Abstract
Aim:
Aripiprazole is an antipsychotic agent to treat schizophrenia, which acts through dopamine D2 partial agonism, serotonin 5-HT1A partial agonism and 5-HT2A antagonism. This study was designed to evaluate the neurobehavioral effects and genotoxic/mutagenic activities of the agent, as well as its effects on lipoperoxidation.
Methods:
Open field and inhibitory avoidance tasks were used. Thirty min before performing the behavioral tasks, adult male CF-1 mice were administered aripiprazole (1, 3 or 10 mg/kg, ip) once for the acute treatment, or the same doses for 5 d for the subchronic treatment. Genotoxic effects were assessed using comet assay in the blood and brain tissues. Mutagenic effects were evaluated using bone marrow micronucleus test. Lipoperoxidation was assessed with thiobarbituric acid reactive substances (TBARS).
Results:
Acute and subchronic treatments significantly decreased the number of crossing and rearing in the open field task. Acute treatment significantly increased the step-down latency for both the short- and long-term memory in the inhibitory avoidance task. Subchronic treatments with aripiprazole (3 and 10 mg/kg) caused significant DNA strain-break damage in peripheral blood but not in the brain. Mutagenic effect was not detected in the acute and subchronic treatments. Nor TBARS levels in the liver were affected.
Conclusion:
Aripiprazole improved memory, but could impair motor activities in mice. The drug increased DNA damage in blood, but did not show mutagenic effects, suggesting that it might affect long-term genomic stability.
Keywords: antipsychotic agent, aripiprazole, locomotion, memory, genotoxic/mutagenic activities, DNA damage, lipoperoxidation
Introduction
Cognitive deficits (attention, executive function, short- and long-term memory) are symptoms observed in patients with schizophrenia1, 2. Several studies have assessed physiopathologic aspects linked to schizophrenia, such as declarative and nondeclarative memory functions, to identify areas of impairment versus preservation3. Similarly, some investigations have evaluated the effect of antipsychotic drugs on cognitive parameters in humans and animals4, 5. Research suggests that schizophrenic patients treated with atypical antipsychotics may perform better in cognitive tasks when compared to patients treated with typical antipsychotics2, 6.
Aripiprazole, 7-{4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butyloxy}-3,3-dihydro-carbostycil (Figure 1), is an atypical antipsychotic drug with distinct properties compared to other efficient antipsychotics4. The drug was developed recently, and it presents a unique pharmacological profile that includes dopamine D2 partial agonism, serotonin 5-HT1A partial agonism, and 5-HT2A antagonism5, 6. Clinical trials have found that aripiprazole was effective in treating the positive, negative, and cognitive symptoms of schizophrenia7. Numerous large scale clinical studies have shown that aripiprazole has a favorable safety and tolerability profile with a relatively low potential for parkinsonism, prolactin elevation, weight gain, QTc prolongation, sedation, tardive dyslinesia, changes in plasma lipid levels, and glucose level elevation8.
Figure 1.
Structure of aripiprazole.
In the experimental context, Nagai et al9 showed that aripiprazole that was administered as either a single dose or as consecutive doses (for 7 d) ameliorated phencyclidine-induced impairment of recognition memory in mice. However, another study showed that aripiprazole impaired the passive-avoidance response at doses near its anti-dopamine ED50 (7.7 mg/kg, as defined by apomorphine stereotypy). At doses lower than those that affected the passive-avoidance response, aripiprazole was unable to reverse the MK-801-induced impairment in the same task4. Therefore, further investigations are necessary to elucidate the effect of aripiprazole on memory.
The aim of the present study was to investigate the effects of aripiprazole on memory and on locomotor and exploratory activities with the inhibitory avoidance and open field tasks as behavioral models. Some drugs that affect memory can induce damage in biomolecules, such as lipids and DNA. Therefore, possible cytotoxic and genotoxic effects were evaluated by measuring lipid peroxidation in the liver and DNA strand breaks in both the peripheral blood and brain tissues after behavioral tasks. Mutagenic effects of aripiprazole were also assessed using the micronucleus frequency in the bone marrow of mice.
Materials and methods
Animals
Male SR-1 mice (163 animals, 2–3 months of age; 30–40 g) from our breeding colony were used. The mice were housed in plastic cages with ad libitum access to water and food, under a 12-h light/dark cycle (lights on at 8:00 AM), and at a constant temperature of 23.0 °C. All experimental procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care. This work was approved by the Ethical Committee of ULBRA.
Drugs and pharmacological procedures
AbilifyTM (Brystol) was used as a source of aripiprazole. Four pills of 20 mg aripiprazole were powdered in a ceramic pestle, and two extractions by steam route were performed with 20% ethanol and ethanol pro analysi (PA). An infrared technique was used to analyze the samples, and the resulting spectrum was compared with the spectrum from the monography.
Aripiprazole was dissolved in saline solution and 5% Tween. The animals were divided in groups and received saline, 5% Tween or 1, 3, or 10 mg/kg doses of aripiprazole. The animals were given only one intraperitoneal (ip) injection (acute treatment) or one injection per day for 5 d (subchronic treatment) as a 0.1 mL/10 g body weight dose. The doses were chosen based on previous reports about the behavioral effects of aripiprazole4. To study the effect that aripiprazole has on memory, the animals received injections 30 min before training in the inhibitory avoidance task.
Neurobehavioral experiments
Open field behavior
The animals were exposed to a 40 cm×50 cm×60 cm open field that was divided into 12 equal squares. The animals were placed in the rear left square, and they were allowed to freely explore the field for 5 min. The animals received the injections 30 min before the test. Crossing of the black lines, rearings performed, and latency to start locomotion were counted and used as measurements of locomotion, exploration and motivation10.
Inhibitory avoidance task
Inhibitory avoidance in rodents is a widely used animal model of aversive learning and memory. A 50 cm×25 cm×25 cm plastic box with a frontal glass wall that had a floor that consisted of parallel 10-mm caliber bronze bars spaced 1 cm from one another was used. The left end of the grid was equipped with a 9-cm wide and 1.5-cm high platform. The mice were placed gently on the platform facing the rear wall, and their latency to step down with all four paws onto the grid was measured. In the training session, after stepping down, the animals received a 0.3-mA, 2-s scrambled foot shock and were immediately withdrawn from the cage. In the test session, either 1.5 h short-term memory (STM) or 24 h long-term memory (LTM) later, the procedure was repeated, but the foot shock was not given. Test session step-down latency was used as a measure of retention. A 180-s upper bound was set up for this measure10.
Genotoxic/mutagenic assays
Comet assay
The alkaline comet assay in peripheral blood and brain tissues was performed as previously described11 but with minor modifications12, 13. Blood samples were collected from a tail blood vessel 3 h and 24 h after the first administration (acute treatment) and 3 h after the last administration (subchronic treatment). The animals were killed by cervical dislocation, and forebrain samples were immediately collected. Each piece of forebrain was finely minced and placed in 0.5 mL of cold phosphate-buffered saline (PBS) to obtain a cell suspension. Brain and blood cell suspensions (5 μL) were embedded in 95 μL of 0.75% low melting point agarose (Gibco BRL) and spread on agarose-precoated microscope slides. After solidification, the slides were placed in lysis buffer (2.5 mol/L NaCl, 100 mmol/L EDTA and 10 mmol/L Tris, pH 10.0) with freshly added 1% Triton X-100 (Sigma) and 10% DMSO for 48 h at 4 °C. The slides were subsequently incubated in freshly prepared alkaline buffer (300 mmol/L NaOH and 1 mmol/L EDTA, pH>13) for 20 min, at 4 °C. An electric current of 300 mA and 25 V (0.90 V/cm) was applied for 15 min to perform DNA electrophoresis. The slides were then neutralized (0.4 mol/L Tris, pH 7.5), stained with silver and analyzed using a microscope. Images of 100 randomly selected cells (50 cells from each of two replicate slides) were analyzed from each animal. The cells were also visually scored according to tail size into five classes, ranging from undamaged (0) to maximally damaged (4), resulting in a single DNA damage score for each animal and consequently for each studied group. Therefore, the damage index (DI) can range from 0 (completely undamaged, 100 cells×0) to 400 (with maximum damage, 100 cells×4)14.
Micronucleus assay
The micronucleus assay was performed according to the US Environmental Protection Agency Gene-Tox Program15. Bone marrow from both femurs was collected after acute and subchronic treatments. The tissue was suspended in fetal calf serum, and smears on the clean glass slides were prepared as described in a previous report16. The slides were air-dried, fixed in methanol, stained in 10% Giemsa and coded for a blind analysis. To avoid false negative results and to obtain a measure of toxicity on bone marrow, the ratio of polychromatic erythrocytes to normochromatic erythrocytes (PCE:NCE) was scored in 1000 cells. The incidence of micronuclei (MN) was observed in 2000 PCE for each animal17.
Lipid peroxidation assay
Thiobarbituric acid reactive substances (TBARS) were used as a marker of lipid peroxidation. After subchronic treatment, the livers were removed, weighed, immediately frozen in liquid nitrogen and stored at -80 oC for ulterior analyses. The frozen tissue was homogenized in 10 volumes (w/v) of phosphate buffer solution (KCl 140 mmol/L, phosphate 20 mmol/L, pH 7.4) in ULTRA-Turrax (IKA-WERK) and centrifuged at 704×g for 10 min. Lipoperoxidation was measured using the TBARS on homogenized tissues, as described by Esterbauer and Cheeseman18. The amount of aldehyde products generated by lipid peroxidation was quantified by the thiobarbituric acid reaction using 3 mg of protein per sample. The results were expressed as nanomoles per milligram of protein. Proteins were determined by the method described by Lowry19.
Statistical analysis
Data from the open field test were expressed as the mean±SEM. These data were analyzed using one-way ANOVA followed by Duncan's test. The analyses of the step-down inhibitory avoidance task were non-parametric because this procedure involved a cutoff score. The data were expressed as medians (interquartile ranges) and analyzed using the Kruskal-Wallis test, followed by the Mann-Whitney test when necessary. Data from the comet assay, micronucleus test and lipid peroxidation assay are expressed as the mean±SD, and statistical significance was determined by one-way ANOVA followed by Tukey's test. In all comparisons, P<0.05 was considered to indicate statistical significance.
Results
Neurobehavioral parameters
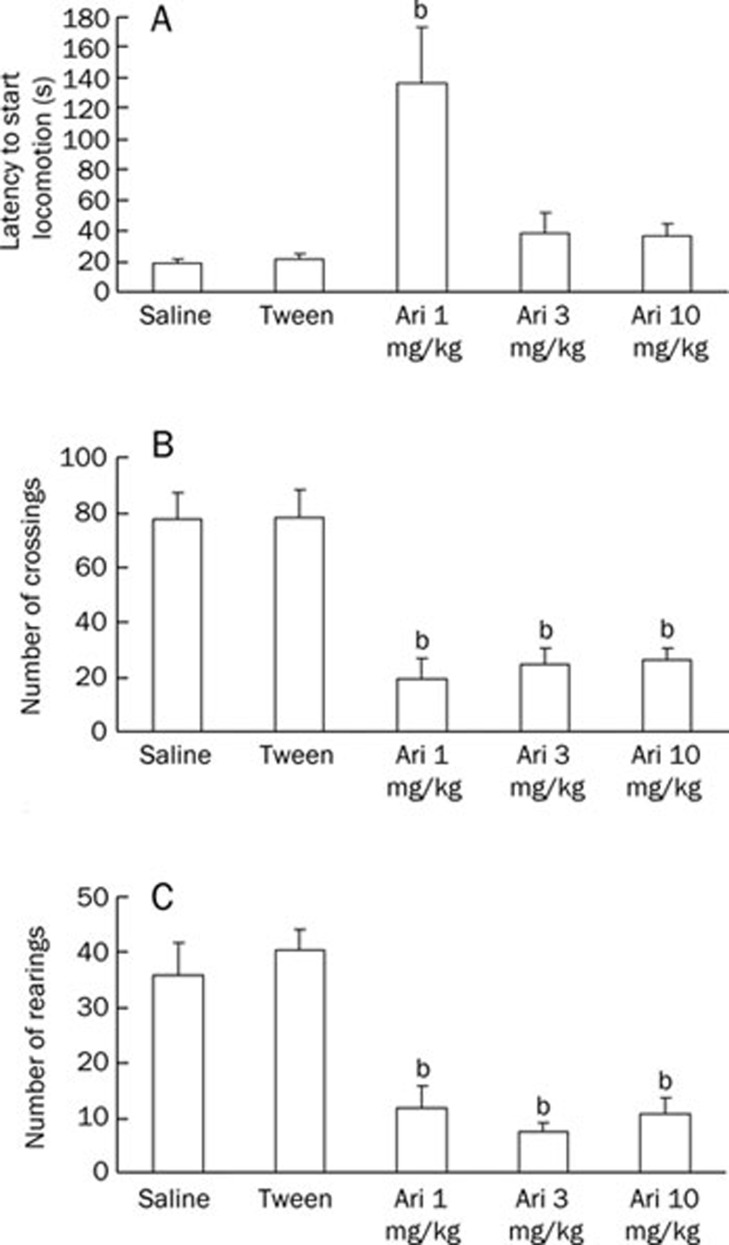
Figure 2 shows the behavioral patterns of mice given saline or aripiprazole (1, 3, or 10 mg/kg) during a 5-min exploration period in an open field after acute treatment. The number of crossings and rearings was lower in the groups that received aripiprazole compared to the control group (P<0.05); however, it was not lower than the Tween group, suggesting that aripiprazole affected the locomotion or exploration of the animals in this task. The latency to start locomotion was different only in the group that received aripiprazole 1 mg/kg (P<0.05).
Figure 2.
Effect of aripiprazole (1, 3, or 10 mg/kg) pretest administration on the following: (A) latency to start locomotion, (B) number of crossings performed and (C) number of rearings performed during a 5-min exploration period of an open field. Animals received an ip injection of saline, Tween or aripiprazole 30 min prior to the locomotory behavior test in the open field (acute treatment). Data are expressed as the mean±SEM. n=10 animals per group. bP<0.05 compared to the saline group; ANOVA/Duncan's test.
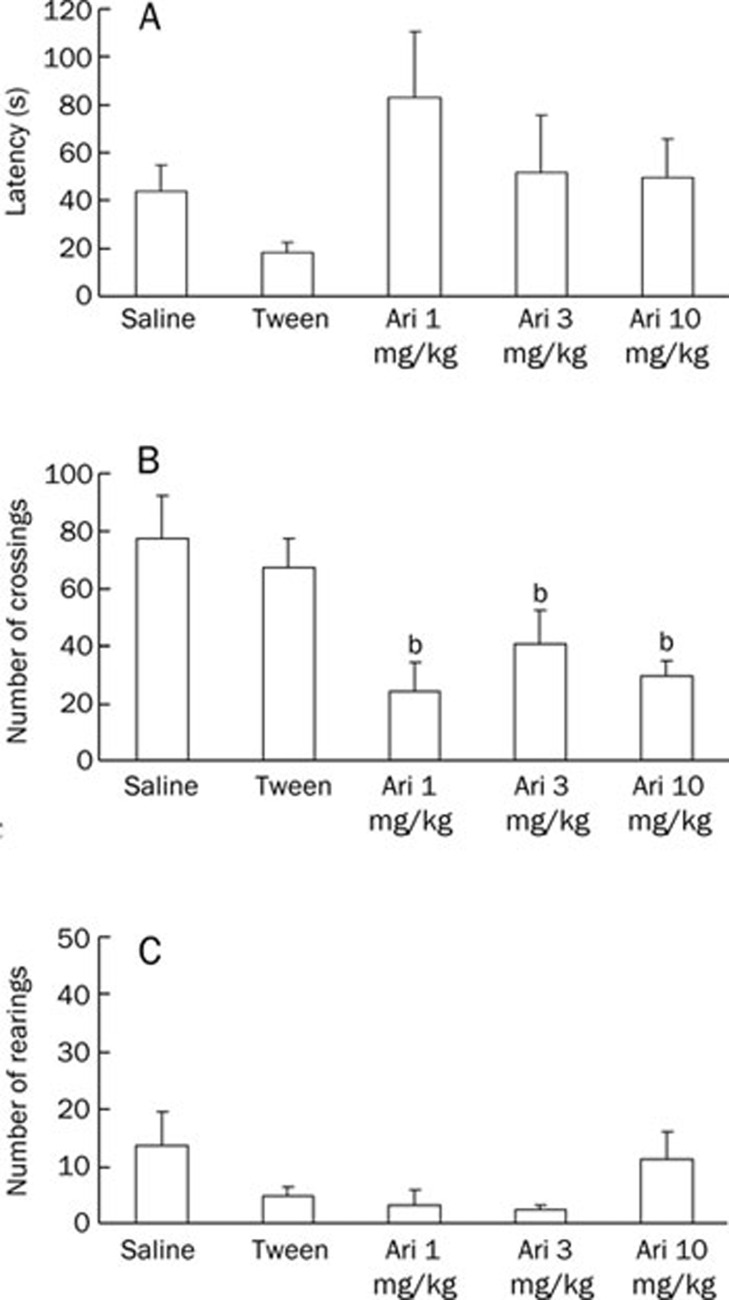
Figure 3 shows the effect of aripiprazole on the open field task after a 5-d treatment. Aripiprazole decreased the crossings performed in all doses tested (P<0.05), although it did not affect rearings (P=0.086) and latency to start locomotion (P=0.182).
Figure 3.
Effect of repeated aripiprazole (1, 3, or 10 mg/kg) administration on the following: (A) latency to start locomotion, (B) number of crossings performed and (C) number of rearings performed during a 5-min exploration period of an open field. Animals received an ip injection of saline, Tween, or aripiprazole for 5 d (subchronic treatment). Behavioral parameters were recorded 30 min after the last administration. Data are expressed as the mean±SEM. n=8–10 animals per group. bP<0.05 compared to the saline group; ANOVA/Duncan's test.
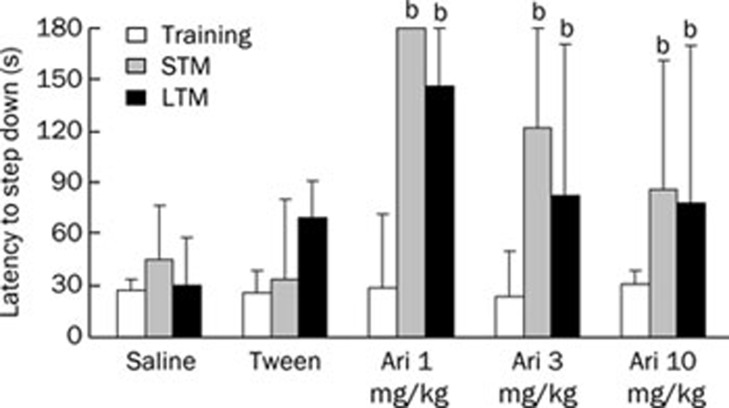
Short- and long-term memory retention of inhibitory avoidance was evaluated in different animals that received aripiprazole (Figure 4). There were no significant differences in training performed among the groups (P=0.819). For both the STM and LTM, aripiprazole increased the step-down latency in all doses tested (P<0.05), which indicates that the drug has the potential to improve the memory of animals in this task after acute treatment.
Figure 4.
Effect of pretraining administration (ip) of saline, Tween or aripiprazole (1, 3, or 10 mg/kg) on STM (1.5 h after training) and LTM (24 h after training) in inhibitory avoidance. n=11–15 animals per group. bP<0.05 compared to the saline group; Kruskal-Wallis/Mann-Whitney test.
Genotoxic parameters
For the acute treatment, aripiprazole did not induce DNA damage in the blood and in brain tissues collected 3 h (blood) and 24 h (blood and brain) after administration (data not shown). However, doses administered for 5 d led to an increase in DI in the peripheral blood of the treated groups compared to the saline or Tween groups (Table 1). In the brain, DI values obtained from groups treated with aripiprazole were not significantly different from those of the controls groups.
Table 1. DNA damage after subchronic treatment with aripiprazole in mice.
| Groups | Blood DI* (mean±SD) | Brain DI (mean±SD) |
|---|---|---|
| Saline | 39±5 | 207±60 |
| Tween | 39±4 | 190±34 |
| Aripiprazole 1 mg/kg | 47±3 | 132±32 |
| Aripiprazole 3 mg/kg | 51±5c | 161±62 |
| Aripiprazole 10 mg/kg | 58±7c | 174±54 |
| Positive control# | 77±27c | 389±6c |
n=5 animals per group. *DI (damage index) can range from 0 (completely undamaged. 100 cells×0) to 400 (with maximum damage 100 cells×4). #Positive control: blood or brain cells from the saline group treated ex vivo with hydrogen peroxide 0.20 mmol/L. cP<0.01 statistically significant difference from the saline and tween groups (ANOVA Tukey's test).
The frequency of micronuclei in the aripiprazole-treated groups was similar to the values obtained for the saline or Tween groups in both the acute and subchronic treatments (Table 2). There was no toxicity in the bone marrow because the PCE:NCE ratio did not decrease significantly in either of the two treatments (data not shown).
Table 2. Frequency of micronucleus in bone marrow of mice after acute and subchronic treatments with aripiprazole.
| Groups | Acute treatment MNPCE in 2000 PCE mean±SD | Subchronic treatment MNPCE in 2000 PCE mean±SD |
|---|---|---|
| Saline | 1.14±0.38 | 1.15±0.37 |
| Tween | 1.20±0.45 | 1.86±1.60 |
| Aripiprazole 1 mg/kg | 1.80±1.79 | 1.43±1.90 |
| Aripiprazole 3 mg/kg | 2.25±1.50 | 1.40±0.89 |
| Aripiprazole 10 mg/kg | 1.40±0.89 | 2.86±2.49 |
| Positive control | 10.0±5.29c | – |
n=5 animals per group. Positive control: cyclophosphamide (25 mg/kg). MNPCE: micronucleated polychromatic erythrocytes (PCE). cP<0.01: statistically significant difference from the saline group (ANOVA Tukey's test).
Lipoperoxidation
Table 3 shows the results of lipid peroxidation evaluated in the liver tissue after subchronic treatment. Aripiprazole did not increase lipid damage in this tissue.
Table 3. TBARS (thiobarbituric acid reactive substances) values (nmol/mg protein) in liver of mice after subchronic treatment with aripiprazole.
| Saline | Tween | Aripiprazole 1 mg/kg | Aripiprazole 3 mg/kg | Aripiprazole 10 mg/kg |
|---|---|---|---|---|
| 0.72±0.23 | 0.65±0.13 | 0.62±0.26 | 0.53±0.13 | 0.42±0.17 |
n=7 animals per group.
Discussion
Neurobehavioral parameters
This work reports the effects of aripiprazole on memory and locomotor activity in mice using the inhibitory avoidance and open field tasks. The experiments showed that this antipsychotic drug was able to decrease the crossings and rearings measured 30 min after only one administration (Figure 2). However, when the animals received aripiprazole for 5 d, a decrease was observed only in the crossing parameter (Figure 3), suggesting that the drug can affect both the locomotor and exploratory activities when administered as an acute dose. The group of animals treated with one dose of aripiprazole (1 mg/kg) exhibited a significant increase in latency time in beginning locomotion in the open field task, which reveals a decrease in motivation. This effect was not observed after 5 d of treatment.
Some works studied the effect of aripiprazole on locomotion and exploration in animals. Used alone, aripiprazole (2.5 and 5 mg/kg) decreased the locomotor activity measured during a 60-min period in mice20. In the same study, aripiprazole antagonized amphetamine- (2 mg/kg) or ethanol-induced (1.75 g/kg) locomotor stimulation21. Therefore, aripiprazole attenuated LY-341495-induced hyperactivity, a metabotropic glutamate receptor antagonist20.
Another study reported that aripiprazole was able to decrease total locomotor activity in a dose-dependent manner, causing marked locomotor suppression 1 h after oral treatment with 0.3 and 1 mg/kg doses4. In the same work, aripiprazole was administered for 7 d, and the locomotor activity was recorded 24 h after the last treatment. Contrasting with the single treatment, repeated treatment with aripiprazole had no effect on locomotor activity.
Here, we observed that a single aripiprazole dose (1, 3, or 10 mg/kg) affected both locomotor and exploratory activities, but after a 5-d treatment, only the crossing parameter was altered. The difference between the behavioral results obtained in the present study and those by Nagai et al9 can be associated with the aripiprazole doses and the animal species used in these treatments.
Phencyclidine [1-(1-phenylcyclohexyl) piperidine hydrochloride (PCP)], a noncompetitive N-methyl-D-aspartate receptor antagonist, impairs the animals' performance in the novel object recognition task. In another study, the single or repeated treatment with aripiprazole, but not haloperidol, revealed that aripiprazole was able to ameliorate the cognitive impairment induced by treatment with PCP. This effect was blocked by co-treatment with dopamine D1 and 5-HT1A antagonist receptors, suggesting that behavior triggered by aripiprazole can be associated with dopamine D1 and serotonin 5-HT1A receptors9.
Enomoto et al5 showed that aripiprazole did not affect animal performance in the Morris water maze and radial-arm maze tests. The authors also observed that the drug showed no ameliorating effect on MK-801-induced impairment of learning and memory in these tasks, which indicated that there was no activity at the NMDA glutamate receptors.
However, 10 mg/kg of aripiprazole, but not 1 or 3 mg/kg, impaired the passive-avoidance response4. These results disagree with what was observed in our study, which shows a memory improvement caused by aripiprazole administration. This can be associated with the differences in the tasks and the administration route (we used the ip route to administer the drugs).
The inhibitory avoidance task reveals how averse an animal may become when faced with some negative stimuli, and it involves associative behavior because the animal has to avoid the shock to the paws. Here, we reported that aripiprazole caused significant changes in STM and LTM in the inhibitory avoidance task, with an improvement in the performance of the animals (longer latency to climb down the platform) when compared to the control group. The inhibitory avoidance task has been used to hypothesize that several biochemical mechanisms may influence memory22, 23. This task is heavily dependent on the hippocampus, where a sequence of molecular events takes place. However, the task is also governed by the events occurring in the entorhinal and parietal cortex because it is also intensely modulated by the basolateral nucleus of the amygdala and influenced by a different sequences of molecular events24. Aripiprazole presents a novel action mechanism, and it is able to modulate several different receptors25. The action mechanism of aripiprazole has not been fully clarified, which may hinder the interpretation of the data obtained in the present study. Serotonin 5-HT1A and 5-HT2A receptors are suggested to play important roles in cognitive functions4. Therefore, the effects on memory shown here might be involved in the actions of aripiprazole on those receptors.
Genotoxic parameters
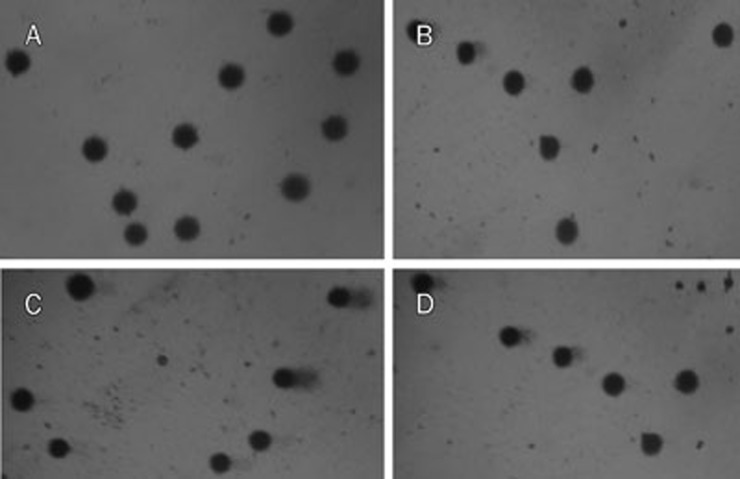
The comet assay was used to detect recent DNA damage, such as single and double strand breaks, alkali-labile sites, DNA-DNA and DNA-protein crosslinks26. Three hours after the last administration in subchronic treatment, blood and brain samples were collected from the same animals that had been tested in the open-field task to conduct the comet assay. An increase in DNA damage in the blood was observed, indicating a genotoxic effect (Table 1). Class 1 damage was the most frequent among the aripiprazole-damaged cells, which is considered a reparable minimal damage (Figure 5).
Figure 5.
Representative images of comets in blood and brain tissues. (A) Blood comets from the control group; (B) Blood comets from the aripiprazole treated group; (C) Brain comets from the control group; (D) Brain comets from the aripiprazole treated group.
The micronucleus test was used to detect clastogenic/aneugenic activities, which leads to an increasing frequency of micronuclei, and suggests mutagenic effects at the chromosomal level15, 26. Aripiprazole has shown positive results in mutagenicity assays27. Here, an increase in the frequency of micronuclei in the PCE of bone marrow was observed, although it was not in a dose-dependent manner and had no statistically significant values (Table 2).
In the brain tissue, aripiprazole did not induce DNA damage in any of the treatments (Table 1), although it caused impairment of neurobehavioral performance. Conversely, DNA damage levels in the aripiprazole-treated groups were lower than in the control groups, though not significantly (Figure 5). Thus, aripiprazole showed weak systemic genotoxicity, inducing DNA damage in the blood and a tendency to protect brain tissue.
This result corroborates previous studies demonstrating the beneficial effects of aripiprazole on neuronal functions. Antipsychotic drugs, such as aripiprazole, consistently increased N-acetylaspartate (NAA) levels, pointing to a usual therapeutic response of increased neuronal viability28. Another study has suggested that aripiprazole inhibits glutamate release from rat prefrontocortical nerve terminals, probably by the activation of dopamine D2 and 5-HT1A receptors, which subsequently results in the reduction in nerve terminal excitability and downstream activation of voltage-dependent Ca2+ channels through a signaling cascade involving PKA. These actions of aripiprazole may contribute to its neuroprotective effect in excitotoxic injury29. In SH-SY5Y human neuroblastoma cells, aripiprazole increased the levels of brain-derived neurotrophic factor (BDNF)-mediated signaling, suggesting that aripiprazole offers neuroprotective effects on human neuronal cells30.
Lipoperoxidation
It is known that aripiprazole is metabolized in the liver by cytochrome P450 3A4 (CYP3A4) and CYP2D6 to dehydroaripiprazole, an active metabolite31, 32. In this sense, aripiprazole was not able to increase the TBARS values, suggesting no induction of lipid peroxidation.
TBARS is a measure of major oxidative degradation products, such as lipid hydroperoxides from unsaturated fatty acids of the membrane, which have been implicated in psychiatric diseases, including schizophrenia33, 34, 35. In the rat brain, aripiprazole has been shown to diminish TBARS in the prefrontal cortex, and it did not alter protein carbonyl content when compared to the control group, indicating that the compound does not induce oxidative damage36. Our results corroborate those findings, suggesting protective effects of aripiprazole on lipids. A small decrease was observed in the TBARS values, but this lacked statistical significance (Table 3). Furthermore, 2.5 mg/kg aripiprazole administered for 28 d has been shown to decrease lipid peroxidation in the brain cortex and plasma in depression-induced rats by chronic mild stress, indicating a protective effect of this drug on oxidative stress37. In another study, aripiprazole increased succinate dehydrogenase activity in the prefrontal cortex, suggesting that it may reverse a possible reduction in metabolism involved in the pathophysiology of neuropsychiatry disorders38.
In conclusion, aripiprazole improved STM and LTM in the inhibitory avoidance task. These findings are in accordance with studies that have shown that atypical antipsychotics may improve cognitive tasks2, 6. Aripiprazole decreased baseline DNA damage in brain tissue, which suggests a neuroprotective effect, and no cytotoxic or mutagenic effects were detected. However, this drug showed a potential to impair motor activity. In addition, the reparable DNA damage in the blood in the subchronic treatment suggests that aripiprazole might affect genomic stability. Therefore, further studies are necessary to assess the molecular mechanisms of aripiprazole on motor, exploratory, and genotoxic activities in chronic treatments to guarantee its safe use.
Author contribution
Jaqueline Nascimento PICADA analyzed the data on genotoxic parameters and wrote the paper. Bruna de Jesus Neto DOS SANTOS performed behavioral tests. Franciele CELSO performed genotoxic tests. Jéssica Dias MONTEIRO performed behavioral tests. Kelly Morais DA ROSA performed behavioral tests. Leandro Rosa CAMACHO performed the extraction of aripiprazole from AbilifyTM (Brystol). Luciana Rodrigues VIEIRA performed TBARS. Taís Madelon FREITAS performed genotoxic testes. Tatiana Grasiela DA SILVA performed genotoxic tests. Viviane Minuzzo PONTES performed behavioral tests. Patrícia PEREIRA analyzed the data on behavioral parameters and wrote the paper.
Acknowledgments
This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul), Brazil.
References
- Harvey PD, Green MF, Keefe RS, Velligan DI. Cognitive functioning in schezophrenia: a consenses on its role in the definition and evaluation of effective treatments for the illness. J Cli Psychiatry. 2004;65:361–72. [PubMed] [Google Scholar]
- Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr Bull. 2006;32:474–85. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Hartzell AM, Izaguirre B, Hamilton AH. Declarative and nondeclarative memory in schizophrenia: what is impaired? What is spared. J Clin Exp Neuropsychol. 2010;32:1017–27. doi: 10.1080/13803391003671166. [DOI] [PubMed] [Google Scholar]
- Ishiyama T, Tokuda K, Ishibashi T, Ito A, Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol. 2007;572:160–70. doi: 10.1016/j.ejphar.2007.06.058. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Ishibashi T, Tokuda K, Ishiyama T, Toma S, Ito A. Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats. Behav Brain Res. 2008;186:197–207. doi: 10.1016/j.bbr.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kern KS, Green MF, Lornblatt BA, Owen JR, McQuade RD, Larson WH, et al. The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology. 2006;187:312–20. doi: 10.1007/s00213-006-0428-x. [DOI] [PubMed] [Google Scholar]
- Kane JM, Larson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63:763–11. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]
- Miller DD, Eudicone JM, Pikalov A, Kim E. Comparative assessment of the incidence and severity of tardive dyskinesia in patients receiving aripiprazole or haloperidol for treatment of schizophrenia: a post hoc analysis. J Clin Psychiatry. 2007;68:1901–6. doi: 10.4088/jcp.v68n1210. [DOI] [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Komei H, Noda Y, Furukawa H, et al. Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology. 2009;202:315–28. doi: 10.1007/s00213-008-1240-6. [DOI] [PubMed] [Google Scholar]
- Pereira P, Tysca D, Oliveira P, da Silva Brum LF, Picada JN, Ardenghi P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol Res. 2005;52:199–203. doi: 10.1016/j.phrs.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi Y, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Picada JN, Flores DG, Zettler CG, Marroni NP, Roesler R, Henriques JAP. DNA damage in brain cells of mice treated with an oxidized form of apomorphine. Mol Brain Res. 2003;114:80–5. doi: 10.1016/s0169-328x(03)00127-x. [DOI] [PubMed] [Google Scholar]
- Pereira P, Oliveira P, Ardenghi P, Rotta LN, Henriques JAP, Picada JN. Neuropharmacological analysis of caffeic acid in rats. Basic Clin Pharmacol Toxicol. 2006;99:374–8. doi: 10.1111/j.1742-7843.2006.pto_533.x. [DOI] [PubMed] [Google Scholar]
- Pereira P, Gianesini J, da Silva Barbosa C, Cassol GF, Von Borowski RG, Kahl VF, et al. Neurobehavioral and genotoxic parameters of duloxetine in mice using the inhibitory avoidance task and comet assay as experimental models. Pharmacol Res. 2009;59:57–61. doi: 10.1016/j.phrs.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Mavournin KH, Blakey DH, Cimino MC, Salamone MF, Heddle JA. The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. A report of the US Environmental Protection Agency Gene-Tox Program. Mutat Res. 1990;239:29–80. doi: 10.1016/0165-1110(90)90030-f. [DOI] [PubMed] [Google Scholar]
- Picada JN, da Silva KV, Erdtmann B, Henriques AT, Henriques JAP. Genotoxic effects of structurally related beta-carboline alkaloids. Mutat Res. 1997;379:135–49. doi: 10.1016/s0027-5107(97)00116-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues CR, Dias JH, Semedo JG, da Silva J, Ferraz AB, Picada JN. Mutagenic and genotoxic effects of Baccharis dracunculifolia (DC) J Ethnopharmacol. 2009;124:321–4. doi: 10.1016/j.jep.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Lowry H, Rosebrough MJ, Farr AL. Protein measurement with the foline reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Bespalov A, Jongen-Rêlo AL, Van Gaalen M, Harich S, Schoemaker H, Gross G. Habituation deficits induced by metabotropic glutamate receptor 2/3 receptor blockade in mice: reversal by antipsychotic drugs. J Pharmacol Exp Ther. 2007;320:944–50. doi: 10.1124/jpet.106.110684. [DOI] [PubMed] [Google Scholar]
- Jerlhag E. The antipsychotic aripiprazole antagonizes the ethanol- and amphetamine-induced locomotor stimulation in mice. Alcohol. 2008;42:123–7. doi: 10.1016/j.alcohol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connections to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Rossato JL, Bonini JS, Coitinho AS, Vianna MR, Medina JH, Cammarota M, et al. Retrograde amnésia induced by drugs acting on different molecular systems. Behav Neurosci. 2004;118:563–8. doi: 10.1037/0735-7044.118.3.563. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Zinn CG, Furini C, Bevilaqua LR, Medina JH, Cammarota M, et al. A link between the hippocampal and the striatal memory systems of the brain. An Acad Bras Cienc. 2006;78:515–23. doi: 10.1590/s0001-37652006000300011. [DOI] [PubMed] [Google Scholar]
- Kessler RM. Aripiprazole: what is the role of dopamine D2 receptor partial agonism. Am J Psychiatry. 2007;164:1310–2. doi: 10.1176/appi.ajp.2007.07071043. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Agurell E, Beevers C, Brendler–Schwaab S, Burlinson B, Clay P, et al. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- Brambilla G, Mattioli F, Martelli A. Genotoxic and carcinogenic effects of antipsychotics and antidepressants. Toxicology. 2009;261:77–88. doi: 10.1016/j.tox.2009.04.056. [DOI] [PubMed] [Google Scholar]
- McLoughlin GA, Ma D, Tsang TM, Jones DN, Cilia J, Hill MD, et al. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res. 2009;8:1943–52. doi: 10.1021/pr800892u. [DOI] [PubMed] [Google Scholar]
- Yang TT, Wang SJ. Aripiprazole and its human metabolite OPC14857 reduce, through a presynaptic mechanism, glutamate release in rat prefrontal cortex: possible relevance to neuroprotective interventions in schizophrenia. Synapse. 2008;62:804–18. doi: 10.1002/syn.20548. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee JG, Ha EK, Choi SM, Cho HY, Seo MK, et al. Differential effects of aripiprazole and haloperidol on BDNF-mediated signal changes in SH-SY5Y cells. Eur Neuropsychopharmacol. 2009;19:356–62. doi: 10.1016/j.euroneuro.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Urichuk L, Prior TI, Dursun S, Baker G. Metabolism of atypical antipsychotics: involvement of cytochrome P450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008;9:410–8. doi: 10.2174/138920008784746373. [DOI] [PubMed] [Google Scholar]
- Waade RB, Christensen H, Rudberg I, Refsum H, Hermann M. Influence of comedication on serum concentrations of aripiprazole and dehydroaripiprazole. Ther Drug Monit. 2009;31:233–8. doi: 10.1097/FTD.0b013e3181956726. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases, therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, et al. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53:56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- Gama CS, Salvador M, Andreazza AC, Lobato MI, Berk M, Kapczinski F, et al. Elevated serum thiobarbituric acid reactive substances in clinically symptomatic schizophrenic males. Neurosci Lett. 2008;433:270–3. doi: 10.1016/j.neulet.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Martins MR, Petronilho FC, Gomes KM, Dal-Pizzol F, Streck EL, Quevedo J. Antipsychotic-induced oxidative stress in rat brain. Neurotox Res. 2008;13:63–9. doi: 10.1007/BF03033368. [DOI] [PubMed] [Google Scholar]
- Eren I, Nazıroglu M, Demirdas A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res. 2007;32:1188–95. doi: 10.1007/s11064-007-9289-x. [DOI] [PubMed] [Google Scholar]
- Streck EL, Rezin GT, Barbosa LM, Assis LC, Grandi E, Quevedo J. Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedeberg Arch Pharmacol. 2007;376:127–33. doi: 10.1007/s00210-007-0178-2. [DOI] [PubMed] [Google Scholar]