Abstract
A spectroscopic and functional analysis of two point-mutated bacteriorhodopsins (BRs) from phototrophic negative halobacterial strains is reported. Bacteriorhodopsin from strain 384 contains a glutamic acid instead of an aspartic acid at position 85 and BR from strain 326 contains asparagine instead of aspartic acid at position 96. Compared to wild-type BR, the M formation in BR Asp85---Glu is accwelerated approximately 10-fold, whereas the M decay in BR Asp96---Asn is slowed down approximately 50-fold at pH6. Purple membrane sheets containing the mutated BRs were oriented and immobilized in polyacrylamide gels or adsorbed to planar lipid films. The measured kinetics of the photocurrents under various conditions agree with the observed photocycle kinetics. The ineffectivity of BR Asp85---Glu resides in the dominance of an inactive species absorbing maximally at approximately 610 nm, while BR Asp96---Asn is ineffective due to its slow photocycle. These experimental results suggest that aspartic acid 96 plays a crucial role for the reprotonation of the Schiff base. Both residues are essential for an effective proton pump.
Full text
PDF
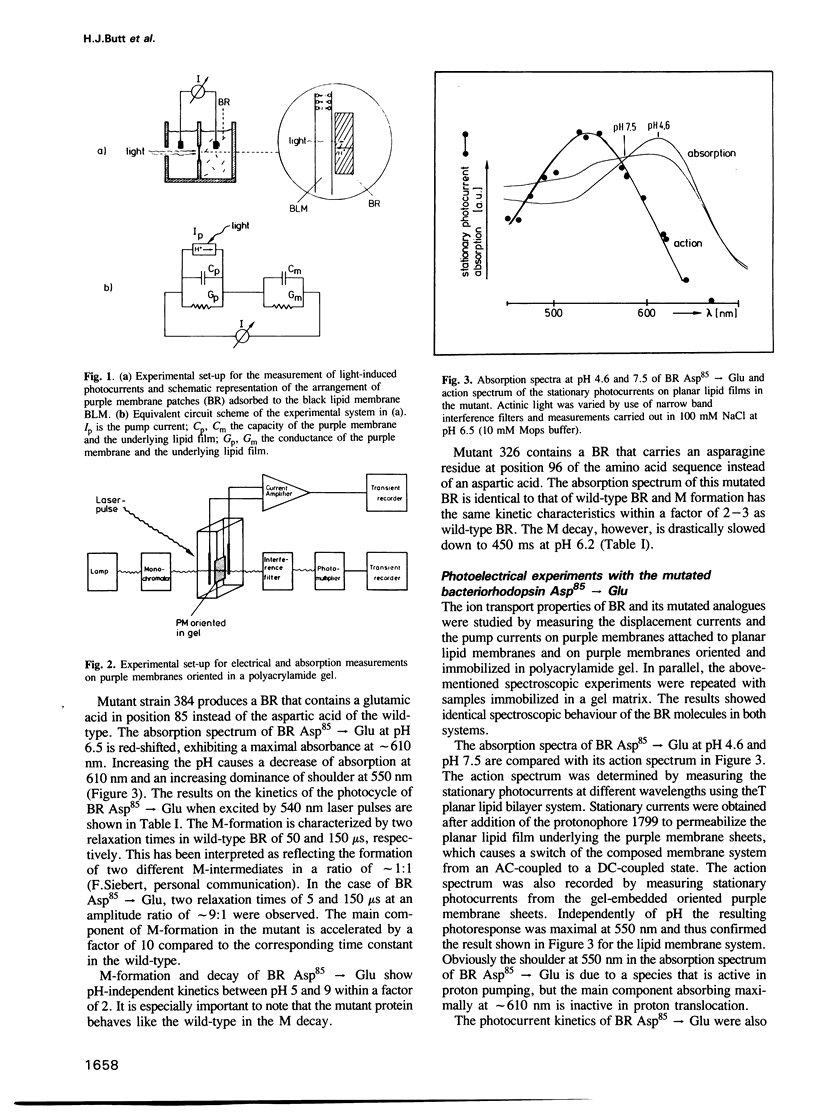
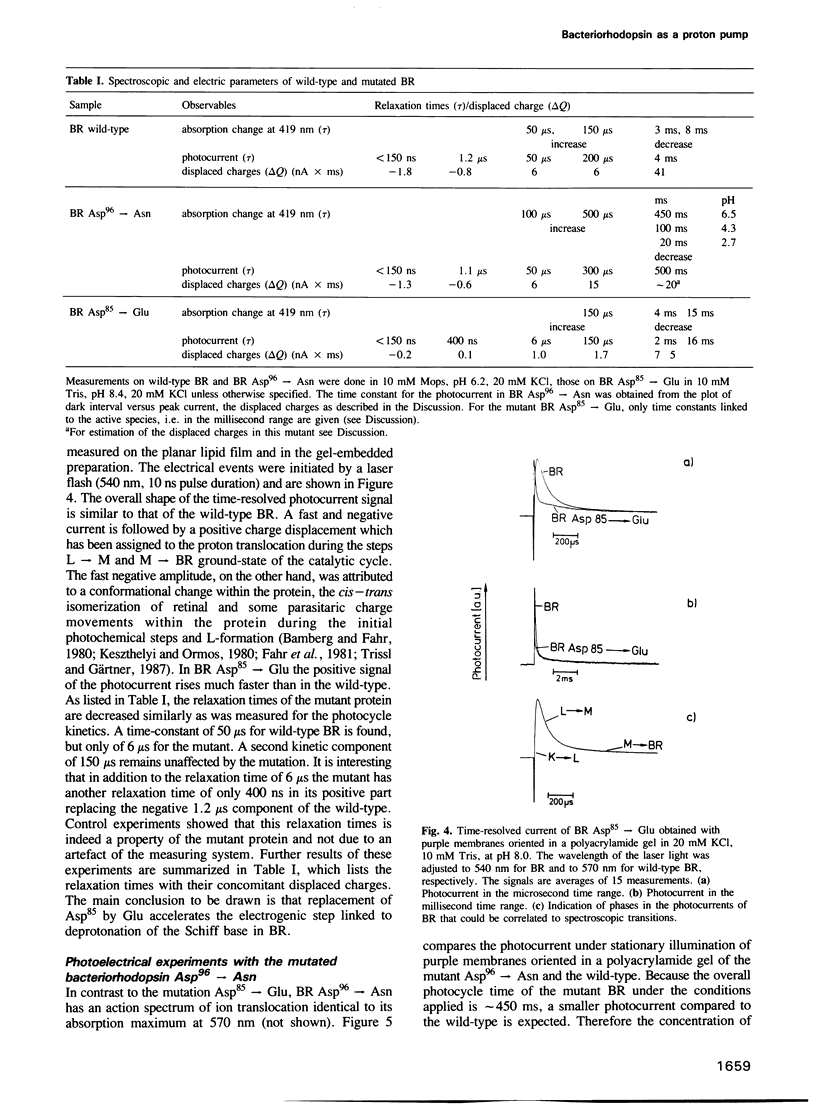
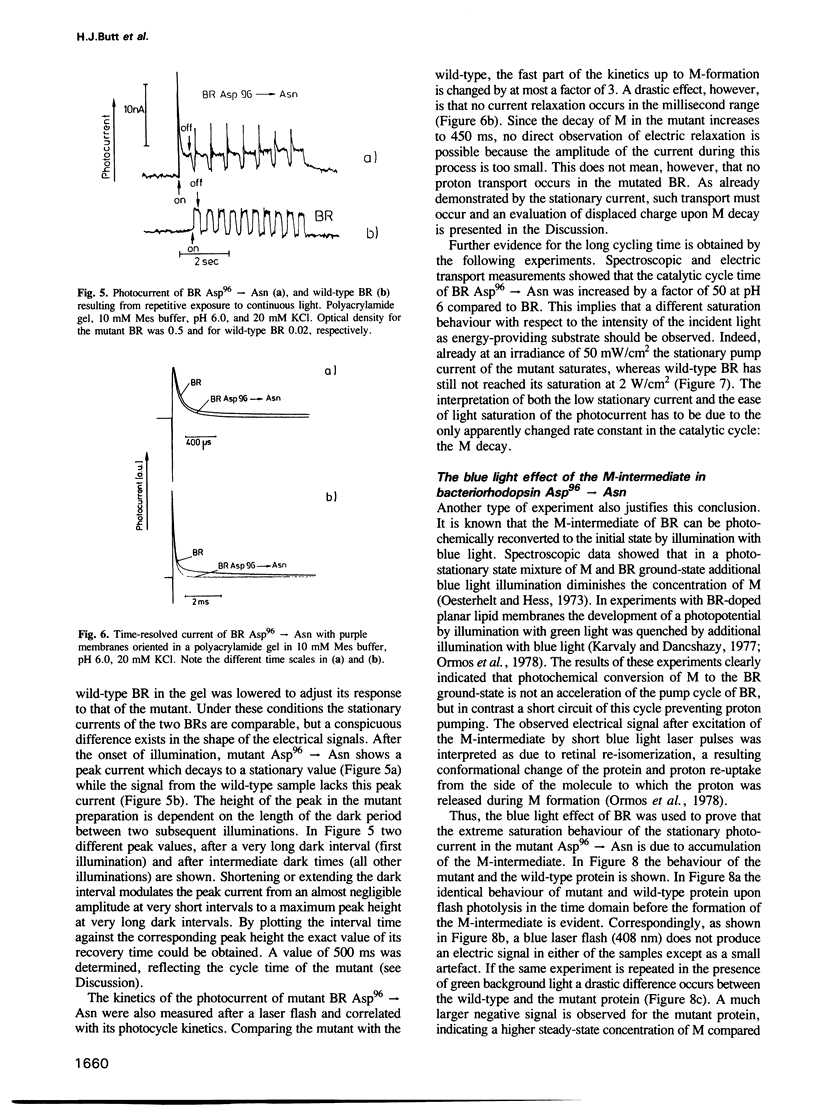
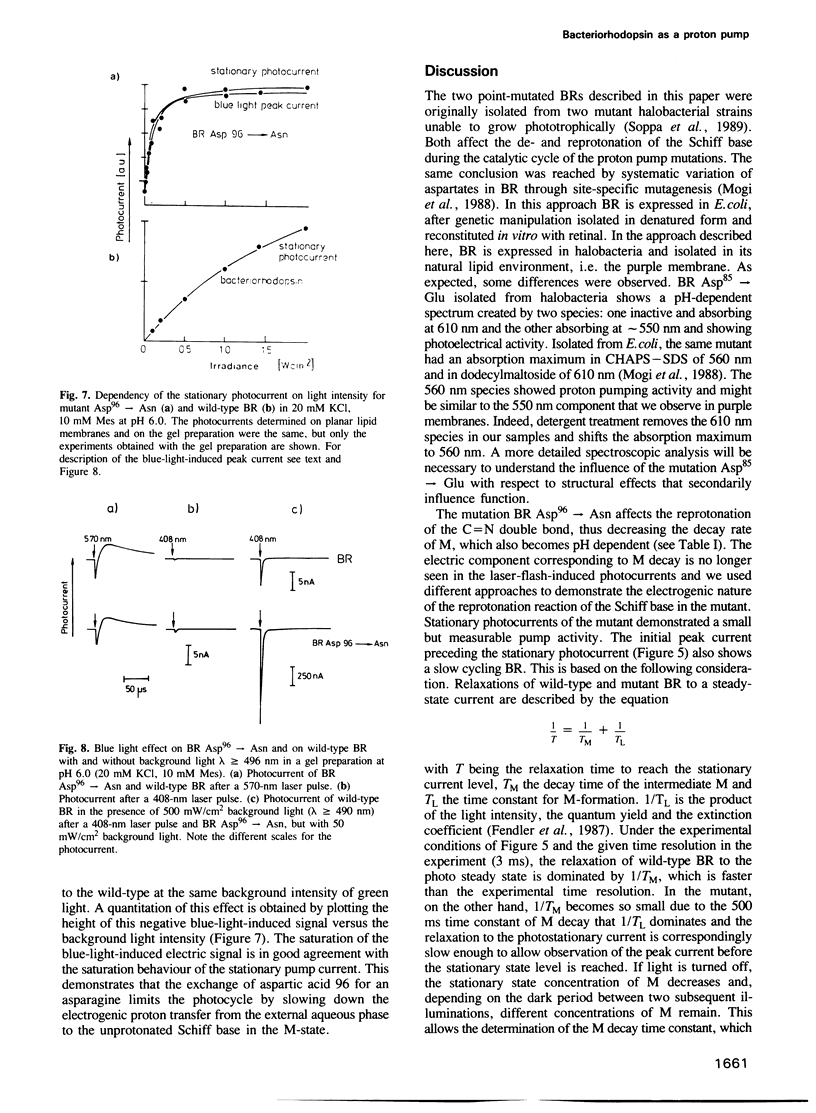

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberg E., Fahr A. Photocurrents induced on black lipid membranes by purple membranes: a method of reconstitution and a kinetic study of the photocurrents. Ann N Y Acad Sci. 1980;358:324–327. doi: 10.1111/j.1749-6632.1980.tb15405.x. [DOI] [PubMed] [Google Scholar]
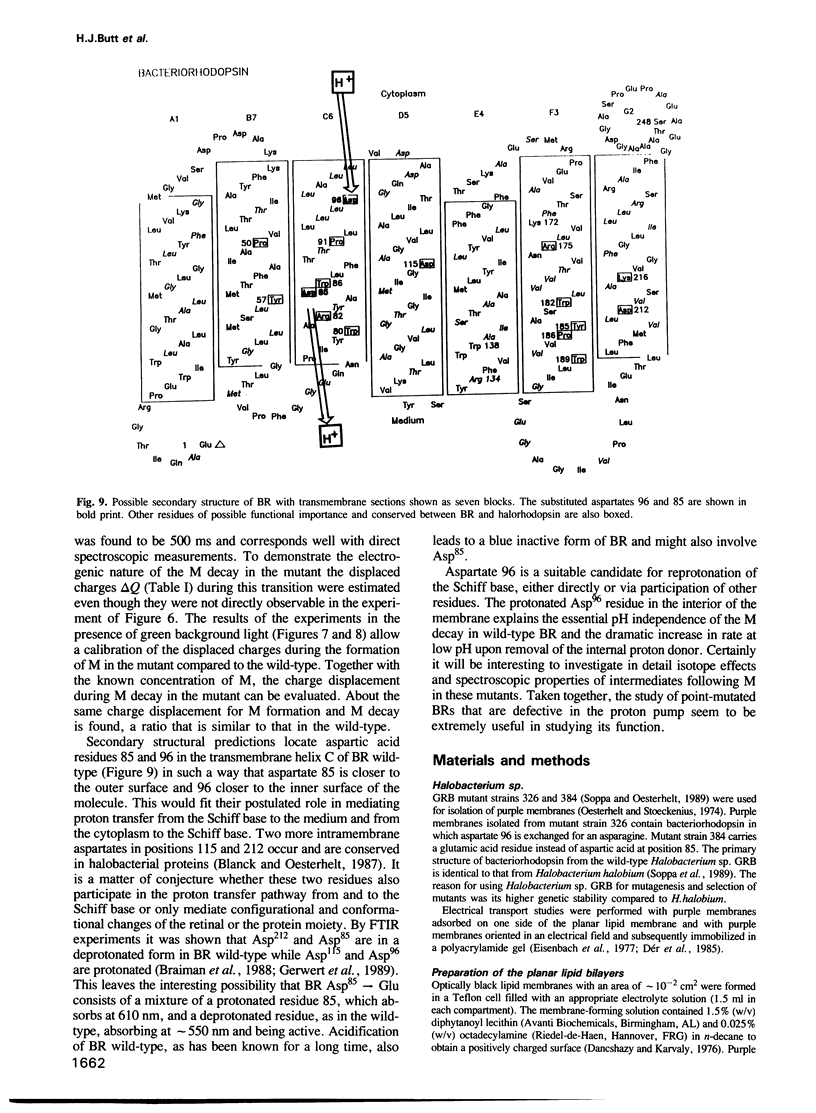
- Blanck A., Oesterhelt D. The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 1987 Jan;6(1):265–273. doi: 10.1002/j.1460-2075.1987.tb04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Karvaly B. Incorporation of bacteriorhodopsin into a bilayer lipid membrane; a photoelectric-spectroscopic study. FEBS Lett. 1976 Dec 15;72(1):136–138. doi: 10.1016/0014-5793(76)80829-0. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Jasaitis A. A., Kaulen A. D., Kondrashin A. A., Liberman E. A., Nemecek I. B., Ostroumov S. A., Semenov AYu, Skulachev V. P. Direct measurement of electric current generation by cytochrome oxidase, H+-ATPase and bacteriorhodopsin. Nature. 1974 May 24;249(455):321–324. doi: 10.1038/249321a0. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Khitrina L. V., Skulachev V. P. Fast stages of photoelectric processes in biological membranes. I. Bacteriorhodopsin. Eur J Biochem. 1981 Jul;117(3):461–470. doi: 10.1111/j.1432-1033.1981.tb06361.x. [DOI] [PubMed] [Google Scholar]
- Dér A., Hargittai P., Simon J. Time-resolved photoelectric and absorption signals from oriented purple membranes immobilized in gel. J Biochem Biophys Methods. 1985 Mar;10(5-6):295–300. doi: 10.1016/0165-022x(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Herrmann T. R., Rayfield G. W. The electrical response to light of bacteriorhodopsin in planar membranes. Biophys J. 1978 Feb;21(2):111–125. doi: 10.1016/S0006-3495(78)85512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvaly B., Dancsházy Z. Bacteriorhodopsin: a molecular photoelectric regulator. Quenching of photovoltaic effect of bimolecular lipid membranes containing bacteriorhodopsin by blue light. FEBS Lett. 1977 Apr 1;76(1):36–40. doi: 10.1016/0014-5793(77)80115-4. [DOI] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ormos P., Dancsházy Z., Karvaly B. Mechanism of generation and regulation of photopotential by bacteriorhodopsin in bimolecular lipid membrane. Biochim Biophys Acta. 1978 Aug 8;503(2):304–315. doi: 10.1016/0005-2728(78)90190-1. [DOI] [PubMed] [Google Scholar]



