Abstract
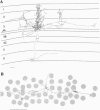
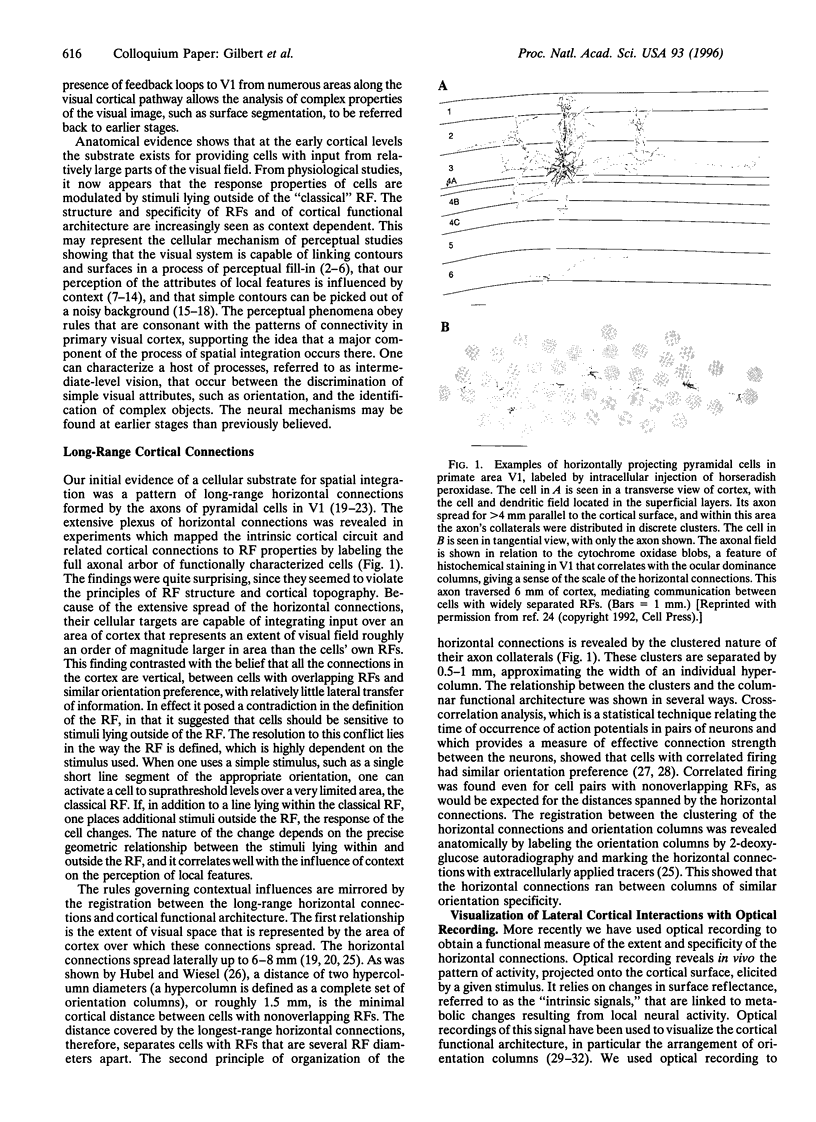
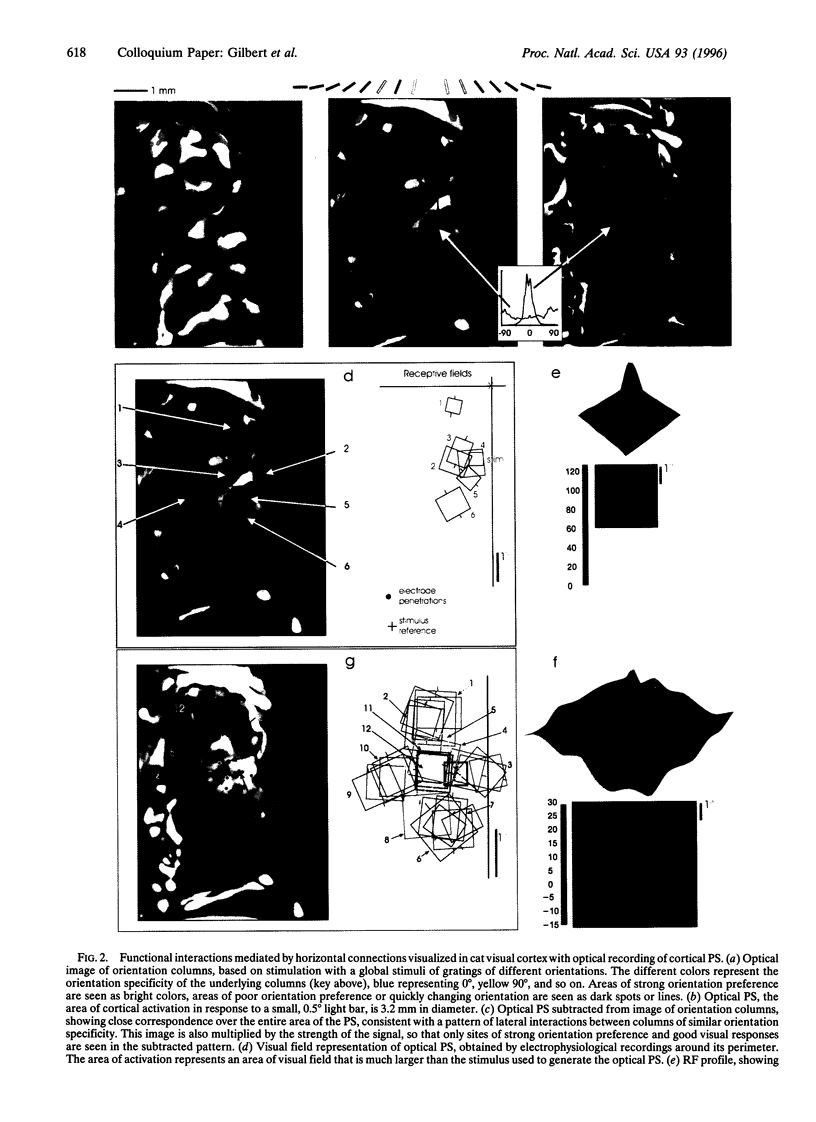
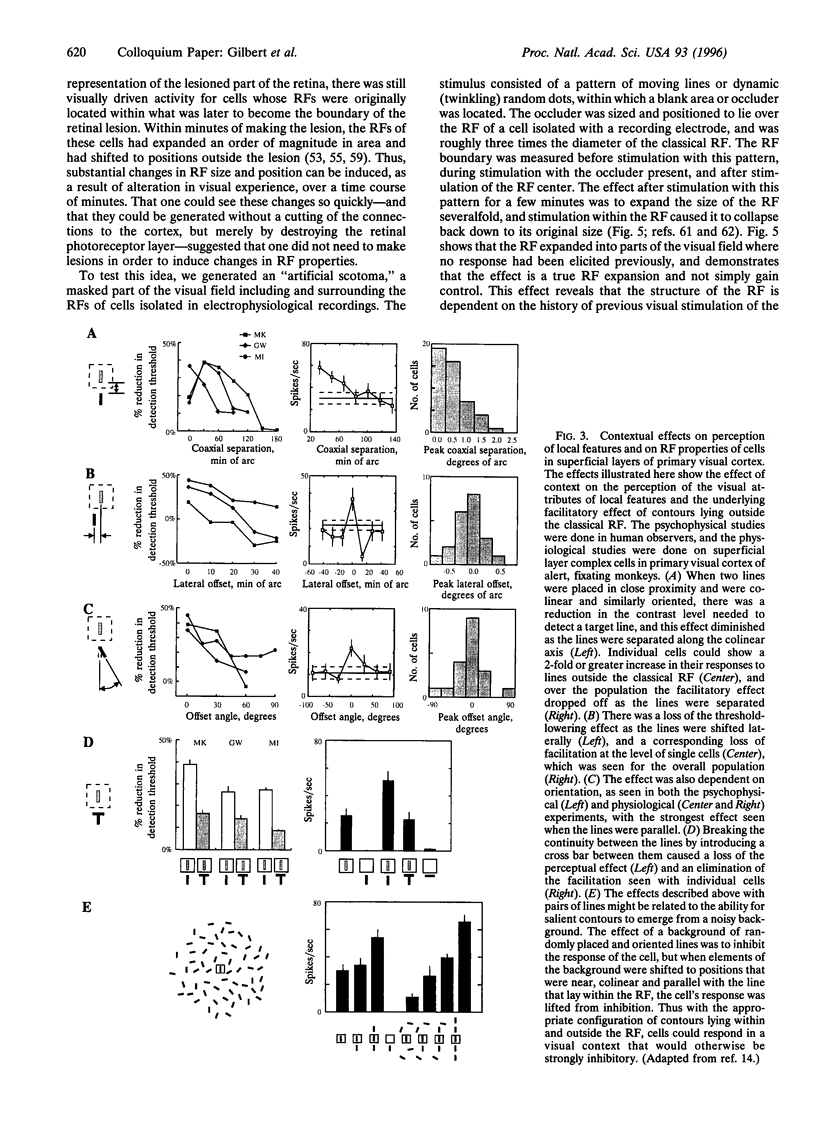
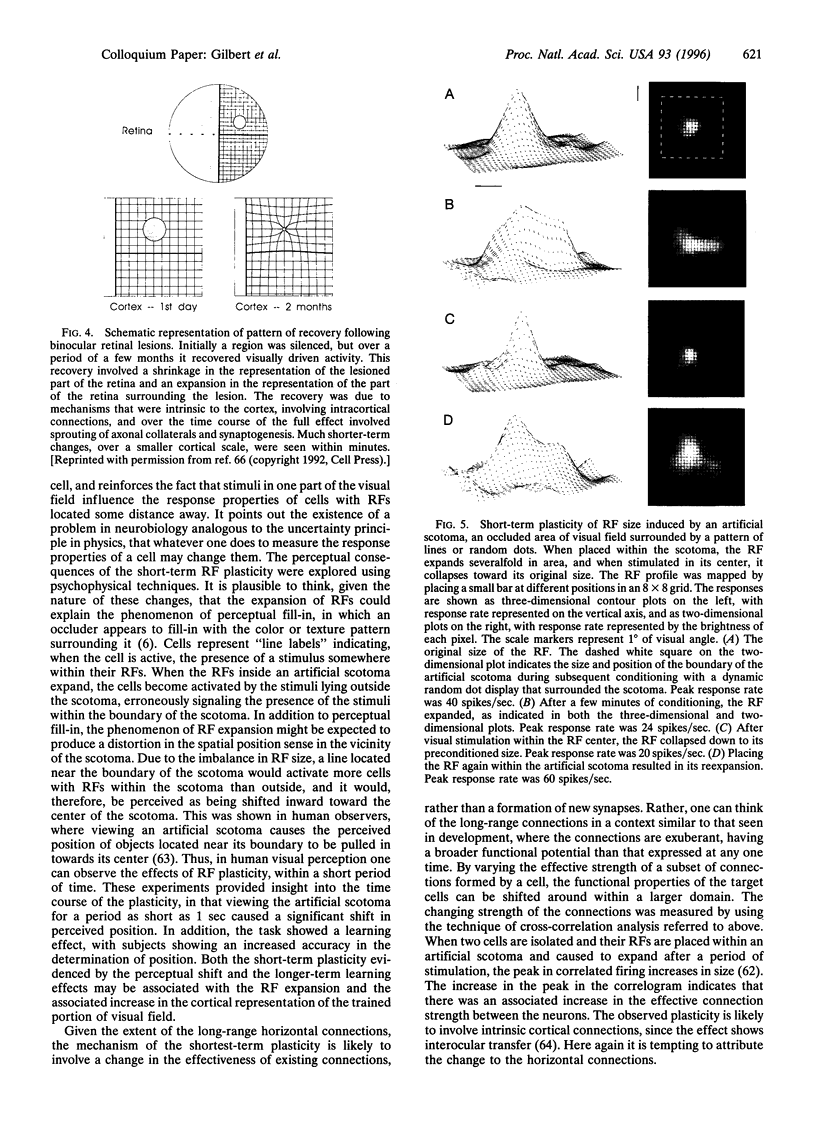
Cells in adult primary visual cortex are capable of integrating information over much larger portions of the visual field than was originally thought. Moreover, their receptive field properties can be altered by the context within which local features are presented and by changes in visual experience. The substrate for both spatial integration and cortical plasticity is likely to be found in a plexus of long-range horizontal connections, formed by cortical pyramidal cells, which link cells within each cortical area over distances of 6-8 mm. The relationship between horizontal connections and cortical functional architecture suggests a role in visual segmentation and spatial integration. The distribution of lateral interactions within striate cortex was visualized with optical recording, and their functional consequences were explored by using comparable stimuli in human psychophysical experiments and in recordings from alert monkeys. They may represent the substrate for perceptual phenomena such as illusory contours, surface fill-in, and contour saliency. The dynamic nature of receptive field properties and cortical architecture has been seen over time scales ranging from seconds to months. One can induce a remapping of the topography of visual cortex by making focal binocular retinal lesions. Shorter-term plasticity of cortical receptive fields was observed following brief periods of visual stimulation. The mechanisms involved entailed, for the short-term changes, altering the effectiveness of existing cortical connections, and for the long-term changes, sprouting of axon collaterals and synaptogenesis. The mutability of cortical function implies a continual process of calibration and normalization of the perception of visual attributes that is dependent on sensory experience throughout adulthood and might further represent the mechanism of perceptual learning.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allman J., Miezin F., McGuinness E. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT). Perception. 1985;14(2):105–126. doi: 10.1068/p140105. [DOI] [PubMed] [Google Scholar]
- Badcock D. R., Westheimer G. Spatial location and hyperacuity: the centre/surround localization contribution function has two substrates. Vision Res. 1985;25(9):1259–1267. doi: 10.1016/0042-6989(85)90041-0. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Interaction effects of visual contours on the discharge frequency of simple striate neurones. J Physiol. 1971 Dec;219(3):659–687. doi: 10.1113/jphysiol.1971.sp009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T., Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991 Oct 3;353(6343):429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
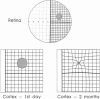
- Chino Y. M., Kaas J. H., Smith E. L., 3rd, Langston A. L., Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Res. 1992 May;32(5):789–796. doi: 10.1016/0042-6989(92)90021-a. [DOI] [PubMed] [Google Scholar]
- Chino Y. M., Smith E. L., 3rd, Wada H., Ridder W. H., 3rd, Langston A. L., Lesher G. A. Disruption of binocularly correlated signals alters the postnatal development of spatial properties in cat striate cortical neurons. J Neurophysiol. 1991 Apr;65(4):841–859. doi: 10.1152/jn.1991.65.4.841. [DOI] [PubMed] [Google Scholar]
- Crane H. D., Piantanida T. P. On seeing reddish green and yellowish blue. Science. 1983 Sep 9;221(4615):1078–1080. doi: 10.1126/science.221.4615.1078. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C., Gilbert C. D. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994 Apr 21;368(6473):737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Das A., Gilbert C. D. Receptive field expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections. J Neurophysiol. 1995 Aug;74(2):779–792. doi: 10.1152/jn.1995.74.2.779. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Hirsch J. A., Wiesel T. N. Lateral interactions in visual cortex. Cold Spring Harb Symp Quant Biol. 1990;55:663–677. doi: 10.1101/sqb.1990.055.01.063. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
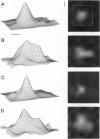
- Grinvald A., Lieke E. E., Frostig R. D., Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci. 1994 May;14(5 Pt 1):2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Gulyás B., Orban G. A., Duysens J., Maes H. The suppressive influence of moving textured backgrounds on responses of cat striate neurons to moving bars. J Neurophysiol. 1987 Jun;57(6):1767–1791. doi: 10.1152/jn.1987.57.6.1767. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen S. J., Skavenski A. A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp Brain Res. 1991;83(3):670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Gilbert C. D. Long-term changes in synaptic strength along specific intrinsic pathways in the cat visual cortex. J Physiol. 1993 Feb;461:247–262. doi: 10.1113/jphysiol.1993.sp019512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J. H., Krubitzer L. A., Chino Y. M., Langston A. L., Polley E. H., Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990 Apr 13;248(4952):229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
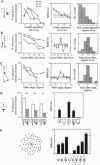
- Kapadia M. K., Gilbert C. D., Westheimer G. A quantitative measure for short-term cortical plasticity in human vision. J Neurosci. 1994 Jan;14(1):451–457. doi: 10.1523/JNEUROSCI.14-01-00451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia M. K., Ito M., Gilbert C. D., Westheimer G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron. 1995 Oct;15(4):843–856. doi: 10.1016/0896-6273(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A., Gilbert C. D., Rivlin P. K., Wiesel T. N. Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol. 1991 Mar 15;305(3):370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J. T., Sur M., Nelson R. J., Felleman D. J. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983 Nov;10(3):639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Nelson R. J., Stryker M. P., Cynader M. S., Schoppmann A., Zook J. M. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984 Apr 20;224(4):591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Pettet M. W., Gilbert C. D. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U., Sagi D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vision Res. 1993 May;33(7):993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Perceptual filling in of artificially induced scotomas in human vision. Nature. 1991 Apr 25;350(6320):699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983 May 20;216(3):303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hikosaka K., Saito H., Yukie M., Fukada Y., Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986 Jan;6(1):134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o D. Y., Frostig R. D., Lieke E. E., Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990 Jul 27;249(4967):417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D. The organization of chromatic and spatial interactions in the primate striate cortex. J Neurosci. 1988 May;8(5):1712–1727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D., Wiesel T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986 Apr;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G., Shimamura K., McKee S. P. Interference with line-orientation sensitivity. J Opt Soc Am. 1976 Apr;66(4):332–338. doi: 10.1364/josa.66.000332. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the domain of disparity signals in human stereoscopic vision. J Physiol. 1986 Jan;370:619–629. doi: 10.1113/jphysiol.1986.sp015954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J., Gerstein G. L. Simulation of dynamic receptive fields in primary visual cortex. Vision Res. 1994 Jul;34(14):1901–1911. doi: 10.1016/0042-6989(94)90314-x. [DOI] [PubMed] [Google Scholar]