Abstract
The concept that specific acupuncture points have salubrious effects on distant target organ systems is a salient feature of Traditional Chinese Medicine (TCM). In this study, we used a multiple‐session experiment to test whether electroacupuncture stimulation at two TCM vision‐related acupoints, UB 60 and GB 37, located on the leg, could produce fMRI signal changes in the occipital regions of the brain, and the specificity of this effect when compared with stimulation at an adjacent non‐acupoint (NAP). Six normal, acupuncture naïve subjects completed the study. Each subject participated in six identical scanning sessions. Voxelwise group analysis showed that electroacupuncture stimulation at both vision‐related acupoints and the NAP produced modest, comparable fMRI signal decreases in the occipital cortex, including the bilateral cuneus, calcarine fissure and surrounding areas, lingual gyrus, and lateral occipital gyrus. Further analysis of fMRI signal changes in occipital cortex showed no significant difference among the three points, UB 60, GB 37, and NAP. Our results thus do not support the view that acupuncture stimulation at vision‐related acupoints induces specific fMRI blood oxygen level dependent (BOLD) signal changes in the occipital cortex. We speculate that cross modal inhibition, produced by needling‐evoked somatosensory stimulation, may account for our finding of BOLD signal decreases in the occipital cortex. Given the complexity of acupuncture systems and brain activity, additional work is required to determine whether functional neuroanatomical correlates of acupoint specificity can be validated by means of brain imaging tools. Hum Brain Mapp 2009. © 2007 Wiley‐Liss, Inc.
Keywords: acupuncture, electroacupuncture, vision‐related acupoint, acupoint specificity, fMRI, brain imaging
INTRODUCTION
The concept that specific acupuncture points have salubrious effects on target organ systems remote from the needling site is a salient feature of the Traditional Chinese Medicine (TCM) acupuncture system. A seminal study published almost 10 years ago by Cho and colleagues [Cho et al., 1998] proposed a conceptual relationship whereby acupoint specificity was mediated via the central neural networks that included the corresponding brain regions. Using fMRI to test their model, Cho's group reported that acupuncture manipulation at acupoints on the leg known to benefit visual system disorders (UB 67‐UB 60) could produce specific fMRI signal changes within the occipital lobes of the brain, whereas sham, non‐acupoints (NAP) could not.
Cho's study received much attention and triggered a rush of experimental replications. Several research groups [Gareus et al., 2002; Li et al., 2003a; Litscher et al., 2004; Parrish et al., 2005; Siedentopf et al., 2002] tried to duplicate this work in various ways. Although most drew similar conclusions, their results sometimes varied in terms of fMRI signal change directionality, for example. Furthermore, only few of these studies included an NAP or non vision‐related acupoint control [Parrish et al., 2005]. The work of Gareus et al. [2002] was a notable exception, reporting in contrast to Cho et al., that acupuncture at a vision‐related acupuncture point (GB 37) could neither directly produce activation in the visual cortex and associated areas, nor modulate fMRI signal changes in the visual cortex evoked by calibrated visual stimulation. Most recently, Cho et al. have published a retraction of their earlier results reporting that, “there is no point specificity, at least for pain and analgesia effects, and that we no longer agree with results in our PNAS article” [Cho et al., 2006].
In this article, we wish to address these uncertainties and contradictions by rigorously testing whether or not stimulation at vision‐related acupoints, when compared with a NAP, can specifically change brain activity in the visual cortex. We will then conjecture possible causes for the disparate findings in the neuroimaging literature on acupoint specificity.
A fundamental characteristic of fMRI data is the relatively large variability in the fMRI signal changes of blood oxygen level dependent (BOLD) response across scanning sessions even within an individual subject [Aguirre et al., 1998; Huettel et al., 2004]. Accordingly in this article, we will also assess the reliability of fMRI signal changes as a means of investigating acupoint specificity, exploring how well a given subject's fMRI patterns remain consistent across scanning sessions. We believe the answers to these questions will not only clarify the relationship between acupuncture needle stimulation at vision‐related acupoints and occipital brain activity, but more importantly, will benefit future functional neuroimaging investigations of acupuncture mechanisms, a research method that is gaining popularity as a tool to explore acupuncture mechanisms [Fang et al., 2004; Hui et al., 2000, 2005; Kong et al., 2002, 2007; Li et al., 2003b, 2004; Napadow et al., 2004; Wu et al., 1999, 2002; Yan et al., 2005; Yoo et al., 2004; Zhang et al., 2003].
In this study, we employed two vision‐related acupoints and one NAP to investigate specificity and reliability of fMRI signal changes in the occipital cortex. To make the data comparable to previous studies, we chose UB 60 as our first acupoint. Prior reports associated stimulation of this point with specific fMRI signal changes in the occipital cortex [Cho et al., 1998; Li et al., 2003a; Parrish et al., 2005]. We chose GB 37 as our second acupoint because it is widely used to treat eye disorders [Lade, 1989; Stux, 1997a], but neither induced nor modulated fMRI BOLD signal change in the occipital cortex according to one previous report [Gareus et al., 2002]. It should be noted that while both GB 37 [Lade, 1989; Stux, 1997a] and UB 60 [Ellis et al., 1988] are mentioned in modern acupuncture texts as treatments for vision‐related disorders, GB 37 is much more frequently indicated in this respect. The NAP was located about 1.5 cm posterior and inferior to the small head of the fibula. According to TCM theory, there is neither an acupoint nor meridian passing through that area [Cheng, 1987; Stux, 1997a].
METHOD AND ANALYSIS
Subjects
Eight healthy, right‐handed, and acupuncture‐naïve subjects (3 males, mean age 29 ± 7 years, mean ± SD) were recruited for this study as approved by the Human Research Committee of the Massachusetts General Hospital. All subjects gave written informed consent after the experimental procedures had been fully explained.
Experimental Procedures
Each subject participated in six identical fMRI scanning sessions. Sessions 1 and 2 were separated by 20–30 min. Sessions 2 and 3 were separated by 3–6 days. After Session 3, the interval between subsequent sessions was 7–21 days. Varied time intervals were chosen as a way to test how time influenced the reliability of fMRI signal changes.
Please see a related publication for further details of the experimental procedures [Kong et al., 2007]. In brief, each fMRI session included four functional scans (5 min each), one functional scan for each of the three points: UB 60 on the right ankle, GB 37 on the right mid‐shin, and the NAP located about 1.5 cm posterior and inferior to the right small head of the fibula (see Fig. 1), and one functional scan of a finger‐tapping task for the purpose of calibration, which was performed at the very beginning for half of the subjects, and at the end for the other half. (The result of finger‐tapping task is not related to this manuscript and thus will not be reported here, but see Kong et al., 2007 for a full description of the results.)
Figure 1.
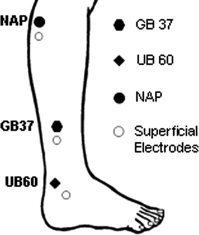
Location of points applied in the experiment: two vision‐related TCM acupuncture points GB 37 on the mid‐shin and UB 60 on the ankle, and one NAP located about 1.5 cm posterior and inferior to the small head of the fibula were chosen in this study.
An ON/OFF block design was used for this study, with “ON” denoting electroacupuncture stimulation. Each scan lasted a total of 5 min; beginning with a 30 s baseline scan; followed by four ON/OFF blocks, with each of the four ON blocks lasting 30 s and the three OFF blocks lasting 30, 60, and 30 s respectively; and ending with an additional 30 s of a baseline scan.
Acupuncture was performed by a single acupuncturist using the same method of point location. During scanning, subjects were told to close their eyes, relax, and focus on the sensations evoked by acupuncture stimulation. In all six sessions, the order of the three points was randomized across the subjects, but kept consistent within the same subject.
Electroacupuncture stimulation (OMS Medical Supplies IC‐1107) was applied on all three points at a frequency of 2 Hz. For UB 60, the superficial electrode was placed about 2 cm anterior and inferior to the same meridian as acupoint UB 62, and for GB 37 the superficial electrode was placed about 2 cm inferior on the same meridian as acupoint GB 38. The superficial electrode was also placed about 2 cm inferior to the NAP (Fig. 1).
To reduce anxiety about the procedures performed during scanning, all subjects were given a brief acupuncture treatment before their first scan session. At the beginning of this acupuncture exposure session, subjects were told that the aim of this study was to investigate brain response reliability to acupuncture stimulation across different acupuncture points. In all, subjects would participate in six identical fMRI scan sessions. At the beginning of each session, subjects were reminded of which session (1–6) we were conducting.
For that introductory session and all subsequent scan sessions, the acupuncturist inserted needles (38‐gauge stainless steel) into the three points and placed superficial electrodes at specific locations inferior to each point (see Fig. 1). The needles were then adjusted to obtain deqi. Deqi is a unique sensation believed to be important for the efficacy of acupuncture treatment and characterized by feelings such as numbness or fullness at the site of stimulation [Cheng, 1987; Stux, 1997b]. Of additional importance here is that sharp pain remained a specific concern and was avoided. Next, the needle, electrode, and electroacupuncture device were connected with a wire lead modified for safe use in the high magnetic field environment of MRI. For each subject, specific stimulation intensity was determined by gradually increasing and adjusting the voltage applied at each point, until the subject reported a mild to moderate sensation of deqi. This was done at the beginning of each session, prior to fMRI scanning. The average intensities (mean ± SD) for the three points were 5.5 ± 2.1 V for GB 37, 4.7 ± 1.7 V for UB 60, and 5.7 ± 2.0 V for the NAP.
After each scan, subjects were asked to quantify their sensations using the Subjective Acupuncture Subjective Sensation Scale (SASS) [Kong et al., 2005]. This scale consists of 11 measures including 9 descriptors of typical acupuncture evoked sensations: stabbing, throbbing, tingling, burning, heaviness, fullness, numbness, soreness, aching, and one blank row for subjects to add their own word(s) if the descriptors had not adequately described the sensations they experienced. For the SASS, subjects also rated their anxiety on a 10 cm scale with the anchor words “none,” “mild,” “moderate,” and “severe” spaced evenly along the continuum.
fMRI Data Acquisition and Analysis
All brain imaging was performed with a 3‐axis gradient head coil in a 3 Tesla Siemens MRI System (Erlagen, Germany) equipped for echo planar imaging. After automated scout and shimming procedures, functional MR images were acquired using gradient echo T2*‐weighted sequence with TR 2,000 ms, TE 40 ms, and a flip angle of 90°. Thirty oblique axial slices (4‐mm thick, 1‐mm skip) oriented parallel to the AC‐PC line were collected to provide whole brain coverage. A high resolution 3D MPRAGE sequence was also collected. During fMRI scanning, Prospective Acquisition Correction (PACE) [Thesen et al., 2000] and an online automatic slice aligning procedure [van der Kouwe et al., 2005] were used to adjust slice position and orientation, ensuring that scanning occurred with the brain registered in a similar position across different scan sessions and subjects.
Because we are primarily interested in occipital cortex brain activity, in this article we will only present fMRI signal changes observed in occipital regions evoked by electroacupuncture stimulation at the three different points. Activations evoked by the finger‐tapping task and acupuncture‐induced activations of brain regions outside occipital lobes are beyond the scope of this article and reported elsewhere [Kong et al., 2007].
Data analysis was performed using SPM2 (Wellcome Department of Cognitive Neurology). Pre‐processing included motion correction in which all functional runs were realigned to the first volume acquired in the scan session, spatial normalization to MNI stereotactic space and spatial smoothing with an 8 mm Gaussian kernel. To screen for subjects with excessive head movement, we set a movement threshold of 2 mm. No subject's head movements exceeded the limit; however, so all data remained eligible for analysis.
Next, a separate general linear model (GLM) was calculated for each session and across each subject with regressors for the difference from baseline for each point. Global signal scaling was applied. At each voxel, low‐frequency noise was removed with a high‐pass filter applied with default values to the fMRI time series.
A group analysis was performed for each point using a random‐effects model across all subjects and experimental sessions. We considered both subjects and sessions as components of measurement uncertainty; therefore, it was appropriate to average our calculations over subjects and sessions and to perform a group analysis (6 subjects × 6 sessions). A one‐way t‐test was used to determine group activation for each point. Since this study focused only on the occipital lobe and was also based on the results from previous studies [Cho et al., 1998; Li et al., 2003a; Litscher et al., 2004; Parrish et al., 2005; Siedentopf et al., 2002], we treated all regions in the occipital lobe as regions of interest (ROI) and thus applied a less strict threshold of P < 0.001 uncorrected with 10 contiguous voxels. To directly compare differences among the three points, a one‐way ANOVA (within‐subject) was also calculated. In the matrix, nonsphericity correction was performed, with replication over subjects, and correlated repeated measures were chosen. A threshold of P < 0.001 uncorrected and with 10 contiguous voxels was used.
To further investigate the influence of acupuncture stimulation at different points, we applied anatomical ROI analysis. This method allows for the interrogation of subtle but consistent global signal changes within a defined anatomical region. The AAL ROI template (anatomically defined by hand on a single brain matched to the MNI/ICBM templates) was used to define anatomical ROIs [Tzourio‐Mazoyer et al., 2002]. According to this template, the lateral surface of the occipital lobe consists of three regions: the superior, middle, and inferior occipital gyrus; and the medial surface of the occipital lobe consists of four regions: the cuneus, calcarine fissure and surrounding cortex (calcarine area), fusiform, and lingual gyrus (Fig. 2 bottom row). The beta values for each individual subject, session, brain region, and electroacupuncture condition (GB 37, UB 60, and NAP) were extracted using the MarsBar region of interest toolbox for SPM (http://marsbar.sourceforge.net/). Finally a paired t‐test was performed to detect the divergence between beta values in different electroacupuncture conditions (GB 37, UB 60, and NAP).
Figure 2.
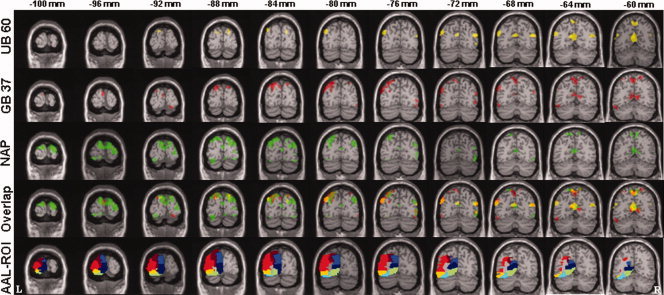
Average fMRI signal decreases evoked by electroacupuncture stimulation at GB 37, UB 60, and NAP overlaid on the T1 MNI signal subject brain (P < 0.001 with 10 contiguous voxels). Top row indicates the average fMRI signal decrease (six subjects by six sessions) evoked by electroacupuncture (EA) at UB 60 (yellow color); Second row indicates fMRI signal changes evoked by EA at GB 37 (red color); third row indicates fMRI signal changes evoked by EA at NAP (green color); fourth row indicates the overlap of fMRI signal decrease by EA at the three points; last row indicates the segmentation of anatomical ROIs: brown, superior occipital gyrus; red, middle occipital gyrus; yellow, inferior occipital gyrus; blue, cuneus; navy blue, calcarine fissure and surrounding cortex (calcarine area); sky blue, fusiform gyrus; green, lingual gyrus. The top line number indicates the y coordinate on the MNI atlas; L indicates left side; R indicates right side.
RESULTS
Behavioral Result
Of the eight volunteers who consented into the study, six (three female) completed all six sessions. Two subjects withdrew from the study after Session 2. Only the data from the subjects who completed all six sessions were analyzed.
Table I presents the SASS descriptor ratings summary for all six subjects in all six sessions, and all three points. The results show that the stimulation on the three points produced approximately equivalent ratings. No subjects reported experiences of sharp pain during the experiment. Furthermore, there were no significant differences on the anxiety ratings evoked by stimulating the three points. On a 0–10 point scale, anxiety ratings were 0.7 for GB 37, 0.8 for UB 60, and 0.6 for NAP.
Table I.
Grand average (mean ± SD), median, and range (median (min, max)) of SASS sensations for UB 60, GB 37, and NAP evoked by electroacupuncture stimulation across all subjects and all sessions on contiguous 0–10 scales
| UB60 | GB 37 | NAP | ||||
|---|---|---|---|---|---|---|
| Mean | Median (range) | Mean | Median (range) | Mean | Median (range) | |
| Soreness | 2.1 ± 2.0 | 2 (0, 6.6) | 2.3 ± 2.4 | 2 (0, 8) | 1.7 ± 2.0 | 0.5 (0, 6.6) |
| Heaviness | 0.7 ± 1.2 | 0 (0, 4) | 0.4 ± 0.8 | 0 (0, 3.3) | 0.6 ± 1.0 | 0 (0, 3) |
| Fullness | 0.5 ± 1.0 | 0 (0, 3) | 0.5 ± 1.1 | 0 (0, 4) | 0.6 ± 1.1 | 0 (0, 4) |
| Numbness | 1.6 ± 1.6 | 1.3 (0, 1.5) | 1.3 ± 1.4 | 1 (0, 5) | 1.4 ± 1.7 | 1 (0, 6.6) |
| Tingling | 4.0 ± 1.9 | 3.3 (0, 6.6) | 3.8 ± 2.3 | 4 (0, 6.6) | 3.1 ± 2.0 | 3.3 (0, 6.6) |
| Aching | 2.3 ± 1.8 | 3 (0, 6) | 2.7 ± 2.4 | 3 (0, 8) | 1.9 ± 1.8 | 2 (0, 5) |
| Burning | 1.3 ± 1.7 | 0 (0, 5) | 1.5 ± 1.6 | 1 (0, 5) | 1.3 ± 1.7 | 0 (0, 5) |
| Throbbing | 3.0 ± 2.6 | 3.3 (0, 7) | 2.8 ± 2.7 | 3.3 (0, 6.6) | 3.5 ± 2.5 | 4.5 (0, 6.6) |
| Stabbing | 2.5 ± 2.4 | 2 (0, 6.6) | 1.6 ± 2.2 | 0.5 (0,6.6) | 2.1 ± 2.0 | 2 (0, 6.6) |
fMRI Results
Group analysis averaged across all subjects and sessions showed no region where fMRI signals increased above threshold when calculating the contrast of electroacupuncture greater than baseline in the occipital lobe. Table II presents the brain regions in the occipital lobe showing BOLD signal decreases during the stimulation of the three points (electroacupuncture less than baseline). Signal decreases were observed in the bilateral cuneus, calcarine fissure and surrounding area, lingual gyrus, and lateral occipital gyrus for all three points.
Table II.
Indices of brain regions with significant fMRI signal decreases evoked by electroacupuncture stimulation at UB 60, GB 37, and NAP
| Condition | Brain region (brodmann area) | Z score | Voxels in cluster | Peak coordinate |
|---|---|---|---|---|
| UB 60 | Bilateral precuneus/cuneus/calcarine fissure and surrounding area/ lingual gyrus (31/17/18) | 4.75 | 867 | −8 −60 20 |
| Right occipital gyrus (19) | 3.53 | 78 | 20 −86 28 | |
| Left occipital gyrus (19) | 3.47 | 48 | −10 −90 32 | |
| Right occipital gyrus (19) | 3.56 | 15 | 50 −72 −2 | |
| GB 37 | Bilateral lingual gyrus/cuneus/ calcarine fissure and surrounding area/precuneus (19/18/17/31) | 6.01 | 1,006 | −8 −50 2 |
| Right precuneus/cuneus (7/19) | 4.57 | 107 | 22 −86 44 | |
| Left cerebellum/fusiform gyrus/ occipital gyrus (19/37/18) | 4.89 | 198 | −50 −62 26 | |
| Right inferior occipital cortex (18) | 3.95 | 82 | 48 −72 −2 | |
| Right fusiform (18/19) | 3.82 | 32 | 48 −78 −12 | |
| Right fusiform (18/19) | 3.73 | 42 | 22 −92 −14 | |
| Bilateral cuneus (18) | 3.73 | 98 | 0 −94 16 | |
| Left fusiform (18/19) | 3.39 | 11 | −32 −76 −14 | |
| NAP | Bilateral cuneus/occipital gyrus/lingual gyrus/calcarine fissure and surrounding area/precuneus (19/18/17/31/7) | 6.04 | 3,070 | −6 −94 34 |
| Left lingual gyrus (18/19) | 4.2 | 89 | −12 −48 −6 | |
| Left cerebellum/lingual gyrus/ calcarine fissure and surrounding area (18/17) | 4.04 | 217 | −24 −90 −12 | |
| Right fusiform gyrus/cuneus/ calcarine fissure and surrounding area (18/17) | 4.0 | 162 | 24 −48 −6 | |
| Right occipital gyrus (19) | 3.88 | 149 | 50 −70 10 | |
| Right cuneus (18) | 3.61 | 54 | 36 −88 14 | |
| Left calcarine fissure and surrounding area (17) | 3.60 | 26 | −8 −96 −10 | |
| Fusiform (18) | 3.38 | 46 | 6 −90 −16 |
Figure 2 shows the fMRI signal decreases evoked by electroacupuncture stimulation at each of the points. Electroacupuncture stimulation at UB 60, GB 37, and NAP all produced widespread fMRI signal deactivation with multiple overlapping regions; though, the extent of NAP‐induced deactivation was greater than the extent of that induced by the other two points. However, at a lower threshold of P < 0.005 uncorrected, the fMRI deactivation patterns evoked by GB 37 and UB 60 were more extensive and similar to that evoked by NAP. This suggests that occipital lobe deactivation patterns between the three points do not significantly differ; but that they induce only minor differences in fMRI signal change.
A voxelwise ANOVA directly comparing electro acupuncture at GB 37, UB 60, and the NAP was additionally performed. Here again, results showed no region above the threshold in the occipital cortex with parameters of P < 0.001 with 10 contiguous voxels. These results demonstrate that although fMRI signal decreases seem more extensive for NAP than for GB 37 and UB 60, as shown in Figure 2, stimulation at the three points did not evoke significant differences in occipital lobe fMRI signal changes.
ROI Analysis
To further investigate the potential influence of acupuncture stimulation at UB 60, GB 37, and NAP on global signal changes in the occipital cortex, we extracted beta values for individual subjects according to the AAL template on all occipital cortices [Tzourio‐Mazoyer et al., 2002]. Paired t‐tests on the beta values of any two points (among UB 60, GB 37, and NAP) in all defined ROIs showed no significant differences (P values ranging from 0.1 to 0.9).
DISCUSSION
We compared brain activation changes in the occipital lobe by stimulating two vision‐related TCM acupoints and one NAP. The group results of our study showed that electroacupuncture stimulation at all three points produced similar fMRI signal decreases in the occipital cortex, including the bilateral cuneus, calcarine fissure and surrounding area, lingual gyrus, and lateral occipital gyrus; neither voxelwise nor anatomical ROI based group analyses showed significant differences among the two TCM acupuncture points (UB 60 and GB 37) and NAP when comparable deqi sensations were elucidated.
Our finding, showing that electroacupuncture stimulation at all three points produced fMRI signal deceases in the group analysis, is partly consistent with the previous report by Cho et al. [1998] and Hui et al. [2005]. In their study, Cho et al. found that of 12 volunteers, acupuncture stimulation at vision‐related points produced fMRI signal increases at the occipital cortex in four subjects, and fMRI signal decreases in eight subjects. Then, they defined subjects with fMRI signal increases as Yin and those with decreases as Yang to explain the contrasting directionality of fMRI signal changes observed in their subjects. However, if we do not employ this post‐hoc definition, the average fMRI signal percent change of all subjects in Cho's study (as indicated in their Table I), is −2.0 ± 8.6, a signal decrease consistent with the results of our own study. fMRI signal decreases at medial occipital regions deriving from acupuncture stimulation at ST 36 were also observed (for subjects reporting typical deqi sensation) in a recent study by Hui et al. [2005].
However, our finding nevertheless conflicts with all other vision‐point studies reporting positive fMRI signal changes [Li et al., 2003a; Litscher et al., 2004; Parrish et al., 2005; Siedentopf et al., 2002].
Several factors may contribute to the discrepant fMRI signal directionality between our work and others'. We speculate that our observed fMRI signal decreases may uniquely represent a cross‐modal interaction [Drevets and Raichle, 1998; Haxby et al., 1994; Kawashima et al., 1995]. According to the theory of cross‐modal interaction, attention can significantly modulate brain activity of different primary sensory modalities: selective attention to one sensory modality can increase activity in cortical regions dedicated to processing attended information, while decreasing the activity in cortical regions which process information for other sensory modalities [Drevets and Raichle, 1998; Haxby et al., 1994; Kawashima et al., 1995]. In line with this theory, Kawashima et al. [1995] found that regional cerebral blood flow decreased in the visual cortex when subjects were required to perform somatosensory tasks (roughness discrimination or tactile matching) in both “eyes open” and “eyes closed” conditions.
In our current study, all subjects were instructed to close their eyes, relax, and pay attention to the sensation evoked by electroacupuncture stimulation during scanning. Then, after each functional run, subjects were required to rate the sensations they experienced during acupuncture stimulation on the SASS scale. It is possible that these explicit instructions in combination with the subsequent rating task uniquely made our electroacupuncture stimulation into a somatosensory task, one that required additional attention to the sensations evoked. As a further support of this view, as reported in a previous manuscript [Kong et al., 2007], we did observed fMRI signal increases in somatosensory related regions including S2, insula and S1.
In contrast, none of the studies reporting positive fMRI signal changes in the occipital cortex [Li et al., 2003a; Litscher et al., 2004; Parrish et al., 2005; Siedentopf et al., 2002] specifically instructed the subject to focus on the sensation under the needle, nor required subjects to rate their sensations after administration.
We speculate that variation in acupuncture administration techniques may have additionally contributed to the signal change discrepancy between our work and others'. In this regard, Hui et al. [2005] have previously shown that standardized needling procedures can nevertheless invoke disparate behavioral and neurobiological responses. In their study, they observed fMRI signal increases at medial occipital regions for subjects reporting deqi sensations mixed with sharp pain, but fMRI signal decreases for subjects reporting only deqi sensations. It is thus possible that the sporadic evocation of pain may have affected overall results in the above investigations [Li et al., 2003a; Litscher et al., 2004; Parrish et al., 2005; Siedentopf et al., 2002], however because few of these studies reported on the detailed sensations evoked by acupuncture stimulation, it is not possible to evaluate this factor.
The inconsistency between our findings and others' may finally derive from the inherent variability in fMRI signal change directionality and amplitude that exists between and even within individuals [Kong et al., 2007]. In our experiment, we found that although group results showed fMRI signal decreases, a single individual could alternately show fMRI signal maintenance, increases, and decreases across various trial runs. Other researchers have reported similar inconsistencies in signal directionality across different individuals and for different modes of acupuncture treatment [Li et al., 2003a].
It has been nearly 10 years since Cho et al. [1998] first attempted to investigate acupuncture point specificity using fMRI, yet validation of the phenomenon through this modality remains undetermined. What this suggests is that the field faces many challenges due to the complexities of acupuncture, the brain, and fMRI.
To begin, acupuncture itself is a very complicated system and differences among acupuncturists, divergent manipulation modes, stimulation parameters, treatment styles, and subjective sensations evoked by acupuncture stimulation (typical deqi sensation vs. sharp pain [Hui et al., 2005]) may all account for some level of incongruity regarding acupoint specificity. It is additionally important to note that acupuncture theory premises that stimulation can produce bidirectional effects to regulate and balance the body's systems [Hammerschlag and Lao, 2001; Yang and Cao, 1987]. Thus, it is not certain that what can be observed in a normal, healthy population necessarily reflects the clinical population. Furthermore, even among patients, acupuncture may not necessarily work always or primarily through the brain.
Second, brain activity itself is very complicated and often difficult to control in experimental settings. For this reason, subtle alterations in experimental paradigm including instruction to the subjects and their cooperation, as well as details of experimental procedure may all influence study findings. It is well‐known in the field of fMRI that the attention level, general arousal, and mood of each individual during scanning can each significantly affect the final results of a study. Accordingly, subtle differences between fMRI signal changes evoked by stimulation at acupoints and NAPs may be masked by psychological conditions such as attention. Taking the case of vision‐related acupoints as an example, the subtle fMRI signal difference between vision‐related and nonvision‐related acupoints/NAPs, if the differences exist, may be obscured by the other physiological phenomenon such as cross‐modal interaction.
Finally, given the relatively low signal to noise ratio, variabilities include variations in MR scanner hardware and software, differences in MRI acquisition sequences, data post‐processing methods [Smith et al., 2005] may also contribute to the inconsistency between various studies.
Before closing, several potential limitations to our study must be addressed. One limitation in interpreting our results is the small sample size in this experiment. Previous studies suggest that a sufficient sample size and number of repeated scans across same subject is necessary to ensure ample power to detect changes in brain activity associated with a task [Huettel et al., 2004]. In our study, we repeated the same procedure six times for each subject. We observed relatively large acupuncture‐induced variability of fMRI signal changes (directionality and amplitude) different scan sessions within the same subjects, this clearly supports the need for multiple repeated sessions across the same subject when investigating brain effects related to acupuncture stimulation [Kong et al., 2007]. To further address this issue, an intraclass correlation coefficient (ICC) analysis was performed on all occipital ROIs across defined in this study. The largest ICC was 0.16, which is rather small, indicating that, this multiple scans on the same subjects provide nearly as much information as would data on different subjects. We also calculated the effective sample size [Scholz and Vangel, 1998] based on the largest ICC (0.16). The results showed that our total 36 sessions (6 subjects by 6 sessions) is approximately equivalent to 20 subjects. Thus, our sample size is at least comparable with previous studies that investigated fMRI signal changes when stimulating vision‐related acupoints. However, we are cautious in our interpretation because we cannot formally accept the null hypothesis (i.e. there is no difference in occipital cortex fMRI signal changes when stimulating vision‐related acupoints and NAP), as one can always increase the sample size.
Another possible limitation to our study was our choice of a baseline. In our experiment, we inserted the needles first and then stimulated them separately in different functional runs. We could have alternatively chosen to insert and stimulate the needles at separate points one by one. In support of our procedures, however, a previous study has shown that needle placement has little or no effect on the brain activation [Hui et al., 2000]. Most importantly, all prior fMRI acupuncture studies of vision points employed a block design, in which acupuncture needle stimulation was typically started (producing deqi) and stopped (diminishing deqi) several times. Here it is the comparison between fMRI signal changes during needle stimulation versus rest (baseline) that reveals which regions of brain activity correlate with acupuncture needle manipulation. Our choice of methods match those used by previous fMRI acupuncture stimulation studies on vision points [Cho et al., 1998; Gareus et al., 2002; Li et al., 2003a; Parrish et al., 2005] as well as other fMRI acupuncture studies [Fang et al., 2004; Hui et al., 2000, 2005; Kong et al., 2002; Li et al., 2004, 2003b; Liu et al., 2004; Napadow et al., 2004; Wu et al., 1999, 2002; Yan et al., 2005; Yoo et al., 2004; Zhang et al., 2003].Thus, we believe our decision to insert all needles first and then stimulate different acupuncture points individually, actually provides the most appropriate baseline, allowing for comparisons across different acupoints.
Finally, although the subjects were told to not open their eyes, it is still possible that they could have opened their eyes and caused fMRI signal changes. Future studies should attempt to assess this directly at the time of the scan.
In summary, we observed similar BOLD signal decreases in occipital cortex evoked by electroacupuncture when stimulating both vision points GB 37 and UB 60, and a NAP. Our inability to find evidence for acupoint specificity does not disprove the neural basis for acupoint specificity. Given the complexity of acupuncture systems and brain activity, additional work is required to determine whether functional neuroanatomical correlates of acupoint specificity can be validated by means of brain imaging tools.
Acknowledgements
The authors would like to thank Dr. Kathleen Hui for her helpful suggestions and comments on the manuscript.
REFERENCES
- Aguirre GK,Zarahn E,D'Esposito M ( 1998): The variability of human, BOLD hemodynamic responses. Neuroimage 8: 360–369. [DOI] [PubMed] [Google Scholar]
- Cheng XN ( 1987): Chinese Acupuncture and Moxibustion. Beijing: Foreign Language Press. [Google Scholar]
- Cho ZH,Chung SC,Jones JP,Park JB,Park HJ,Lee HJ,Wong EK,Min BI ( 1998): New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA 95: 2670–2673. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cho ZH,Chung SC,Lee HJ,Wong EK,Min BI ( 2006): Retraction. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA 103: 10527. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Drevets WC,Raichle ME ( 1998): Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implicaitons for interactions between emotion and cognition. Cognit Emotion 12: 352–385. [Google Scholar]
- Ellis A,Wiseman N,Boss K ( 1988): Fundamentals of Chinese Acupuncture. Brookline, MA: Paradigm. [Google Scholar]
- Fang JL,Krings T,Weidemann J,Meister IG,Thron A ( 2004): Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology 46: 359–362. [DOI] [PubMed] [Google Scholar]
- Gareus IK,Lacour M,Schulte AC,Hennig J ( 2002): Is there a BOLD response of the visual cortex on stimulation of the vision‐related acupoint GB 37? J Magn Reson Imaging 15: 227–232. [DOI] [PubMed] [Google Scholar]
- Hammerschlag R,Lao L ( 2001): Future direction for research on the physiology of acupuncture In: Stux G,Hammerschlag R, editors. Clinical Acupuncture–Scientific Basis. Berlin: Springer‐Verlag; pp 211–219. [Google Scholar]
- Haxby JV,Horwitz B,Ungerleider LG,Maisog JM,Pietrini P,Grady CL ( 1994): The functional organization of human extrastriate cortex: A PET‐rCBF study of selective attention to faces and locations. J Neurosci 14(11): 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel AS,Song AW,McCarthy G ( 2004): Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates; pp 217–251. [Google Scholar]
- Hui KK,Liu J,Makris N,Gollub RL,Chen AJ,Moore CI,Kennedy DN,Rosen BR,Kwong KK ( 2000): Acupuncture modulates the limbic system and subcortical gray structures of the human brain: Evidence from fMRI studies in normal subjects. Hum Brain Mapp 9: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK,Liu J,Marina O,Napadow V,Haselgrove C,Kwong KK,Kennedy DN,Makris N ( 2005): The integrated response of the human cerebro‐cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 27: 479–496. [DOI] [PubMed] [Google Scholar]
- Kawashima R,O'Sullivan BT,Roland PE ( 1995): Positron‐emission tomography studies of cross‐modality inhibition in selective attentional tasks: closing the “mind's eye”. Proc Natl Acad Sci USA 92: 5969–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J,Ma L,Gollub RL,Wei J,Yang X,Li D,Weng X,Jia F,Wang C,Li F,Li R,Zhuang D ( 2002): A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI‐4 Hegu) in normal subjects reveals differential brain activation between methods. J Altern Complement Med 8: 411–419. [DOI] [PubMed] [Google Scholar]
- Kong J,Fufa DT,Gerber AJ,Rosman IS,Vangel MG,Gracely RH,Gollub RL ( 2005): Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. J Pain 6: 55–64. [DOI] [PubMed] [Google Scholar]
- Kong J,Gollub RL,Webb JM,Kong JT,Vangel MG,Kwong K ( 2007): Test‐retest study of fMRI signal change evoked by electro‐acupuncture stimulation. Neuroimage 34: 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lade A ( 1989): Point images and functions In: Lade A,editor. Acupuncture Points Images and Functions. Seattle: Eastland Press; pp 27–311. [Google Scholar]
- Li G,Cheung RT,Ma QY,Yang ES ( 2003a): Visual cortical activations on fMRI upon stimulation of the vision‐implicated acupoints. Neuroreport 14: 669–673. [DOI] [PubMed] [Google Scholar]
- Li G,Liu HL,Cheung RT,Hung YC,Wong KK,Shen GG,Ma QY,Yang ES ( 2003b): An fMRI study comparing brain activation between word generation and electrical stimulation of language‐implicated acupoints. Hum Brain Mapp 18: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G,Huang L,Cheung RT,Liu SR,Ma QY,Yang ES ( 2004): Cortical activations upon stimulation of the sensorimotor‐implicated acupoints. Magn Reson Imaging 22: 639–644. [DOI] [PubMed] [Google Scholar]
- Litscher G,Rachbauer D,Ropele S,Wang L,Schikora D,Fazekas F,Ebner F ( 2004): Acupuncture using laser needles modulates brain function: First evidence from functional transcranial Doppler sonography and functional magnetic resonance imaging. Lasers Med Sci 19: 6–11. [DOI] [PubMed] [Google Scholar]
- Liu WC,Feldman SC,Cook DB,Hung DL,Xu T,Kalnin AJ,Komisaruk BR ( 2004): fMRI study of acupuncture‐induced periaqueductal gray activity in humans. Neuroreport 15: 1937–1940. [DOI] [PubMed] [Google Scholar]
- Napadow V,Makris N,Liu J,Kettner NW,Kwong KK,Hui KK ( 2004): Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp 24: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish TB,Schaeffer A,Catanese M,Rogel MJ ( 2005): Functional magnetic resonance imaging of real and sham acupuncture. Noninvasively measuring cortical activation from acupuncture. IEEE Eng Med Biol Mag 24: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz F,Vangel M ( 1998): Tolerance bounds and Cpk confidence bounds under batch effects In: Kahle W,von Collani E, Franz J,Jensen U, editors. Advances in Stochastic Models for Reliability, Quality and Safety. Boston: Birkhauser Boston; pp 361–379. [Google Scholar]
- Siedentopf CM,Golaszewski SM,Mottaghy FM,Ruff CC,Felber S,Schlager A ( 2002): Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci Lett 327: 53–56. [DOI] [PubMed] [Google Scholar]
- Smith SM,Beckmann CF,Ramnani N,Woolrich MW,Bannister PR,Jenkinson M,Matthews PM,McGonigle DJ ( 2005): Variability in fMRI: a re‐examination of inter‐session differences. Hum Brain Mapp 24: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stux G ( 1997a): Channels, organs, and points In: Stux G, Pomeranz B, editors. Basics of Acupuncture. Berlin: Springer‐Verlag; pp 84–194. [Google Scholar]
- Stux G ( 1997b): Technique of acupuncture In: Stux G,Pomeranz B, editors. Basics of Acupuncture. Berlin: Springer‐Verlag; pp 202–213. [Google Scholar]
- Thesen S,Heid O,Mueller E,Schad LR ( 2000): Prospective acquisition correction for head motion with image‐based tracking for real‐time fMRI. Magn Reson Med 44: 457–465. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJ,Benner T,Fischl B,Schmitt F,Salat DH,Harder M,Sorensen AG,Dale AM ( 2005): On‐line automatic slice positioning for brain MR imaging. Neuroimage 27: 222–230. [DOI] [PubMed] [Google Scholar]
- Wu M‐T,Hsieh J‐C,Xiong J,Yang P‐C,Pan H‐B,Chen Y‐CI,Tsai G,Rosen BR,Kwong KK ( 1999): Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain preliminary experience. Radiology 212: 133–141. [DOI] [PubMed] [Google Scholar]
- Wu MT,Sheen JM,Chuang KH,Yang P,Chin SL,Tsai CY,Chen CJ,Liao JR,Lai PH,Chu KA,Pan HB,Yang CE ( 2002): Neuronal specificity of acupuncture response: A fMRI study with electroacupuncture. Neuroimage 16: 1028–1037. [DOI] [PubMed] [Google Scholar]
- Yan B,Li K,Xu J,Wang W,Li K,Liu H,Shan B,Tang X ( 2005): Acupoint‐specific fMRI patterns in human brain. Neurosci Lett 383: 236–240. [DOI] [PubMed] [Google Scholar]
- Yang JS,Cao YM ( 1987): Aupuncture Point. Shanghai: Shanghai Science and Technology Press; 181p. [Google Scholar]
- Yoo SS,Teh EK,Blinder RA,Jolesz FA ( 2004): Modulation of cerebellar activities by acupuncture stimulation: Evidence from fMRI study. Neuroimage 22: 932–940. [DOI] [PubMed] [Google Scholar]
- Zhang WT,Jin Z,Cui GH,Zhang KL,Zhang L,Zeng YW,Luo F,Chen AC,Han JS ( 2003): Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: A functional magnetic resonance imaging study. Brain Res 982: 168–178. [DOI] [PubMed] [Google Scholar]


