficient mouse colony of the Animal Resources Core of the Sidney Kimmel Comprehensive Cancer Center which is supported by an NIH-NCI Comprehensive Cancer Center Support Grant (P30 CA006973). These studies were supported by NIH-Prostate SPORE Grant (P50 CA058236). JP Michiel Sedelaar was supported by a research grant from the Dutch Cancer Foundation.
Footnotes
The present address of J.P. Michiel Sedelaar is Department of Urology, Radboud University, Nijmegen Medical Center, Nijmegen, The Netherlands
REFERENCES
- 1.Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92:1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- 2.Bartke A, Dalterio S. Evidence for episodic secretion of testosterone in laboratory mice. Steroids. 1975;26:749–756. doi: 10.1016/0039-128x(75)90107-5. [DOI] [PubMed] [Google Scholar]
- 3.van Steenbrugge GJ, Groen M, de Jong FH, Schroeder FH. The use of steroid-containing silastic implants in male nude mice: Plasma hormone levels and the effect of implantation on the weights of ventral prostate and seminal vesicles. Prostate. 1984;5:639–647. doi: 10.1002/pros.2990050610. [DOI] [PubMed] [Google Scholar]
- 4.Redding TW, Schally AV. Inhibition of the pituitary-gonadal axis in nude mice by continuous administration of LHRH agonists and antagonists. J Endocrinol. 1990;126:309–315. doi: 10.1677/joe.0.1260309. [DOI] [PubMed] [Google Scholar]
- 5.Jeyaraj DA, Grossman Petrusz P. Altered bioavailability of testosterone in androgen-binding protein-transgenic mice. Steroids. 2005;70:704–714. doi: 10.1016/j.steroids.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Mukai M, Dong Q, Hardy MP, Kiyokawa H, Peterson RE, Cooke PS. Altered prostatic epithelial proliferation and apoptosis, prostate development, and serum testosterone in mice lacking cyclin-dependent kinase inhibitors. Biol Reprod. 2005;73:951–958. doi: 10.1095/biolreprod.105.040980. [DOI] [PubMed] [Google Scholar]
- 7.Weng Y, Xie F, Xu L, Zagorevski D, Spink DC, Ding X. Analysis of testosterone and dihydrotestosterone in mice tissues by liquid chromatography-electrospray ionization-tandem mass spec-trometry. Anal Biochem. 2010;402:121–128. doi: 10.1016/j.ab.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohr BA, Guay AT, O’Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: Results from the Massachusetts Male Aging Study. Clin Endocrinol. 2005;62:64–73. doi: 10.1111/j.1365-2265.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- 9.Plymate SR, Tenover JS, Bremmer WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated nonsex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 10.Diver MJ. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem. 2006;43:3–12. doi: 10.1258/000456306775141803. [DOI] [PubMed] [Google Scholar]
- 11.Morales A, Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. Aging Male. 202(5):74–86. [PubMed] [Google Scholar]
- 12.Sodergrad R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 13.Mendel CM. the free hormone hypothesis: A physiological based mathematical mode. Endrocrin Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 14.Janne M, Deol HK, Power GA, Yee SP, Hammond GL. Human sex hormone-binding globulin in transgenic mice. Mol Endocrinol. 1998;12:123–136. doi: 10.1210/mend.12.1.0050. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 17.Isaacs JT, D’Antonio JM, Chen S, Antony L, Dalrymple SP, Ndikuyeze GH, Luo J, Denmeade SR. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72:1491–1505. doi: 10.1002/pros.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho CK, Stoddart M, Walton M, Anderson RA, Beckett GJ. Calculated free testosterone in men: Comparison of four equations and with free androgen index. Ann Clin Biochem. 2006;43:389–397. doi: 10.1258/000456306778520115. [DOI] [PubMed] [Google Scholar]
- 19.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Neilson Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart study and applied to three geographically distinct cohorts. J Clin Endrocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedelaar JPM, Isaacs JT. Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. Prostate. 2009;69:1724–1729. doi: 10.1002/pros.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schroeder FH. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50:857–861. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]
- 22.Luu-The V, Pelletier G, Labrie F. Quantitative appreciation of steroidogenicgene expression in mouse tissues: New roles for type 2 5alpha-reductase, 20alpha-hydroxysteroid dehydrogenase and estrogen sulfotransferase. J Steroid Biochem Mol Biol. 2005;93:269–276. doi: 10.1016/j.jsbmb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Morote J, Orsola A, Planas J, Trilla E, Raventos CX, Cecchini L, Catalan R. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–1295. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 24.Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: Implications for clinical decision making. Urology. 2000;56:1021. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 25.Attard G, Reid A, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W, de Bono JS. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JP, Hwang K, Singh H, Wang D, Reynolds CP, Curley RW, Williams SC, Maurer BJ, Kang MH. Fenretinide metabolism in humans and mice: Utilizing pharmacological modulation of its metabolism to increase systemic exposure. Br J Pharmacol. 2011;163:1263–1275. doi: 10.1111/j.1476-5381.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bronson FH. Mammalian reproduction: An ecological perspective. Biol Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Bartke A, Amador AG, Chandrashekar V, Klemmcke HG. Seasonal differences in testicular receptors and steroidogenesis. J Steroid Biochem. 1987;27:581–587. doi: 10.1016/0022-4731(87)90357-8. [DOI] [PubMed] [Google Scholar]
- 29.Eick GN, Thornton JW. Evolution of steroid receptors from an estrogen-sensitive ancestral receptor. Mol Cell Endocrinol. 2011;334:31–38. doi: 10.1016/j.mce.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishioka J, Hara S, Isaacs JT, Tomura A, Nishikawa K, Kageyama Y. Suppression of mutant androgen receptors by flutamide. Int J Urol. 2009;16:516–521. doi: 10.1111/j.1442-2042.2009.02284.x. [DOI] [PubMed] [Google Scholar]
- 32.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: The saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–321. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Kuwahara S, Mizukami T, Omura M, Hagihara M, Iinuma Y, Shimizu Y, Tamada H, Tsukamoto Y, Nishida T, Sasaki F. Seasonal changes in the hypothalamo-pituitary-testes axis of the Japanese wood mouse (Apodemus speciosus) Anat Rec. 2000;260:366–372. doi: 10.1002/1097-0185(20001201)260:4<365::AID-AR50>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Schradin C. Seasonal changes in testosterone and corticosterone levels in four social classes of a desert dwelling sociable rodent. Horm Behav. 2008;53:573–579. doi: 10.1016/j.yhbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Attardi B, Ohno S. Androgen and estrogen receptors in the developing mouse brain. Endocrinology. 1976;99:1279–1290. doi: 10.1210/endo-99-5-1279. [DOI] [PubMed] [Google Scholar]