Abstract
In the present study, we examined the profile of psychiatric symptoms in boys with fragile X syndrome (FXS) using a parent report instrument. In addition, by comparing boys with FXS to boys with nonsyndromic autism spectrum disorder (ASD) utilizing multiple matching strategies, we examined between-group differences in the types of psychiatric symptoms observed and in the strength of their concurrent associations. Across all matching strategies, symptoms of manic/hyperactive behaviors and general anxiety were more frequently reported for boys with FXS than for boys with nonsyndromic ASD. Results also indicated a positive association between social avoidance and general anxiety in FXS that was stronger than that observed in nonsyndromic ASD across all matching strategies. Theoretical and treatment implications are discussed.
1. Introduction
Fragile X syndrome (FXS) is the leading inherited cause of intellectual disability (Crawford, Acuna, & Sherman, 2001) and is the second only to Down syndrome as a genetic cause of intellectual disability. The syndrome results from an expansion of the cytosine-guanine-guanine (CGG) sequence of nucleotides within the FMR1 gene on the X chromosome to more than 200 repeats (Oostra & Willemson, 2003). This expansion typically leads to the reduction or absence of FMRP, the protein normally produced by the FMR1 gene, which is essential for synaptic plasticity and experience-dependent learning (Bassall & Warren, 2008). In addition to cognitive impairments, a variety of behavioral difficulties have a high comorbidity with FXS, especially in males, including hyperactivity and attentional difficulties (e.g., Baumgardner, Reiss, Freund, & Abrams, 1995; Cornish, Scerif, & Karmiloff-Smith, 2007; Scerif, Longhi, Cole, Karmiloff-Smith, & Cornish, 2012; Turk, 1998;) and anxiety and withdrawal (e.g., Bregman, Leckman, & Ort, 1988; Cordeiro, Ballinger, Hagerman, & Hessl, 2011; Kau, Reider, Payne, Meyer, & Fruend, 2000). Moreover, the vast majority of males with FXS are likely to display some behaviors that are characteristically observed in individuals with nonsyndromic autism spectrum disorder (ASD), that is, individuals with ASD for whom there is no known etiology (Bailey et al., 2004; Baumgardner et al., 1995; Hartley et al., 2011). In the present study, we sought to clarify the behavioral symptom profile of FXS and understand the ways in which this profile is similar to and different from that of nonsyndromic ASD. Such data are critical to efforts to develop targeted treatments, especially those that have utility for both FXS and nonsyndromic ASD. Given the moderating effects of the second unaffected X chromosome in females, males with FXS are typically more severely affected across domains of behavioral functioning (Mazzocco, 2000). Thus, the present study focused on males only.
Recent advances in neurobiology have increased our understanding of the pathophysiology of FXS, leading to the development of pharmacological treatments targeting core deficits of this disorder. Such treatments have been shown to rescue many phenotypic features in the FMR1 knock out (KO) mouse and other animal models of FXS (Bagni, Tassone, Neri, & Hagerman, 2012; Berry-Kravis et al., 2011; Bhakar, Dölen, & Bear, 2012; Dölen, Carpenter, Ocain, & Bear, 2010; Hagerman, Lauterborn, Au, & Berry-Kravis, 2012). Positive effects of several pharmacological agents have been observed in human clinical trials as well, including those involving minocycline (Dziembowska et al., 2013: Leigh et al., 2013), sertraline (Indah Winarni et al., 2012), AFQ056 (an mGluR5 antagonist) (Jacquemont et al., 2010), and arbaclofen (a GABAB agonist) (Berry-Kravis et al., 2012). More generally, the symptom overlap between FXS and nonsyndromic ASD has led to the hope that targeted treatments benefitting FXS will also benefit those with nonsyndromic ASD (Gurkan & Hagerman, 2012). Despite initial positive results and the optimism generated among scientists and families, there have been many disappointments in human clinical trials as well. In general, results for humans with FXS have been modest relative to the impressive findings with the KO mouse. Moreover, the extension of drugs with positive findings for FXS to individuals with nonsyndromic ASD has not always shown benefit, with arbaclofen being a recent high-profile “failure” for nonsyndromic ASD (Pollock, 2013).
There are several reasons for the pattern of human study findings. First, although the impairments that define FXS have been well described for many behavioral domains, there remains much that we do not understand about the phenotype, including the extent to which different behavioral symptoms associated with the FXS phenotype emerge from the same or different underlying neural mechanisms. As a result, decisions about which behavioral endpoints to select for use in clinical trials have often been based on clinical intuition rather than being hypothesis driven. It would be useful, therefore, to understand which symptoms of the FXS phenotype are strongly correlated with one another, which would suggest common underlying mechanisms, and which symptoms are not correlated, which would suggest different underlying mechanisms. Second, despite the findings of shared behavioral symptoms between FXS and nonsyndromic ASD, there is emerging evidence suggesting that the same behavioral symptoms may reflect different underlying mechanisms in the two conditions (Gallagher & Hallahan, 2012; McDuffie, Thurman, Hagerman, & Abbeduto, in press; Wolff, Bodfish, Hazlett, Lightbody, Reiss, & Piven, 2012). If this is the case, the expectation of finding targeted treatments that are equally efficacious across the disorders might not be reasonable. Direct studies comparing behavioral profiles across FXS and nonsyndromic ASD have been few and existenting studies do not adequately account for other developmental characteristics that differ between the two disorders, such as IQ, which can complicate interpretation of such cross-syndrome comparisons.
The current study was designed to compare the behavioral profiles observed in FXS to the profiles observed in nonsyndromic ASD. To begin to identify characteristics that may be unique to the behavioral phenotype associated with FXS, we examined the psychiatric symptom profile of boys with FXS relative to comparison groups of same-aged children with a diagnosis of nonsyndromic ASD. In the present study, we use the term “nonsyndromic ASD” to refer to boys who display symptoms of autism that are frequent and/or severe enough to exceed behavioral criteria for a research classification of ASD, but for whom a comorbid genetic diagnosis of FXS or other known etiology has been ruled out. To inform our understanding of whether the same behavioral symptoms reflect similar or different underlying psychological mechanisms, we examined concurrent associations across domains of psychiatric symptoms as well as concurrent associations between these symptoms and other domains of functioning across the two disorders. Studies such as these are vital to understanding the extent to which targeted treatments may be similarly efficacious in FXS and nonsyndromic ASD.
1.1 Symptoms associated with an Autism Spectrum Disorder
Behavioral characteristics typically associated with the presentation of an ASD are frequently observed in males with FXS. As many as 90% of males with FXS are described as displaying at least some behavioral symptoms of an ASD; furthermore, when utilizing standard caseness criteria for research classification of ASD (Risi et al., 2006), it is estimated that 60% of males with FXS have symptoms that are frequent and severe enough to warrant a comorbid diagnosis of ASD (e.g., Harris et al., 2008). Despite these findings, there is disagreement regarding how to interpret the presence or absence of behaviors typically associated with a classification of ASD in the context of an individual with FXS. Currently, there is much debate within the field regarding whether the behavioral symptoms typically interpreted to represent core domains of autism symptomatology are: (1) the result of the same underlying neurological/psychological impairments that affect individuals with nonsyndromic ASD or (2) superficially similar behaviors but arise from different underlying psychological/neurological impairments. Given recent findings of neuroimaging studies suggesting potentially important structural and functional differences between the brains of individuals with FXS and those with nonsyndromic ASD (for review see Gallagher & Hallahan, 2012), research focused on elucidating the similarities and differences between the two disorders and examining at what levels these differences exist is of particular importance. Direct comparisons between appropriately matched FXS and ASD samples are necessary to discern where the behavioral and neurophysiological boundaries lay between these two disorders.
To date, many comparisons between individuals with FXS and individuals with nonsyndromic ASD have focused on the behaviors central to a diagnosis of ASD, examined as either a function of symptom severity and/or categorical diagnostic metrics. Recent findings have begun to identify between-syndrome differences between boys with FXS and boys with nonsyndromic ASD when matched on age and categorical diagnostic status (Wolff et al., 2012) and when matched on age and a continuous metric of autism symptom severity (McDuffie et al., in press). McDuffie and colleagues, for example, recently found that boys with FXS are less impaired in social smiling, gesture use, and offering to share, and more impaired in complex mannerisms than are boys with nonsyndromic ASD, whether matched on age and diagnostic status or autism symptoms severity (McDuffie et al., in press). These results suggest that important differences in social-affective, communicative, and restricted, repetitive behaviors may differentiate boys with FXS from those with ASD and may have important implications for the application of targeted treatments. In order to understand the extent to which symptoms overlap or discriminate between the FXS and nonsyndromic ASD phenotypes, it is necessary for empirical studies to go beyond evaluating the similarities/differences in autism symptoms by directly comparing symptom profiles in other domains of functioning, particularly domains that are frequently implicated in both phenotypes.
Two domains of functioning that are frequently noted as problematic for individuals with FXS are hyperactivity/inattention and anxiety. Increased symptom presentation in these domains is also observed for individuals with nonsyndromic ASD (de Bruin, Ferdinand, R. F., Meester, S., de Nijs, P. F. A., & Verheij, F., 2007; Gjevik, Eldevik, Fjaeran-Granum, & Sponhein, 2011; Leyfer et al., 2006; Mattila et al., 2010; Simonoff et al., 2008); yet, direct comparisons of the disorders on these domains have yet to be conducted. In the current study, we examined of the profile of symptoms and the interrelations among these symptom domains to shed light on whether similar behavioral challenges reflect the same underlying mechanisms in these two neurodevelopmental disorders.
1.2 Hyperactivity and Attentional Difficulties
Males with FXS often display significant hyperactivity as well as attentional difficulties. For example, in their sample of 14 males with FXS (chronological (CA) range: 3 – 27 years), Bregman, Leckman, and Ort (1988) found that all participants demonstrated significant inattention and hyperactivity and 13/14 participants demonstrated clinically significant impulsivity problems. Fryns, Jacobs, Kleczkowska, and van den Berghe (1984) reported similar findings for a sample of 21 males between 2 and 21 years of age, describing “hyperkinetic behavior with restlessness, overactivity, agitation and poor, superficial attention (Fryns et al., 1984, pg. 133)” as the most salient behavioral characteristics of their sample. Sansone et al. (2012) compared a factor analysis of Aberrant Behavior Checklist-Community (ABC-C; Aman & Sigh, 1994) data from individuals with FXS to the original norming data for individuals with general intellectual disability. They found that in the case of FXS, fewer items loaded onto the Hyperactivity factor than had been observed for individuals with general intellectual disability. Items loading on this factor were more explicitly related to elevated activity in the sample of individuals with FXS. For individuals with general intellectual disability, items related to disruptive behavior, impulsivity and inattentiveness/distractibility also loaded on the Hyperactivity factor, whereas for participants with FXS, these items loaded on Irritability and Lethargy/Withdrawal factors.
More generally, multiple studies have compared the rates of hyperactivity, attention problems, and impulsivity in FXS to rates found in other clinical groups. Baumgardner et al. (1995) found that children with FXS between 3 and 16 years of age (mean CA = 8.7 years, SD = 4.3), earned higher maternal ratings of hyperactivity, measured by the Parent-Aberrant Behavior Checklist (ABC; Krug, Arick, Almond, 1993) than did a group of participants of mixed etiology ID matched on CA and similar in IQ. Furthermore, a greater proportion of children with FXS met DSM-III-R criteria for attention deficit/hyperactivity disorder than did controls of mixed etiology ID. Turk (1998) reported a similar pattern of findings when comparing children with FXS to children with Down syndrome (DS) and children with nonsyndromic developmental disability matched on developmental age. The children with FXS in Turk’s study earned higher maternal ratings of restlessness and hyperactivity, as measured by the Children’s Behavior Checklist (CBCL; Achenbach & Edelbrock, 1983), than did the children with DS or children with developmental delay. Not all studies have been able to replicate this finding (e.g., Einfeld, Hall, & Levy; 1991; Kau et al., 2000); however, there are several possible explanations for the discrepant findings across studies including the CA of the participants as well as the measures used to assess hyperactivity.
Despite the increased risk for presenting with these symptoms, no studies to date directly compare symptoms of hyperactivity, attention problems, and impulsivity between FXS and nonsyndromic ASD. Consequently, we do not know the extent to which these symptoms influence the similarities and differences observed between the two phenotypes. By exploring the between-group similarities and differences that exist in the hyperactivity and attentional domain and interrelations across domains, we can learn whether their similar behavioral presentations/difficulties reflect the same underlying mechanisms in these two neurodevelopmental disorders.
1.3 Anxiety/Withdrawal
Males with FXS also are often described as presenting with significant anxiety, gaze aversion, and relative to individuals with other ID syndromes (e.g., Bregman, Leckman, & Ort, 1988; Cordeiro et al., 2011; Einfeld, Tonge, and Florio, 1994; Kau et al., 2000). For example, in their factor analysis of the ABC-C data from individuals with FXS, Sansone et al. (2012) identified a sixth factor for their FXS sample that was not observed for individuals with general intellectual disability in the original norming sample. Sansone et al. argued that this factor, referred to as Social Avoidance, captured core aspects of the FXS phenotype related to gaze avoidance, social escape behaviors, and social anxiety. Cordeiro et al. (2011), using a diagnostic clinical interview assessing the presence of DSM-IV anxiety disorders, found that 86.2% of their sample met diagnostic criteria for at least one anxiety disorder, most frequently specific phobia and social phobia. Cordeiro et al. found that rates of anxiety disorders were higher in FXS relative to the general population (Shaffer et al., 1996), children with nonsyndromic ID (Dekker & Koot, 2003), and children with Williams syndrome (Leyfer, Woodruff-Borden, & Mervis, 2009), a neurogenetic syndrome in which the rate of General Anxiety Disorder is reported as more than double the rate observed in the general population. In contrast, multiple studies have also reported no difference to decreased rates of Withdrawal (Baumgardner et al., 1995; Kau et al., 2000) and antisocial behavior (Einfeld, Tonge, & Florio, 1994) relative to IQ-matched participants with mixed etiology ID.
Although the observation of higher ratings of anxiety, gaze aversion, and avoidance behaviors would seem to be inconsistent with lower ratings of withdrawal and antisocial behaviors, these findings correspond to the clinical descriptions of FXS found in the literature and have aided our characterization of the behavioral phenotype observed in males with FXS. A broad range of social difficulties is observed in males with FXS; in addition, the social/behavioral profile in FXS has been characterized as “a portrait of sharp contrasts (Bailey & Nelson, 1995, pg. 240).” On the one hand, “these individuals generally are perceived to engage in prosocial behavior, desire social relationships, and form social attachments (Bailey & Nelson, 1995, pg. 240).” On the other hand, individuals with FXS frequently present with social/gaze avoidance and anxiety, a behavioral presentation that potentially contributes to the frequent reports of symptom overlap between individuals with FXS and those with nonsyndromic ASD. Thus, this domain of functioning is especially important to examine relative to individuals with nonsyndromic ASD as it may provide insights into the neuropsychological underpinnings of many of the social/affective symptoms of ASD observed in FXS.
This clinical presentation is consistent with existing research from typical development, which demonstrates that although sociability and shyness may be linked, these two factors are distinguishable (Cheek & Buss, 1981); that is, one can demonstrate a preference for/interest in interacting with people but still experience discomfort engaging within the social interaction. Given the interest in elucidating the similarities and differences between the FXS and ASD phenotypes, we are brought to an important empirical question yet to be addressed; namely, what role does anxiety play in the similarities and differences observed between these phenotypes? By comparing the profile of symptoms of anxiety and their interrelations with other domains of functioning, the present study may shed light on whether these symptoms reflect the same underlying mechanisms in these two neurodevelopmental disorders. This information can be used as a first step in exploring the role of anxiety in the development of the FXS and nonsyndromic ASD behavioral phenotypes, thereby potentially shedding light on the similarities and differences in the developmental trajectories of these two behavioral phenotypes.
1.4 Research Questions
In the current study, we examined the presence and profile of psychiatric symptoms in boys with FXS based upon maternal ratings of these symptoms. In addition, we examined whether between-syndrome differences existed in the types of psychiatric symptoms observed when comparing boys with FXS to boys with nonsyndromic ASD. Finally, we investigated the extent to which between-syndromes differences existed in the associations among psychiatric symptom domains as well as in the associations of these symptoms with other aspects of behavioral functioning. It is important to recognize that a number of methodological challenges arise when focusing on comparisons across neurodevelopmental disorders (e.g., Mervis & Klein-Tasman, 2004). For example, when comparing individuals with FXS to those with nonsyndromic ASD, large and significant differences are observed both in nonverbal reasoning ability and in the presence of the core symptoms of autism, with males with FXS demonstrating, on average, more limited nonverbal reasoning ability and better social functioning than males with nonsyndromic ASD (McDuffie et al., in press; Wolff et al., 2013). Additionally, a negative association has been observed between NVIQ and severity of autism symptoms in FXS (Lewis et al., 2006). To control for these differences, and to allow for a more careful characterization of between-group similarities and differences, the present study utilized three alternate matching strategies resulting in subsamples of participants who were matched groupwise on: (a) CA; (b) CA and NVIQ; and, (c) CA, NVIQ, and autism symptom severity. Thus, the current study addressed the following research questions:
For boys with FXS, what is the observed profile of maternal ratings of current psychiatric symptoms across symptom domains?
Are there between-group differences in maternal ratings of current psychiatric symptoms? Do the observed group differences vary according to the matching strategy used?
Do between-group differences exist in the pattern of associations across psychiatric symptom domains? Do the observed group differences vary according to the matching strategy used?
Do between-group differences exist in the concurrent associations between psychiatric symptoms and other child characteristics (i.e., CA, nonverbal reasoning ability, and autism symptom severity)? Do the observed group differences vary according to the matching strategy used?
2. Method
2.1 Participants
Participants were drawn from a larger study of word learning in males with FXS and males with nonsyndromic ASD. Inclusion in the larger study required participants in both diagnostic groups to meet the following criteria: (a) between 4 and 10 years of age, (b) nonverbal mental age between 2;0 and 5;11, (c) native English speaker with parents who are fluent English speakers, (d) speech is the primary means of communication, (e) no sensory or physical impairments that would limit participation in the project (based on parent report), (f) a pure tone, air-conduction threshold of 30 dB HL or better in each ear (averaged across 500, 1000, and 2000 Hz), and (g) lived at home with the biological mother. In addition, participants with FXS were required to provide a report documenting diagnosis of FMR1 full mutation (i.e., >200 CGG repeats, with or without mosaicism). Participants with nonsyndromic ASD were included in the present study if: (1) parents provided evidence documenting that genetic testing had been completed to rule out a diagnosis of FXS; (2) a project physician conducted dysmorphology and neural exam and ruled out other possible syndromic causes of ASD (e.g., Rett’s syndrome, tuberous sclerosis); (3) the participant received a calibrated autism severity score of at least four on the Autism Diagnostic Observation Schedule (ADOS; Gotham, Pickles, & Lord, 2009); and (4) a classification of ASD according to the criteria outlined by Risi et al. (2006) on the Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, A., & Lord, C., 2003). Project staff who had completed research reliability training for these measures administered both the ADOS and the ADI-R. Use of these criteria resulted in 43 participants with FXS and 55 participants with nonsyndromic ASD available for selection in the present study.
Inclusion in the present project required participants to have completed the Leiter International Performance Scale (Leiter), the ADOS, and the Anxiety, Depression, and Mood Scale (ADAMS; all described below). As a result, seven participants (2 FXS, 5 nonsyndromic ASD) were excluded for missing Leiter data and two participants (both nonsyndromic ASD) were excluded for missing ADOS data. For the participants with FXS, those who met these criteria were included as the full sample of participants (Full Sample) in the present study. For the participants with nonsyndromic ASD, a subsample of the 49 participants who met these criteria was selected, utilizing the sampling procedures outlined by Mervis and John (2008), to create the Full Sample group of children with nonsyndromic ASD who matched the Full Sample children with FXS on CA (t(80) = .52, p = .60).
The participants with FXS in the Full Sample were 41 males aged 4.06 – 10.63 years (M = 7.24, SD = 2.04). The racial composition of this sample was 85% White, 7.5% Asian, 2.5% African-American, and 5.0% bi-racial (both children were Native American/White). Of the participants with FXS in the Full Sample, 10% were of Hispanic origin. The 41 participants with nonsyndromic ASD in the Full Sample ranged in age from 4.02 – 10.99 years (M = 7.46, SD = 1.76). The racial composition of this group was 80% White, 5% Asian, 10% African-American, and 5% bi-racial (Native American/White). Of the participant with nonsyndromic ASD in the Full Sample, 12.5% were of Hispanic origin.
As seen in Table 1, although the two diagnostic groups in the Full Sample were well matched on CA, males with FXS demonstrated significantly lower NVIQ standard scores (SS; measured via the Leiter) and autism symptom severity scores (measured via the ADOS) than did the males with nonsyndromic ASD. To address this issue, a subsample of participants was selected (CA-IQ Matched Sample), utilizing the sampling procedures outlined by Mervis and John (2008), to create groups of children with FXS or nonsyndromic ASD matched on both CA and NVIQ SS (n = 30/group). Between-group comparisons demonstrated that the males with FXS in the CA-IQ Matched Sample, however, still earned significantly lower autism symptom severity scores than did the males with nonsyndromic ASD (Table 1). Consequently, a final subsample of participants (CA-IQ-Autism Symptom Matched Sample) was generated, utilizing the Mervis and John (2008) sampling procedures, to create a group of males with FXS and a group of males with nonsyndromic ASD matched on CA, NVIQ SS, and autism symptom severity (n = 16/group). Descriptive statistics regarding CA, NVIQ SS, and autism symptom severity score as well as between-group comparisons on the matching variables for all three samples are presented in Table 1. The utilization of multiple matching procedures when conducting between-group comparisons was designed to allow us to elucidate more fully the similarities and differences between males with FXS and males with nonsyndromic ASD in psychiatric symptoms, with more confidence in findings that emerged regardless of matching strategy.
Table 1.
Descriptive Statistics for Matching Variables as a Function of Sample
| Variables | FXS Mean (SD) | Nonsyndromic ASD Mean (SD) | p-value |
|---|---|---|---|
| Full Sample (FXS: n = 41/group)
|
|||
| CA | 7.17 (2.06) | 7.40 (1.78) | .60 |
| Leiter NVIQ SS | 61.85 (17.45) | 71.73 (18.25) | .01* |
| ADOS Autism Symptom Severity | 5.98 (2.44) | 8.02 (1.48) | <.001** |
|
| |||
| CA-IQ Matched Sample (n = 30/group)
|
|||
| CA | 7.25 (2.17) | 7.53 (1.81) | .600 |
| Leiter NVIQ SS | 64.73 (13.82) | 65.17 (13.91) | .904 |
| ADOS Autism Symptom Severity | 5.80 (2.46) | 8.00 (1.51) | < .001** |
|
| |||
| CA-IQ-Autism Symptom Matched Sample (n = 16/group)
|
|||
| CA | 7.76 (2.38) | 8.03 (1.94) | .726 |
| Leiter NVIQ SS | 58.50 (11.64) | 60.19 (14.82) | .723 |
| ADOS Autism Symptom Severity | 7.19 (1.91) | 7.31 (1.62) | .843 |
p < .01,
p < .001
2.2 Measures
2.2.1 Anxiety, Depression, and Mood Scale (ADAMS, Esbensen, Rojahn, Aman, & Ruedrich, 2003)
The ADAMS is a 28-item informant questionnaire designed to be used as a screener for psychiatric disorders in individuals with intellectual disability. Behaviors are rated on a 4-point Likert scale describing the severity of each problem behavior. Higher scores indicate increased severity of problem. The ADAMS yields five subscale scores: Manic/Hyperactive Behavior, Depressed Mood, Social Avoidance, General Anxiety, and Obsessive/Compulsive Behavior. Biological mothers were the respondents for the present study. The measure was normed on a sample of individuals with intellectual disabilities of a wide age range. The measure has been shown to demonstrate good internal consistency with alpha coefficients ranging from .75 - .83, with a mean of .80.
2.2.2 The Leiter International Performance Scale – Revised (Leiter; Roid & Miller, 1997)
The Leiter is an individually and nonverbally administered standardized measure of nonverbal intelligence that indexes fluid reasoning, visualization, visuospatial memory, and attention. Only the subtests comprising the Brief IQ version of this assessment were administered; namely, Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. Mean IQ for the Leiter standardization sample is 100, with a standard deviation of 15.
2.2.3 Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999)
The ADOS is a semi-structured play-based interaction with an examiner in which the examiner creates specific interactive contexts in which the participant’s social, communication and repetitive behaviors can be observed. The ADOS consists of four modules, each designed for individuals at a particular expressive language level. Calibrated severity scores, which allow comparisons across different ADOS modules, were computed based on the algorithm provided by Gotham, Pickles, and Lord (2009) and served as the metric for autism symptom severity in the present study.
2.3 Procedures
Participants were drawn from a larger study of word learning in males with FXS and males with nonsyndromic ASD. Biological mothers completed several questionnaires including the ADAMS. Children completed a battery of assessments including the Leiter and the ADOS. The entire test battery was administered over the course of two consecutive days.
3. Results
The number of items comprising each subscale varied across the five ADAMS subscales. As such, a mean rating was computed for each subscale and utilized in all analyses. Furthermore, examination of the data indicated that the distributions for mean rating scores for the Manic/Hyperactive Behavior, Depressed Mood, Social Avoidance, and Obsessive/Compulsive Behavior subscales violated the parametric assumption of normality. Non-parametric tests, therefore, were utilized in all analyses.
3.1 Psychiatric Symptom Profile for the Full Sample of Males with FXS
The first research question was focused on whether psychiatric symptoms, as represented by mean rating scores on the five ADAMS subscales, varied significantly across subscales for the Full Sample of males with FXS. Results of a Friedman’s Analysis of Variance (ANOVA) indicated that mean ratings varied significantly as a function of subscale (χ2(4) = 85.97, p < .001). Results of follow-up Wilcoxon tests (αfw = .005) indicated that mean maternal rating on the Manic/Hyperactive Behavior scale was significantly higher than mean rating on all other subscales (rs ≥ .52, ps < .003). In addition, the mean rating on the Depressed Mood scale was significantly lower than the mean ratings for all other subscales (rs ≥ .50, ps < .001). No other significant differences between subscales were observed.
3.2 Between-Group Differences - Presence of Psychiatric Symptoms
The second research question was focused on whether or not differences in psychiatric symptoms were evident between males with FXS and males with nonsyndromic ASD based upon the three matching strategies utilized to assign participants to subsamples. Mann Whitney U tests were used to address this research question in all comparisons.
3.2.1 Full CA matched sample
Results of comparisons for the Full Sample (n = 41/group) indicated that males with FXS earned a significantly higher mean rating on the General Anxiety subscale (U = 1086.5, z = 2.29, p = .02, r = .25) and a significantly lower mean rating on the Obsessive/Compulsive Behavior subscale (U = 607.0, z = -2.18, p = .03, r = .24) than did CA-matched males with nonsyndromic ASD (see Figure 1). In addition, a trend was observed for mean rating on the Manic/Hyperactive Behavior subscale to be higher for males with FXS than for CA-matched males with nonsyndromic ASD, (U = 1050.0, z = 1.95, p = .05, r = .22).
Figure 1.
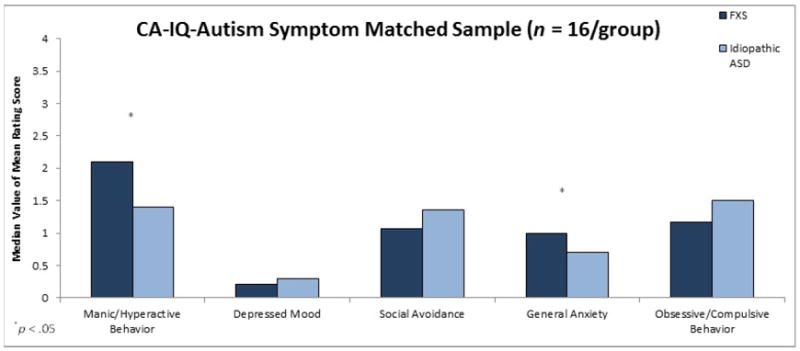
Maternal mean ratings on ADAMS subscales as a function of diagnostic group for Full Sample matched on CA.
3.2.2 CA-NVIQ matched sample
Results of comparisons for the CA-NVIQ Matched Sample (n = 30/group) indicated that males with FXS earned a significantly higher mean rating on both the Manic/Hyperactive Behavior subscale (U = 596.00, z = 2.17, p = .03, r = .28) and the General Anxiety subscale (U = 603.50, z = 2.28, p = .02, r = .29) than did males with nonsyndromic ASD matched on CA and NVIQ (see Figure 2).
Figure 2.
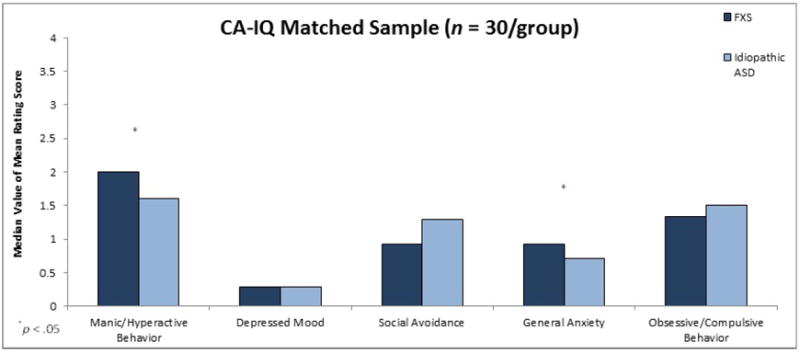
Maternal mean ratings on ADAMS subscales as a function of diagnostic group for CA-NVIQ Matched Sample.
3.2.3 CA-NVIQ-Autism Severity matched sample
Finally, the between-group analyses were conducted for the CA-NVIQ-Autism Symptom Matched Sample (n = 16/group). Results of these comparisons once again showed that that males with FXS earned a significantly higher mean rating for the Manic/Hyperactive Behavior (U = 190.50, z = 2.37, p = .02, r = .42) and the General Anxiety (U = 187.50, z = 2.25, p = .02, r = .40) subscales than did males with nonsyndromic ASD who were matched on CA, NVIQ and autism severity.
3.3 Patterns of Associations between Psychiatric Symptoms: Between Group Differences
The third research question was focused on whether between-group differences were evident in the concurrent associations across the psychiatric symptoms represented by the ADAMS subscales. Using Spearman correlation coefficients, concurrent within-group associations were evaluated as a function of matching strategy. An adjusted significance level was used to control for multiple comparisons (αfw = .01). Between-group comparisons were then conducted to evaluate differences in the strengths of any inter-correlations reaching the adjusted significance level between boys with FXS and boys with nonsyndromic ASD.
3.3.1 Fragile X syndrome
For the Full Sample of males with FXS, there were significant associations between all of the ADAMS subscales with two exceptions. Symptoms of both Social Avoidance and Obsessive/Compulsive behaviors failed to meet the adjusted criterion for a significant association with Manic/Hyperactive symptoms. Strong associations (r > .60) were observed between the mean rating of General Anxiety and the mean ratings of both Depressed Mood and Social Avoidance. These associations remained significantly and strongly associated for the CA-NVIQ matched and the CA-NVIQ-Autism Severity matched samples of males with FXS (see Table 2).
Table 2.
Spearman Correlation Coefficients across ADAMS Subscale Ratings for Males with FXS as a function of Matching Sample
| Depressed Mood | Social Avoidance | General Anxiety | Obsessive/Compulsive Behavior | |
|---|---|---|---|---|
| Full Sample FXS (n = 41) | ||||
| Manic/Hyperactive Behavior | r = .53** | r = .34+ | r = .58** | r = .39+ |
| Depressed Mood | r = .42* | r = .67** | r = .46* | |
| Social Avoidance | r = .69** | r = .43* | ||
| General Anxiety | r = .53** | |||
|
| ||||
| CA-IQ Matched Sample FXS (n = 30) | ||||
| Manic/Hyperactive Behavior | r = .51* | r = .35 | r = .59* | r = .43+ |
| Depressed Mood | r = .50* | r = .71** | r = .47* | |
| Social Avoidance | r = .74** | r = .49* | ||
| General Anxiety | r = .47** | |||
|
| ||||
| CA-IQ-Autism Symptom Matched Sample FXS (n = 16) | ||||
| Manic/Hyperactive Behavior | r = .53+ | r = .46 | r = .64* | r = .53+ |
| Depressed Mood | r = .74* | r = .91** | r = .36 | |
| Social Avoidance | r = .81** | r = .44 | ||
| General Anxiety | r = .50+ | |||
p < .05,
p < .01,
p < .001
3.3.2 Nonsyndromic Autism Spectrum Disorder
For the Full Sample of males with nonsyndromic ASD, the associations between Manic/Hyperactive Behavior and both Social Avoidance and Obsessive/Compulsive behavior reached criterion for significance (αfw = .01). In addition, significant associations were observed between Depressed Mood and General Anxiety and between Social Avoidance and Obsessive/Compulsive behavior. These associations remained significantly associated, with moderate to strong strength (rs >.50), in the CA-NVIQ matched sample. The only significant association that remained in the CA-NVIQ-Autism Severity matched sample was that between mean ratings of Social Avoidance and Obsessive/Compulsive Behavior (see Table 3).
Table 3.
Spearman Correlation Coefficients across ADAMS Subscale Ratings for Males with Nonsyndromic ASD as a function of Matching Sample
| Depressed Mood | Social Avoidance | General Anxiety | Obsessive/Compulsive Behavior | |
|---|---|---|---|---|
| Full Sample Nonsyndromic ASD (n = 41) | ||||
| Manic/Hyperactive Behavior | r = .27 | r = .52* | r = .39+ | r = .56** |
| Depressed Mood | r = .35+ | r = .55** | r = .32+ | |
| Social Avoidance | r = .15 | r = .54** | ||
| General Anxiety | r = .28 | |||
|
| ||||
| CA-IQ Matched Sample Nonsyndromic ASD (n = 30) | ||||
| Manic/Hyperactive Behavior | r = .12 | r = .54* | r = .38+ | r = .61** |
| Depressed Mood | r = .37+ | r = .58* | r = .36 | |
| Social Avoidance | r = .21 | r = .66** | ||
| General Anxiety | r = .30 | |||
|
| ||||
| CA-IQ-Autism Symptom Matched Sample Nonsyndromic ASD (n = 16) | ||||
| Manic/Hyperactive Behavior | r = -.16 | r = .50+ | r = .55+ | r = .43 |
| Depressed Mood | r = .39 | r = .12 | r = .45 | |
| Social Avoidance | r = .18 | r = .66* | ||
| General Anxiety | r = .28 | |||
p < .05,
p < .01,
p < .001
3.3.3 Between-Syndrome Comparisons
A Fisher r-to-z transformation was used to determine if significant between-syndrome differences were observed in the strengths of association between different psychiatric symptom domains. Between-group comparisons were only conducted in instances where discrepant associations were observed; that is, in instances where (a) an association between psychiatric symptom domains met a p-value adjusted for multiple significance tests (p < .01) for one diagnostic group; and (b) failed to meet an unadjusted criteria for a significant association (p < .05) in the other diagnostic group. In this way, we strategically restricted the number of potential comparisons to include only those in which the pattern of association was most marked in one group and was weak in the comparison group.
In the comparison utilizing the Full Samples, a significant between-group difference in association strength was observed in only one comparison: the correlation between General Anxiety and Social Avoidance was significantly stronger for males with FXS than it was for males with nonsyndromic ASD (z = 3.04, p = .002). In the comparison utilizing the CA-NVIQ matched sample, as observed in the previous matching sample, the correlation between General Anxiety and Social Avoidance was significantly stronger for males with FXS than it was for males with nonsyndromic ASD (z = 2.71, p = .003). Finally, in the comparison utilizing the CA-NVIQ-Autism Severity matching sample two significant between-group differences in association strength were observed. First, as observed in the previous matching samples, the correlation between General Anxiety and Social Avoidance was significantly stronger for males with FXS than it was for males with nonsyndromic ASD (z = 2.41, p = .016). In addition, the correlation between General Anxiety and Depressed Mood was significantly stronger in males with FXS than it was for males with nonsyndromic ASD (z = 3.59, p < .001).
3.4 Developmental Correlates of Psychiatric Symptoms
The fourth research question was focused on concurrent correlations between maternal ratings of psychiatric symptoms and child characteristics; specifically, CA, NVIQ, and autism symptom severity. Concurrent associations, as a function of matching subsample, were first examined within each diagnostic group. Analyses were then conducted to determine if between-syndrome differences existed in the strengths of association across all matching samples.
3.4.1 Fragile X Syndrome
As seen in Table 4, none of the associations between CA, NVIQ, and autism symptom severity and the ADAMS subscales met the study criterion for a significant association in any of the matching samples.
Table 4.
Spearman Correlation Coefficients between CA, NVIQ, and Autism Symptom Severity and ADAMS Subscale Ratings for Males with FXS as a function of Matching Sample
| ADAMS Subscale | CA | Leiter NVIQ SS | ADOS Autism Symptom Severity |
|---|---|---|---|
| Full Sample FXS (n = 41) | |||
| Manic/Hyperactive Behavior | r = .28 | r = -.26 | r = .28 |
| Depressed Mood | r = .20 | r = .05 | r = -.03 |
| Social Avoidance | r = .17 | r = -.19 | r = .19 |
| General Anxiety | r = .32 | r = -.08 | r = .20 |
| Obsessive/Compulsive Behavior | r = .20 | r = .14 | r = -.02 |
|
| |||
| CA-IQ Matched Sample FXS (n = 30) | |||
| Manic/Hyperactive Behavior | r = .22 | r = -.30 | r = .15 |
| Depressed Mood | r = .19 | r = -.06 | r = -.05 |
| Social Avoidance | r = .25 | r = -.23 | r = .36 |
| General Anxiety | r = .31 | r = -.25 | r = .36 |
| Obsessive/Compulsive Behavior | r = .17 | r = -.14 | r = .18 |
|
| |||
| CA-IQ-Autism Symptom Matched Sample FXS (n = 16) | |||
| Manic/Hyperactive Behavior | r = .36 | r = -.17 | r = .26 |
| Depressed Mood | r = .30 | r = -.10 | r = .05 |
| Social Avoidance | r = .26 | r = -.16 | r = .16 |
| General Anxiety | r = .21 | r = -.02 | r = .22 |
| Obsessive/Compulsive Behavior | r = .16 | r = -.08 | r = .17 |
p < .01
3.4.2 Nonsyndromic Autism Spectrum Disorder
As seen in Table 5, none of the associations between CA, NVIQ, and autism symptom severity and the ADAMS subscales met study criterion for a significant association in any of the matching samples.
Table 5.
Spearman Correlation Coefficients between CA, NVIQ, and Autism Symptom Severity and ADAMS Subscale Ratings for Males with Nonsyndromic ASD as a function of Matching Sample
| ADAMS Subscale | CA | Leiter NVIQ SS | ADOS Autism Symptom Severity |
|---|---|---|---|
| Full Sample Nonsyndromic ASD (n = 41) | |||
| Manic/Hyperactive Behavior | r = -.21 | r = -.14 | r = .22 |
| Depressed Mood | r = .11 | r = -.27 | r = .12 |
| Social Avoidance | r = -.12 | r = -.31 | r = .27 |
| General Anxiety | r = .07 | r = .01 | r = -.04 |
| Obsessive/Compulsive Behavior | r = -.20 | r = -.26 | r = .03 |
|
| |||
| CA-IQ Matched Sample Nonsyndromic ASD (n = 30) | |||
| Manic/Hyperactive Behavior | r = -.17 | r = -.07 | r = .11 |
| Depressed Mood | r = .17 | r = -.25 | r = .12 |
| Social Avoidance | r = -.22 | r = -.01 | r = .05 |
| General Anxiety | r = .22 | r = -.02 | r = -.07 |
| Obsessive/Compulsive Behavior | r = -.23 | r = -.29 | r = -.03 |
|
| |||
| CA-IQ-Autism Symptom Matched Sample FXS (n = 16) | |||
| Manic/Hyperactive Behavior | r = .003 | r = .11 | r = -.12 |
| Depressed Mood | r = .26 | r = -.56 | r = .06 |
| Social Avoidance | r = -.22 | r = .02 | r = .10 |
| General Anxiety | r = .21 | r = -.12 | r = -.22 |
| Obsessive/Compulsive Behavior | r = -.10 | r = -.09 | r = .00 |
p < .01
3.4.3 Between-Syndrome Comparisons
Across all three matching samples, none of the tested associations met study criterion for a significant association; therefore, there was no need examine between-syndrome differences in association strength.
4. Discussion
The overall aim of the current study was to contribute to a more nuanced understanding of how profiles of psychiatric symptoms may be shared or differ between boys with FXS, the leading inherited cause of intellectual disability, and boys with nonsyndromic ASD. These two syndromes are often considered to have substantial behavioral overlap given that the vast majority of boys with FXS also display symptoms that are diagnostic of ASD. Additionally, individuals with either diagnosis are at increased risk of presenting with other psychiatric symptoms. Investigation of the similarities and differences in symptoms of psychopathology and in their interrelations across domains may shed light on the psychological/neural underpinnings of shared symptomatology in these two disorders.
4.1 Psychiatric Profiles for Males with FXS
The first aim of this study was to examine the presence and profile of psychiatric symptoms in boys with FXS, based on maternal report. Our results indicated that manic/hyperactive behaviors were the most problematic area of psychiatric symptoms reported for these boys. Manic/Hyperactive behaviors were reported more frequently than symptoms of depressed mood or symptoms in the domains of obsessive/compulsive behaviors, general anxiety, or social avoidance, which did not differ significantly from one another. Finally, symptoms of depressed mood were the least likely to be reported for boys with FXS.
These results are in general agreement with the observational research literature describing the behavioral characteristics of boys with FXS. This literature reveals that these individuals face challenges arising from inattention and hyperactivity, anxiety, and socially avoidant behaviors (e.g., Baumgardner et al., 1995; Bregman, Leckman, & Ort, 1988; Cordeiro et al., 2011; Cornish, Scerif, & Karmiloff-Smith, 2007; Kau et al., 2000; Scerif et al., 2012; Turk, 1998). As has been proposed for children without intellectual disability, it seems likely that behavioral challenges resulting from inattention and hyperactivity in males with FXS would have serious downstream consequences for developmental outcomes by negatively impacting the ability of these males to learn from the environment (e.g., Blackman, Westervelt, Stevenson, & Welch, 1991; Normandeau & Guay, 1998) and to successfully navigate social interactions (e.g., DuPaul, McGoey, Eckert, & VanBrakle, 2001; Kingery, Erdley, Marshall, Whitaker, & Reuter, 2010).
4.2 Between-Group Differences in Maternal Ratings of Psychiatric Symptoms
Prior research indicates that behaviors typically associated with the presence of an ASD are frequently observed in individuals with FXS (e.g., Baumgardner et al., 1995; Bailey, Hatton, Mesibov, Ament, & Skinner, 2000; Hartley et al., 2001; Rogers, Wehner, & Hagerman, 2001). In addition, we know that psychiatric symptoms, particularly inattention/hyperactivity, are frequently observed in individuals with nonsyndromic ASD (e.g., Leyfer et al., 2006; Simonoff et al., 2008). Given these observations, comparison of psychiatric symptoms between boys with FXS and boys with nonsyndromic ASD can provide insight as to where the boundaries lie between these two phenotypes.
In the current study, comparisons utilizing participant groups matched on CA provided information regarding the similarities and differences between boys with FXS and those with nonsyndromic ASD in samples that were representative of the larger populations of each disorder. This between-group analysis indicated that, when matched on CA, symptoms of general anxiety and manic/hyperactive behavior were more frequently reported by mothers of boys with FXS than by mothers of boys with nonsyndromic ASD. Conversely, obsessive-compulsive behaviors were more frequently reported by mothers of boys with nonsyndromic ASD. Thus, the full sample comparison begins to reveal important differences in the behavioral phenotypes that characterize the two disorders. These distinctions confirm the descriptive literature in terms of the presence of hyperactivity and inattention and general anxiety as defining features of FXS (e.g., Baumgardner et al., 1995; Bregman, Leckman, & Ort, 1988; Cordeiro et al., 2011; Cornish, Scerif, & Karmiloff-Smith, 2007; Kau et al., 2000; Scerif et al., 2012; Turk, 1998).
In the process of thoroughly examining between-group differences in psychiatric symptoms, however, two additional phenotypic characteristics must be considered. First, as seen in the current sample, large and significant differences are observed between boys with FXS and boys with nonsyndromic ASD with regard to nonverbal cognitive ability, with boys with FXS demonstrating more limited ability than do boys with nonsyndromic ASD. Second, boys with FXS in the current study demonstrated milder symptoms of autism, on average, than did boys with nonsyndromic ASD, although there was considerable overlap in the range of these symptoms across the full participant sample.
Between-group differences in nonverbal cognitive ability and autism symptomology may confound the interpretation of any other potential group differences. To address this problem, the present study utilized two additional matching strategies to facilitate a more careful consideration of how profiles of psychiatric symptoms may be shared or may differ between boys with FXS and boys with nonsyndromic ASD. Specifically, the first strategy controlled for differences in NVIQ while the second controlled for both NVIQ and autism symptom severity.
Interestingly, across all group-matching strategies, mothers of boys with FXS more frequently reported symptoms of general anxiety and manic/hyperactive behaviors than did mothers of boys with nonsyndromic ASD. The finding of robust between-group differences in these two behavioral domains supports the conclusion that inattention and hyperactivity as well as anxiety are more likely to characterize the behavioral phenotype of boys with FXS even when comparing boys with FXS to boys with nonsyndromic ASD who have the same level of nonverbal ability and severity of autism symptoms. One can speculate on the downstream developmental effects of these two characteristics, which are likely to adversely affect the ways in which boys with FXS interact with objects and people and learn from their social and physical environments over time. That is, boys who -- from early in development -- have fewer or briefer opportunities to engage with and learn from their surroundings and social partners because they are inattentive, hyperactive, and anxious, are likely to display cumulative deficits in both conceptual and social/affective learning over time. As such, these between-syndrome differences may be important in terms of understanding how the behavioral phenotype of FXS emerges and to what extent the developmental trajectory overlaps with that of individuals with diagnoses of nonsyndromic ASD. Of course, longitudinal data are needed to fully address cause and effect relationships.
4.3 Patterns of Associations
The remaining aims of our study focused on exploring patterns of within-group associations. Our analyses revealed similar patterns of findings regardless of the matching strategy used. No significant associations were observed, in either diagnostic group, between psychiatric symptom domains and CA, NVIQ, or autism symptom severity across all three matching samples. With regard to correlations between psychiatric symptom domains for boys with FXS, the strongest patterns of association (generally rs > .60) were observed for maternal reports of general anxiety with all other psychiatric symptom domains. For boys with nonsyndromic ASD, the strongest patterns of significant association (generally, rs > .50) were observed between maternal ratings of social avoidance and obsessive/compulsive behaviors, and between general anxiety and depressed mood. Additionally, the correlations between social avoidance and general anxiety were strong and significant for all samples of boys with FXS, but these same associations failed to reach significance for any of the samples with nonsyndromic ASD. Moreover, the positive association between social avoidance and general anxiety was significantly stronger in males with FXS than it was for males with nonsyndromic ASD, and this significant between-group difference was observed across all of the matched samples. Interestingly, only ratings of general anxiety, and not social avoidance, were observed to differ significantly between males with FXS and males with nonsyndromic ASD. One may speculate that social avoidance, as measured by the ADAMS, represents a different underlying construct in FXS than in nonsyndromic ASD. Specifically, we hypothesize that social anxiety underlies social avoidance in FXS but that some other construct contributes to social avoidance in nonsyndromic ASD.
Some additional support for this hypothesis may be found in a study assessing the construct validity of the ADAMS in a group of individuals with FXS, which included both males and females of varying ages (M = 12.78 years; range: 5.00 – 33.30 years; Cordeiro et al. 2010). These researchers compared the ADAMS with the Anxiety Disorders Interview Schedule for DSM-IV (ADIS; Silverman & Albano, 2004), a semi-structured parent interview designed to differentially diagnose anxiety disorders and assess their severity in children and adolescents. Results indicated that the ADAMS Social Avoidance subscale was significantly associated with DSM-IV symptoms of two social anxiety disorders (i.e., Social Phobia and Selective Mutism) as measured by the ADIS. Conversely, the ADAMS General Anxiety subscale was significantly correlated with multiple anxiety disorders, both social and nonsocial, as measured by the ADIS. That is, for individuals with FXS, the ADAMS domain of General Anxiety seems to represent a less well-defined group of anxiety symptoms, whereas the Social Avoidance domain of the ADAMS maps closely onto two specific DSM-IV diagnoses, both representing social anxiety disorders. However, there are no currently published studies examining the construct validity of the ADAMS in a group of individuals with nonsyndromic ASD. In contrast to the findings for individuals with FXS, we speculate that the ADAMS Social Avoidance subscale does not affiliate closely with social anxiety disorders for individuals with nonsyndromic ASD, but rather maps onto another disorder, possibly characterized by obsessive-compulsive behaviors. The findings of the current study and our own clinical impressions support this proposal.
These findings highlight an important consideration in the debate on how to interpret symptoms of ASD in FXS. When individuals experience anxiety within social interactions, they are likely to behave in ways that often interfere with social interactions, such as talking less, making less eye contact, and engaging in more nervous gestures (Cheek & Buss, 1981). Given the increased rates of anxiety in FXS relative to children with nonsyndromic ASD, and the interrelation between anxiety and social avoidance, which was observed in FXS but not in nonsyndromic ASD, we can speculate as to the extent to which these differences in anxiety influence other the similarities and differences observed between the two phenotypes. In particular, some of the symptoms typically thought to indicate autistic symptomatology in FXS may actually be attributed to general anxiety. This finding seems to correspond to our clinical impression of a qualitative difference in social impairment in FXS relative to ASD and our empirical findings of less impairment in skills such as smiling to share enjoyment and use of communicative gestures for boys with FXS relative to age and ability matched boys with nonsyndromic ASD (McDuffie et al., in press). Continued investigation of this line of research is important; not only for understanding both disorders, but also for understanding if the expectation of finding targeted treatments to be similarly efficacious across the two disorders is reasonable.
4.4 Conclusions and Future Directions
To date, most comparisons of the behavioral phenotypes associated with FXS and with nonsyndromic ASD have focused on the behaviors identified as being central to a diagnosis of autism and the prevalence of an ASD diagnosis among those with FXS. Moreover, many studies have excluded individuals with FXS who met criteria for an ASD diagnosis and other studies have compared individuals based only on categorical criteria. Although males with FXS may display symptoms of ASD, they also may differ in important ways from individuals with nonsyndromic ASD. Furthermore, as functioning in one cognitive or psychosocial domain can, in interaction with other factors, influence functioning in another domain to shape the final developmental outcome (e.g., Fidler, Lunkenheirmer, & Hahn, 2011; Karmiloff-Smith, 1998); these differences may be key in understanding whether similar behavioral features have different developmental origins in FXS and nonsyndromic ASD. Thus, investigations focused on documenting the similarities and differences that exist between FXS and nonsyndromic ASD in aspects of functioning other than simply symptoms of autism are needed to demarcate the boundaries between these two neurodevelopmental disorders and their underlying psychological and neural mechanisms.
In the present study, we examined the presence and profile of psychiatric symptoms in boys with FXS based upon maternal ratings. In addition, we examined whether between-group differences existed in the types of psychiatric symptoms observed when comparing boys with FXS to boys with nonsyndromic ASD. Finally, we investigated the extent to which between-syndrome differences existed in the associations across psychiatric symptom domains as well as the associations of these symptoms with other aspects of behavioral functioning. Results indicated that manic/hyperactive behaviors were the most problematic area of psychiatric symptoms reported for boys with FXS. When compared to boys with nonsyndromic ASD, symptoms of manic/hyperactive behaviors and general anxiety were significantly more likely to be reported by mothers of boys with FXS than by mother of boys with nonsyndromic ASD, regardless of the matching strategy used to control for CA, nonverbal cognitive ability, and/or autism symptoms. In addition, our results indicated a positive association between social avoidance and general anxiety in FXS that was significantly stronger than this same association for males with nonsyndromic ASD, again across all of our matching samples. These findings suggest that anxiety is a key factor inhibiting or impeding interpersonal functioning in boys with FXS and raises the possibility that this may be a key difference in the development of the respective phenotypes of FXS and nonsyndromic ASD. These findings also suggest that different treatments may ultimately be needed to treat the social impairments of individuals with FXS and individuals with ASD.
When first developed, the factor structure of the ADAMS was determined based upon performance of a large group of individuals with IDD attributable a wide variety of etiologies. It is not yet known whether this same factor structure would continue to emerge based upon the performance of more homogeneous groups of individuals with IDD (i.e., with a single diagnosis or genetic cause for their condition). Additionally, the construct validity of the original ADAMS subscales has been evaluated for individuals with FXS using the ADIS (Cordeiro et al., 2011), such an examination of has not be conducted for individuals with nonsyndromic ASD and is needed to evaluate our hypothesis that the ADAMS Social Avoidance scale represents a different underlying constellation of behaviors in boys with nonsyndromic ASD than it does in boys with FXS.
In addition, it is important for future studies to consider the role of anxiety in the development of the FXS behavioral phenotype, particularly in identifying the role of anxiety in the development of social cognition and its influence on the interpersonal functioning of boys with FXS. Furthermore, recent research has implicated the GABAergic neurotransmitter system in the expression of anxiety disorders (Lydiard, 2002). Additionally, mouse models of FXS have demonstrated down regulation in the GABAA signaling system (D’Hulst et al., 2008; Olmos-Serrano et al., 2010). Based upon these suggestive findings, future studies should explore the similarities and differences in the GABA signaling systems between FXS and nonsyndromic ASD.
Finally, the present study utilized a parent report measure of psychiatric symptoms. Although this measure provides quantifiable information regarding the presence and severity of behaviors, it was not designed to evaluate DSM symptoms for specific diagnoses. Future studies should evaluate whether the same pattern of findings is observed utilizing DSM-based interviews to measure psychiatric symptoms. Finally, due to the differences observed between males and females with FXS in terms of prevalence as well as severity of affectedness, the present study focused on only males. Future studies should evaluate whether the same pattern of findings are observed in females with FXS or nonsyndromic ASD.
Figure 3.
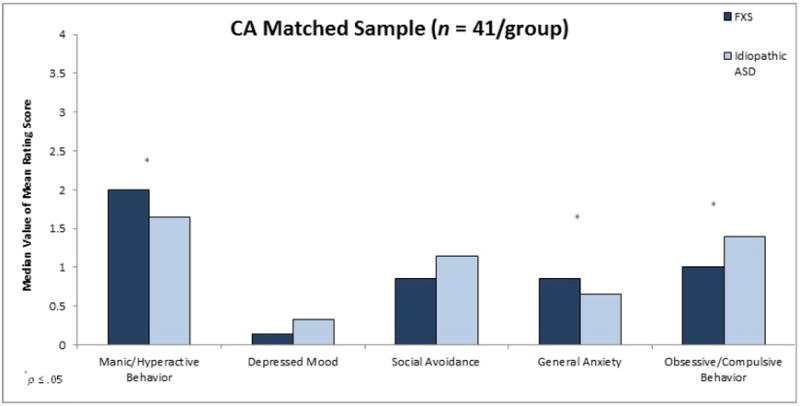
Maternal Mean ratings on ADAMS subscales as a function of diagnostic group for CA-NVIQ-Autism Symptom Severity Matched Sample.
Highlights.
We compare parental report of psychiatric symptoms in boys with fragile X syndrome or nonsyndromic autism spectrum disorder.
Use of multiple matching strategies allowed for a more careful consideration of between group similarities and differences.
Ratings of hyperactivity and anxiety were higher in fragile X syndrome than in nonsyndromic autism spectrum disorder.
Social avoidance was related to general anxiety in fragile X syndrome but not nonsyndromic autism spectrum disorder.
Acknowledgments
This research was supported by grant R01 HD054764 from the National Institute of Child Health and Human Development. We wish to thank the children and their families for their participation in this study. We also thank Sara Kover, Ashley Oakes, David Benjamin, Susan Harris, Beth Goodlin-Jones, Claire Hauser, Sara Lifson, Eileen Haebig, and Cecilia Compton for assisting with data collection and Susen Schroeder for coordinating all study visits. Leonard Abbeduto has received financial support to develop and implement outcome measures for fragile X syndrome clinical trials from F. Hoffman-LaRoche, Ltd., Roche TCRC, Inc., and Neuren Pharmaceuticals Limited. Randi J. Hagerman has received funding from Novartis, Roche Pharmaceuticals, Curemark, Forest, and Seaside Therapeutics to carry out treatment studies in FXS and autism.
Footnotes
No other authors have financial disclosures to make.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist and revised Revised Child Behavior Profile. Burlington, VT: Queen City Printers; 1983. [Google Scholar]
- Aman MG, Singh NN. Aberrant Behavior Checklist-Community Supplementary Manual. E. Aurora, NY: Slosson Educational Publications; 1994. [Google Scholar]
- Bagni C, Tasonne F, Neri G, Hagerman R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. American Society for Clinical Investigation. 2012;122:4314–4322. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30:49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Nelson D. The nature and consequences of fragile X syndrome. Mental Retardation and Developmental Disability Research Reviews. 1995;4:238–244. [Google Scholar]
- Bailey DB, Jr, Roberts JE, Hooper SR, Hatton DD, Mirrett PL, Roberts JE, Scaaf JM. Research on fragile X syndrome and autism: Implications for the study of genes, environments, and developmental language disorders. In: Rice ML, Warren SF, editors. Developmental Language Disorders: From Phenotypes to Etiologies. Mahwah, NJ: Lawrence Erlbaum; 2004. pp. 121–150. [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardner TL, Reiss AL, Freund LS, Abrams MT. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics. 1995;95:744–752. [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Hagerman RJ, et al. Effects of STX209 (Arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: A randomized, controlled, phase 2 trial. Science in Translational Medicine. 2012;4:152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses. Annual Review of Neuroscience. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman JA, Westervelt VD, Stevenson R, Welch A. Management of preschool children with Attention Deficit-Hyperactivity Disorder. Topics in Early Childhood Special Education. 1991;11:91–104. [Google Scholar]
- Bregman JD, Leckman JF, Ort SI. Fragile X syndrome: Genetic predisposition to psychopathology. Journal of Autism and Developmental Disorders. 1988;18:343–354. doi: 10.1007/BF02212191. [DOI] [PubMed] [Google Scholar]
- Cheek JM, Buss AH. Shyness and sociability. Journal of Personality and Social Psychology. 1981;41:330–339. [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, Nijs PFA, Verheij F. High rates of psychiatric co-morbidity in PDD-NOS. Journal of Autism and Developmental Disorders. 2007;37:877–886. doi: 10.1007/s10803-006-0215-x. [DOI] [PubMed] [Google Scholar]
- Dekker MC, Koot HM. DSM-IV disorders in children with borderline to moderate intellectual disability. I: Prevalence and impact. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:915–922. doi: 10.1097/01.CHI.0000046892.27264.1A. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Heulens I, Brouwer JR, Willemson R, De Geest N, Reeve SP, Kooy RF, et al. Expression of GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Research. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- Dölen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacology & Therapeutics. 2010;127:78–93. doi: 10.1016/j.pharmthera.2010.02.008. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, McGoey KE, Eckert TL, VanBrakle J. Preschool children with Attention-Deficit/Hyperactivity Disorder: Impairments in behavioral, social, and social functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, Durbin-Johnson B, Hagerman RJ, Tassone F, et al. High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. American Journal of Medical Genetics. 2013;161:1897–1903. doi: 10.1002/ajmg.a.36023. [DOI] [PubMed] [Google Scholar]
- Einfeld S, Hall W, Levy F. Hyperactivity and fragile X syndrome. Journal of Abnormal Child Psychology. 1991;19:253–262. doi: 10.1007/BF00911230. [DOI] [PubMed] [Google Scholar]
- Einfeld SL, Tonge BJ, Florio T. Behavioural and emotional disturbance in fragile X syndrome. American Journal of Medical Genetics. 1994;51:386–391. doi: 10.1002/ajmg.1320510417. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, Ruedrich S. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders. 2003;33:617–629. doi: 10.1023/b:jadd.0000005999.27178.55. [DOI] [PubMed] [Google Scholar]
- Fidler DJ, Lunkenheimer E, Hahn L. Emerging behavioral phenotypes and dynamic systems theory. International Review of Research on Developmental Disabilities. 2011;40:17–42. [Google Scholar]
- Fryns JP, Jacobs J, Kleczkowska A, van den Berghe H. The psychological profile of the fragile X syndrome. Clinical Genetics. 1984;25:131–134. doi: 10.1111/j.1399-0004.1984.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Gallagher A, Hallahan B. Fragile X-associated disorders: A clinical overview. Journal of Neurology. 2012;259:401–413. doi: 10.1007/s00415-011-6161-3. [DOI] [PubMed] [Google Scholar]
- Gevik E, Eldevik S, Fjæran-Granum T, Sponheim E. Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41:761–769. doi: 10.1007/s10803-010-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan CK, Hagerman RJ. Targeted treatments in autism and fragile X syndrome. Research in Autism Spectrum Disorders. 2012;6:1311–1320. doi: 10.1016/j.rasd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatments. In: Denman RB, editor. Modeling Fragile X Syndrome. New York: Springer; 2012. pp. 297–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Hagerman RJ, et al. Autism profiles of males with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, Bailey DB., Jr Exploring the adult life of men and women with fragile X syndrome: Results from a national survey. American Journal of Intellectual and Developmental Disorders. 2011;1:16–35. doi: 10.1352/1944-7558-116.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indah Winarni T, Chonchaiya W, Adams E, Au J, Rivera SM, Nguyen DV, Hagerman RJ. Sertraline may improve language developmental trajectory in young children with fragile X syndrome: A retrospective chart review. Autism Research and Treatment, 2012. 2012:8. doi: 10.1155/2012/104317. article ID 104317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Gomez-Mancilla B, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Science in Translational Medicine. 2011;3:64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kau ASM, Reider EE, Payne L, Meyer WA, Freund L. Early behavioral signs of psychiatric phenotypes in fragile X syndrome. American Journal on Mental Retardation. 2000;105:266–299. doi: 10.1352/0895-8017(2000)105<0286:EBSOPP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kingery JN, Erdley CA, Marshall KC, Whitaker KG, Reuter TR. Peer experiences of anxious and socially withdrawn youth: An integrative review of the developmental and clinical literature. Clinical Child and Family Psychology Review. 2010;13:91–128. doi: 10.1007/s10567-009-0063-2. [DOI] [PubMed] [Google Scholar]
- Krug DA, Arick JR, Almond PJ. Autism Behavior Checklist. Austin, TX: Pro-Ed; 1993. [Google Scholar]
- Leigh MJ, Nguyen DV, Mu Y, Winarni TI, Schneider A, Chechi T, Hagerman RJ, et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2013;34:147–155. doi: 10.1097/DBP.0b013e318287cd17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, Schroeder S. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research. 2006;50:532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Lainhart JE, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Woodruff-Borden J, Mervis CB. Anxiety disorders in children with Williams syndrome, their mothers, and their siblings: Implications for the etiology of anxiety disorders. Journal of Neurodevelopmental Disorders. 2009;1:4–14. doi: 10.1007/s11689-009-9003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorders. The Journal of Clinical Psychiatry. 2002;64:21–27. [PubMed] [Google Scholar]
- Mattila M-L, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, Moilanen I, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: A community- and clinic-based study. Journal of Autism and Developmental Disorders. 2010;40:1080–1093. doi: 10.1007/s10803-010-0958-2. [DOI] [PubMed] [Google Scholar]
- Mazzocco MMM. Advances in research on the fragile X syndrome. Mental Retardation and Developmental Disabilities Research Review. 2000;6:96–106. doi: 10.1002/1098-2779(2000)6:2<96::AID-MRDD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, Abbeduto L. Current symptoms of autism in males with fragile X syndrome. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-013-2013-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, John AE. Vocabulary abilities in children with Williams syndrome: Strengths, weaknesses, and relation to visuospatial construction ability. Journal of Speech, Language, and Hearing Research. 2008;51:967–982. doi: 10.1044/1092-4388(2008/071). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Methodological issues in group-matching designs: α levels for control variable comparisons and measurement characteristics of control and target variables. Journal of Autism and Developmental Disorders. 2004;34:7–17. doi: 10.1023/b:jadd.0000018069.69562.b8. [DOI] [PubMed] [Google Scholar]
- Normandeau S, Guay F. Preschool behavior and first-grade school achievement: The mediational role of cognitive self-control. Journal of Educational Psychology. 1998;90:111–121. [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufman WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. The Journal of Neuroscience. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, Willemson R. A fragile X balance: FMR1 expression levels. Human Molecular Genetics. 2003;12:249–257. doi: 10.1093/hmg/ddg298. [DOI] [PubMed] [Google Scholar]
- Pollack A. An experimental drug’s bitter end. The New York Times. 2013 Jun 6; http://www.nytimes.com/2013/06/07/business/an-experimental-drugs-bitter-end.html?pagewanted=all&_r=0.
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH, Jr, Leventhal BL, Pickles A, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner E, Hagerman R. The behavioral phenotype in fragile X: Symptoms in very young children with fragile x syndrome, nonsyndromic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scales-Revised. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufman WE, Hessl D, et al. Psychometric study of the Aberrant Behavior Checklist in fragile X syndrome and implications for targeted treatment. Journal of Autism and Developmental Disorders. 2012;42:1377–1392. doi: 10.1007/s10803-011-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Delineation of early attentional control difficulties in fragile X syndrome: Focus on neurocomputational changes. Neuropsychologia. 2007;45:1889–1898. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, Cornish K. Attention across modalities as a longitudinal predictor of early outcomes: The case of fragile X syndrome. Journal of Child Psychology and Psychiatry. 2012;53:614–650. doi: 10.1111/j.1469-7610.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- Schaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Regier DA, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the epidemiology of child and adolescent mental disorders study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule. San Antonio: Graywind, a Division of the Psychological Corporation; 20041996. [Google Scholar]
- Siminoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Turk J. Fragile X syndrome and attentional deficits. Journal of Applied Research in Intellectual Disabilities. 1998;11:175–191. [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1324–1332. doi: 10.1016/j.jaac.2012.09.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


