Abstract
Rationale
Involvement of metabotropic glutamate 5 (mGlu5) receptors has been suggested in the reinforcing effects of psychostimulants. However, little is known about the role of these receptors in psychostimulant withdrawal.
Objectives
The role of mGlu5 receptors was assessed in the anhedonic and somatic aspects of psychostimulant withdrawal.
Methods
Anhedonia was assessed with the discrete-trial current-intensity intracranial self-stimulation (ICSS) procedure after the termination of cocaine (180 mg/kg/day, salt, 3 days, IP) or nicotine (40 mg/kg/day, base, 28 days, SC) administration via osmotic minipumps in mGlu5 receptor knockout (mGluR5-/-) and wildtype (mGluR5+/+) mice. Somatic signs were assessed during nicotine withdrawal. The effects of the nicotinic acetylcholine receptor antagonist mecamylamine on ICSS thresholds were assessed during chronic nicotine administration.
Results
Nicotine-treated mGluR5+/+ and mGluR5-/- mice demonstrated similar threshold elevations during mecamylamine-precipitated withdrawal compared with their saline-treated counterparts. During spontaneous nicotine and cocaine withdrawal, thresholds in drug-withdrawing mGluR5+/+, but not mGluR5-/-, mice were elevated up to 72 h of nicotine/cocaine withdrawal and then returned to baseline, indicating attenuation of withdrawal-induced anhedonia in mGluR5-/- mice. Nicotine-withdrawing mGluR5+/+, but not mGluR5-/-, mice showed increases in somatic signs compared with saline-treated counterparts.
Conclusions
mGlu5 receptor null mutation attenuates the anhedonic and somatic effects of psychostimulant withdrawal. This attenuated withdrawal in mGluR5-/- mice may result from lack of drug-induced adaptations in mGlu5 receptor function that may occur in mGluR5+/+ mice with chronic drug administration. Thus, these results suggest involvement of mGlu5 receptors in psychostimulant dependence, and mediation of anhedonic and somatic signs of psychostimulant withdrawal.
Keywords: Intracranial self-stimulation, Somatic signs, Anhedonia, mGluR5, Mice
The administration of drugs of abuse, including cocaine and nicotine, increases glutamatergic neurotransmission in brain structures implicated in the regulation of reward processes, such as the dorsal striatum (McKee and Meshul 2005; Perez de la Mora et al. 1991), nucleus accumbens (NAc; Pierce et al. 1996; Reid et al. 2000; Saellstroem Baum et al. 2006; Smith et al. 1995), and ventral tegmental area (VTA; Kalivas and Duffy 1995; 1998; Schilstrom et al. 2000). Moreover, drug-induced adaptations in glutamatergic neurotransmission have been suggested to be involved in the development of drug dependence (Hao et al. 2010; Kalivas and Volkow 2005; Kenny and Markou 2004; Liechti and Markou 2008).
Numerous studies have demonstrated a role for the mGlu5 receptor in the rewarding effects of several drugs of abuse. One of the first demonstrations of the involvement of the mGlu5 receptor in the reinforcing effects of drugs of abuse was from Chiamulera and colleagues who demonstrated that mice lacking the mGlu5 receptor did not self-administer cocaine nor display cocaine-induced hyperlocomotion (Chiamulera et al. 2001). After this original observation in knockout mice, the attenuating effects of mGlu5 receptor blockade on drug self-administration have been demonstrated extensively with the use of the mGlu5 receptor antagonists 2-methyl-6-(phenylethynyl)-pyridine hydrochloride (MPEP) and 3-([2-methyl-4-thiazol]ethynyl)pyridine (MTEP) for cocaine (Backstrom and Hyytia 2006; Kenny et al. 2005; Kenny et al. 2003; Kumaresan et al. 2009; Martin-Fardon et al. 2009; Paterson et al. 2003; Tessari et al. 2004), nicotine (Bespalov et al. 2005; Liechti and Markou 2007; Paterson and Markou 2005; Paterson et al. 2003; Tessari et al. 2004), and ethanol (Backstrom et al. 2004; Cowen et al. 2005; Schroeder et al. 2005) in rats. Furthermore, consistent with the findings of Chiamulera and colleagues in mGlu5 receptor mutant mice, MPEP suppressed cocaine- and nicotine-induced hyperlocomotion (Herzig and Schmidt 2004; McGeehan et al. 2004; Tessari et al. 2004; Veeneman et al. 2010).
Localization studies have indicated high abundance of mGlu5 receptors in brain sites involved in reward processes, including the striatum and NAc (Lu et al. 1999; Shigemoto et al. 1993; Testa et al. 1994), further supporting the involvement of mGlu5 receptors in brain reward function. Moderate to low expression levels of mGlu5 receptors were also identified in other brain regions, including the olfactory tubercle, hippocampus, neocortex, subthalamic nucleus, entopeduncular nucleus, globus pallidus, ventral pallidum, and substantia nigra (Lu et al. 1999; Shigemoto et al. 1993; Testa et al. 1994).
Studies suggest an important role for the mGlu5 receptor in the reinforcing and hyperlocomotive effects of psychomotor stimulant compounds, but little is known about the potential function of these receptors in psychostimulant dependence and withdrawal, the focus of the present studies. One of the primary symptoms of nicotine and cocaine withdrawal syndromes in humans is depressed mood and anhedonia, with anhedonia defined as the inability to experience pleasure from rewarding stimuli (American Psychiatric Association 2000; Hughes 2007). Anhedonia can be assessed reliably and quantitatively in rodents using the intracranial self-stimulation (ICSS) procedure, in which the anhedonic depression-like mood is reflected in elevations of brain reward thresholds (Markou and Koob 1991). Withdrawal from chronic nicotine administration elevated brain reward thresholds in C57Bl/6J mice in the ICSS procedure (Johnson et al. 2008; Stoker et al. 2008). Similarly, cocaine withdrawal elevated ICSS thresholds in C57BL/6J mice (Stoker and Markou 2011). Studies that investigated the effect of mGlu5 receptor antagonism on ICSS thresholds during nicotine and cocaine withdrawal in rats using the mGlu5 receptor antagonist MPEP demonstrated elevations in ICSS thresholds in both drug-withdrawing and control rats (Harrison et al. 2002; Kenny et al. 2005; Liechti and Markou 2007). These findings suggest that blockade of mGlu5 receptors negatively regulates brain reward function both under baseline conditions and during nicotine withdrawal, thereby exacerbating the anhedonic aspects of nicotine withdrawal.
The somatic signs of withdrawal occur in both humans and rodents after termination of chronic administration of nicotine and cocaine (American Psychiatric Association 2000). Nicotine withdrawal in humans results in various somatic signs, including bradycardia, gastrointestinal discomfort, nausea, headache, increased appetite, drowsiness, sweating, and dizziness (American Psychiatric Association 2000; Hughes et al. 1984; Shiffman 1979). Somatic signs observed during nicotine withdrawal in mice include body shakes, head shakes, paw tremor, scratching, and grooming (Balerio et al. 2004; Damaj et al. 2003; Isola et al. 1999; Salas et al. 2004; Semenova et al. 2003a; Stoker et al. 2008). In rodents, these somatic signs of withdrawal are not observed after the cessation of cocaine administration (Watkins et al. 2000). Antagonism of the mGlu5 receptor by MPEP administration exacerbated the somatic signs of nicotine withdrawal in rats (Liechti and Markou 2007).
The present study assessed the effects of null mutation of the mGlu5 receptor on the anhedonic aspects of spontaneous withdrawal from nicotine and cocaine. The somatic aspects of nicotine withdrawal were also assessed in these mice. Nicotine and cocaine withdrawal were induced in mice null for the mGlu5 receptor (mGluR5-/-) and their wildtype counterparts (mGluR5+/+) by the removal of osmotic minipumps that contained nicotine, cocaine or saline. The anhedonic aspects of nicotine/saline and cocaine/saline withdrawal were subsequently assessed using the ICSS procedure, and the somatic signs of nicotine withdrawal were assessed using observational methods. In addition, ICSS thresholds were assessed after the precipitation of nicotine withdrawal by administering the nonselective nAChR antagonist mecamylamine in nicotine-dependent mice.
Materials and Methods
Subjects
The mGlu5 receptor null line (Grm5tm1Rod) was generated as described previously (Jia et al. 1998). The null line was backcrossed to the C57BL/6J strain for at least 10 generations, and the breeders were kindly donated to our laboratory by Novartis Pharma, Switzerland. Knockout and wildtype littermates were bred in our laboratory from heterozygous breeding pairs. Upon weaning, the mice were maintained in groups of 2-4 until the electrode implantation surgery, after which the mice were housed individually. The subjects were 8- to 12-week-old males at the beginning of the experiments. The animals were housed in a humidity- and temperature-controlled animal facility on a 12 h/12 h (lights off at 7:00 AM) reverse light/dark cycle with ad libitum access to food and water except during testing. Behavioral testing was conducted during the dark phase of the light/dark cycle (unless otherwise required by the experimental design). All experiments were in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council's Guide for Care and Use of Laboratory Animals, and were approved by the University of California San Diego Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO, USA) dissolved in sterile 0.9% saline solution was infused through subcutaneous (s.c.) osmotic minipumps for 28 days (model 2004 Alzet, Palo Alto, CA, USA). Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD, USA) dissolved in saline was infused through intraperitoneal (i.p.) osmotic minipumps for 3 days (model 1003D, Alzet, Palo Alto, CA, USA). Mecamylamine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) dissolved in saline was injected subcutaneously in a volume of 0.1 ml/10 g. All drug doses are reported as the salt, with the exception of nicotine, the doses of which are reported as the weight of the base because of conventions in the field.
Minipump and ICSS electrode implantation were performed using aseptic surgery techniques as described previously (Stoker et al. 2008; Stoker and Markou 2011). Details about ICSS apparati, and training and testing in the ICSS procedure, as well as assessment of somatic signs of nicotine withdrawal, are also described in detail in these same publications.
Experimental design
Experiment 1: Effects of chronic cocaine administration and withdrawal on performance in the ICSS procedure in mGluR5-/- and mGluR5+/+ mice
mGluR5-/- (n = 15) and mGluR5+/+ (n = 16) mice were prepared with stimulating electrodes in the lateral hypothalamus and trained in the ICSS procedure. After establishment of stable ICSS performance (defined as ≤ 10% variation in brain reward thresholds over three consecutive test sessions), the mice were prepared with 3-day i.p. minipumps that delivered saline or 180 mg/kg/day cocaine (salt) and tested once daily. ICSS thresholds and response latencies during cocaine administration and withdrawal are expressed as the percentage of the baseline value (5-day average before cocaine administration). After 72 h of cocaine/saline administration, the minipumps were removed, and mice were tested in the ICSS procedure 3, 6, 8, 12, 24, 28, 48, 52, 72, 76, 96, 100 h after pump removal and once daily thereafter.
Experiment 2: Effects of chronic nicotine administration and nAChR antagonist-precipitated and spontaneous nicotine withdrawal on ICSS performance and somatic signs in mGluR5-/- and mGluR5+/+ mice
Naive mGluR5-/- (n = 30) and mGluR5+/+ (n = 26) mice were prepared with stimulating electrodes in the lateral hypothalamus and trained in the ICSS procedure. After establishment of stable ICSS performance, mice were prepared with 28-day s.c. minipumps that delivered saline or 40 mg/kg/day nicotine base and tested once daily. Mecamylamine hydrochloride was injected s.c. on days 9, 11, 13, and 15 of nicotine/saline exposure using a within-subjects Latin square design for the factor Dose (0, 1.5, 3, and 6 mg/kg, salt). Mice were tested in the ICSS procedure immediately after mecamylamine/saline injection. ICSS thresholds and response latencies after mecamylamine/saline administration are expressed as the percentage of the baseline values obtained on the previous day. Mice were habituated to the cylinders for somatic sign observations on days 25 and 27 of nicotine/saline exposure. On day 29, the minipumps were removed, and mice were tested in the ICSS procedure 3, 6, 8, 12, 16, 24, 28, 48, 52, 72, 76, 96, and 100 h after pump removal. At 24 h post-pump removal, the ICSS session was followed by a 20 min observation of somatic signs.
Statistical analyses
All analyses were performed using the PASW 18 Statistical Package (SPSS, Chicago, IL, USA). The data were analyzed using appropriate two- and three-way analyses of variance (ANOVA), with Genotype and Cocaine/Saline Exposure and Nicotine/ Saline Exposure as the between-subjects factors and Administration Day, Withdrawal Day, and Mecamylamine Dose as the within-subjects factors. Newman-Keuls post hoc analyses followed statistically significant interaction effects in the ANOVAs. The level of significance was set at 0.05. ICSS thresholds and response latencies are expressed as the percentage of baseline values for the ANOVAs. Baseline values were established as the following for the different experiments: for mecamylamine-precipitated withdrawal, baseline values were the values obtained during the previous day's ICSS session; for nicotine/saline, cocaine/saline administration, and cocaine/saline withdrawal, baseline values were defined as the mean values obtained during the last five ICSS daily sessions before pump implantation (Table 1); for spontaneous nicotine/saline withdrawal, baseline values were defined as the mean of the values obtained during the last five ICSS daily sessions before pump removal (Table 1). ICSS thresholds with chronic nicotine administration were combined for analysis (days 16 + 17, days 18 + 19, days 20 + 21, days 22 + 23, days 24 + 25, and days 26 + 27) to provide robust and reliable estimates of the effects of nicotine/saline administration. For ICSS withdrawal, data time-points of early withdrawal were also analyzed using the mean of two sequential time-points (3 + 6 h, 8 + 12 h, 24 + 28 h, 48 + 52 h, 72 + 76 h, and 96 + 100 h). For further analysis of the overall effect of cocaine/nicotine withdrawal on ICSS thresholds, the area-under-the-curve (i.e., the sum of the thresholds of all withdrawal time-points) was analyzed by two-way ANOVA, with Genotype and Cocaine/ Saline Exposure or Nicotine/ Saline Exposure as the between-subjects factors.
Table 1.
Mean baseline thresholds (μA) of the last five daily ICSS sessions before pump implantation/removal used to express the data as a percentage of baseline for the statistical analyses. Data are expressed as mean ± SEM
| Mean threshold values (μA) of the last five daily ICSS sessions before cocaine/saline pump implantation (Experiment 1) | |||
|
| |||
| mGluR5+/+ saline (n = 9) | 69.79 | ± | 8.06 |
| mGluR5+/+ cocaine (n = 7) | 65.95 | ± | 4.04 |
| mGluR5-/- saline (n = 7) | 57.25 | ± | 4.50 |
| mGluR5-/- cocaine (n = 8) | 71.61 | ± | 12.60 |
| Mean threshold values (μA) of the last five daily ICSS sessions before nicotine/saline pump implantation used for analysis of nicotine administration data (Experiment 2) | |||
|
| |||
| mGluR5+/+ saline (n = 10) | 65.67 | ± | 7.86 |
| mGluR5+/+ nicotine (n = 16) | 73.96 | ± | 6.08 |
| mGluR5-/- saline (n = 12) | 87.20 | ± | 6.54 |
| mGluR5-/- nicotine (n = 18) | 82.26 | ± | 5.91 |
| Mean threshold values (μA) of the last five daily ICSS sessions before nicotine/saline pump removal used for analysis of nicotine withdrawal data (Experiment 2) | |||
|
| |||
| mGluR5+/+ saline (n = 10) | 58.29 | ± | 6.32 |
| mGluR5+/+ nicotine (n = 16) | 60.35 | ± | 5.04 |
| mGluR5-/- saline (n = 12) | 73.34 | ± | 5.64 |
| mGluR5-/- nicotine (n = 18) | 75.46 | ± | 6.30 |
Results
Experiment 1: Effects of chronic cocaine administration and withdrawal on performance in the ICSS procedure in mGluR5-/- and mGluR5+/+ mice
Chronic cocaine administration
Chronic administration of cocaine did not induce lowering of brain reward thresholds (Fig. 1A). While an ANOVA on the chronic cocaine/saline administration data detected a statistically significant Genotype × Cocaine/ Saline Exposure × Administration Day interaction (F2,54 = 3.283, p < 0.05), the instability of thresholds at the 4 h post-pump implantation time-point was most likely the cause of this interaction effect. Post hoc analyses revealed that ICSS thresholds in cocaine-treated mGluR5+/+ and saline-treated mGluR5-/- mice were elevated compared with their respective counterparts 4 h after the surgical procedure. The latency to turn the wheel manipulandum remained unaffected during cocaine administration (data not shown).
Figure 1.
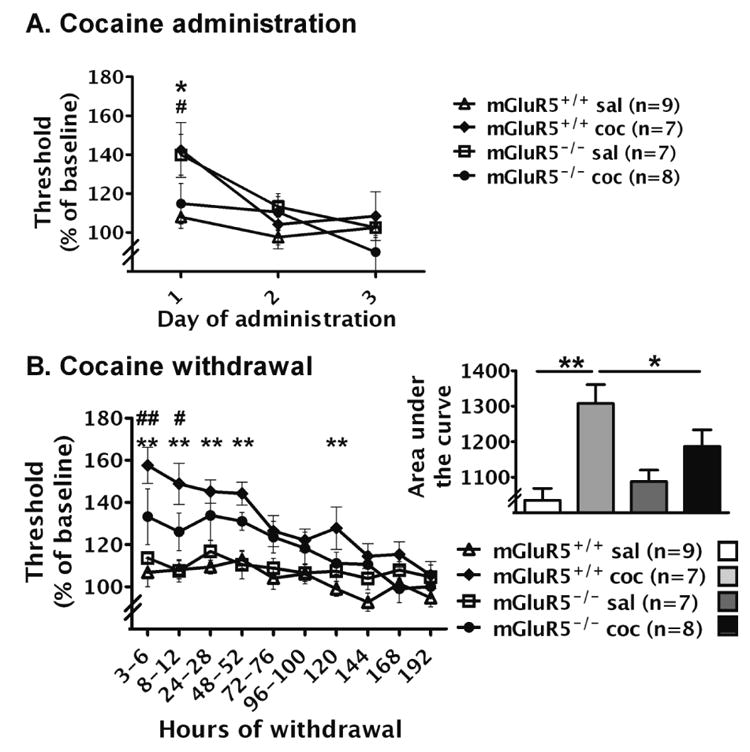
Effects of chronic cocaine/saline administration and withdrawal on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. The data are expressed as a percentage of baseline thresholds (mean ± SEM). (A) Effects of chronic cocaine/saline administration (180 mg/kg/day, base, 3 days) on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. Asterisk indicates a significant difference between saline- and cocaine-treated mGluR5+/+ mice (*p < 0.05). Pound sign indicates a significant difference between saline- and cocaine-treated mGluR5-/- mice (#p < 0.05). (B) Effects of cocaine/saline withdrawal on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. Asterisks (**p < 0.01) indicate a significant differences between cocaine- and saline-treated mGluR5+/+ mice at a specific time-point using Newman-Keuls post hoc comparisons after a significant Genotype × Cocaine/Saline Exposure interaction effect. Thresholds in cocaine-withdrawing mGluR5+/+ mice were significantly elevated compared with their saline-treated counterparts at 3-6 h, 8-12 h, 24-28 h, 48-52 h, and 120 h after pump removal (p < 0.01). Pound signs (#p < 0.05, ##p < 0.01) indicate significant differences between cocaine-treated mGluR5+/+ and mGluR5-/- mice at 3-6 h (p < 0.01) and 8-12 h (p < 0.05). Inset to B: Area-under-the-curve calculated as the sum of threshold values 3-120 h post-pump removal. Newman-Keuls post hoc analysis after a significant Genotype × Nicotine/Saline Exposure interaction demonstrated a significant elevation of thresholds in cocaine-withdrawing mGluR5+/+ mice compared with cocaine-withdrawing mGluR5-/- mice (*p < 0. 05) and saline-treated mGluR5+/+ mice (**p < 0.01).
Cocaine withdrawal
During early cocaine withdrawal, ICSS thresholds in mGluR5+/+ and mGluR5-/- mice were significantly elevated compared with their respective saline-withdrawing counterparts (Fig. 1B). Moreover, cocaine-withdrawing mGluR5+/+ mice demonstrated significant threshold elevations compared with mGluR5-/- mice withdrawing from chronic cocaine, revealed by statistically significant Cocaine/Saline Exposure × Withdrawal Day (F9,243 = 0.952, p < 0.001) and Genotype × Cocaine/ Saline Exposure (F1,27 = 4.324, p < 0.05) interaction effects, and significant main effects of Cocaine Saline Exposure (F1,27 = 19.841, p < 0.001) and Withdrawal Day (F9,243 = 14.062, p < 0.001). However, no Genotype × Cocaine/Saline Exposure × Withdrawal Day interaction was found. Pre-planned comparisons demonstrated significant threshold elevations in cocaine-withdrawing mGluR5+/+ mice compared with saline-treated mGluR5+/+ mice 3-6 h, 8-12 h, 24-28 h, 48-52 h, and 120 h after pump removal (p < 0.01), and significant attenuation of withdrawal-induced threshold elevations in cocaine-treated mGluR5-/- mice compared with cocaine-treated mGluR5+/+ 3-6 h (p <0.01) and 8-12 h (p < 0.05) after pump removal. The attenuation of cocaine withdrawal in mGluR5-/- mice was also demonstrated by the analysis of the area-under-the-curve (Fig. 1B inset), which yielded a significant Cocaine/Saline Exposure × Genotype interaction (F1,27 = 4.324, p < 0.05) and a significant main effect of Genotype (F1,27 = 19.841, p < 0.001). Post hoc analyses of the area-under-the-curve data revealed significant threshold elevations during cocaine withdrawal in mGluR5+/+ mice compared with cocaine-withdrawing mGluR5-/- mice (p < 0.05) and saline-treated mGluR5+/+ mice (p < 0.01). Thresholds in saline-withdrawing mGluR5+/+ and mGluR5-/- mice did not differ from each other at any time-point and were close to 100% of baseline. The latency to turn the wheel manipulandum remained unaffected during cocaine withdrawal (data not shown).
Experiment 2: Effects of chronic nicotine administration and nAChR antagonist-precipitated and spontaneous nicotine withdrawal on ICSS performance and somatic signs in mGluR5-/- and mGluR5+/+ mice
Chronic nicotine administration
During chronic nicotine administration, ICSS thresholds in nicotine-treated mGluR5+/+ and mGluR5-/- mice did not differ significantly from their saline-treated counterparts (Fig. 2A). A significant Genotype × Nicotine/Saline Exposure × Administration Day interaction was found (F9,468 = 1.903, p < 0.05), but the post hoc analyses did not reveal significant differences in ICSS thresholds between the experimental groups at any time point. No other statistically significant main or interaction effects were observed. The latency to turn the wheel manipulandum remained unaffected during nicotine administration (data not shown).
Figure 2.
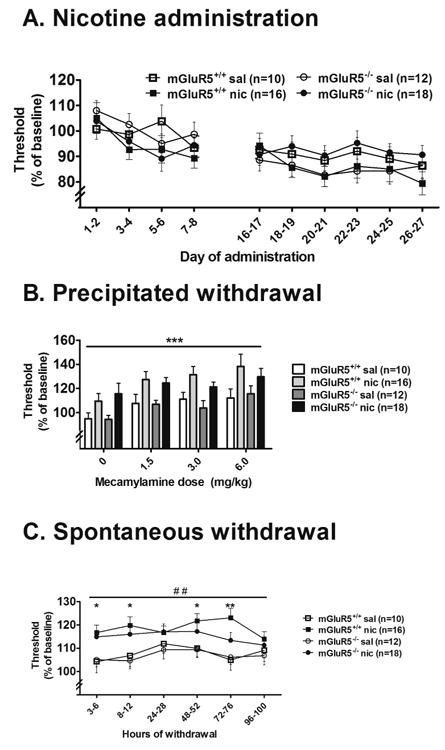
Effects of chronic nicotine/saline administration, and precipitated and spontaneous nicotine withdrawal on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. The data are expressed as a percentage of baseline thresholds (mean ± SEM). (A) Effects of chronic nicotine/saline administration (40 mg/kg/day, base, 28 days) on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. ANOVA indicated a significant Genotype × Nicotine/Saline Exposure × Administration Day interaction, but post hoc analyses did not reveal significant differences in ICSS thresholds between the experimental groups at any time point. (B) Effects of mecamylamine-precipitated nicotine/saline withdrawal on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. Asterisks (**p < 0.01) indicate a significant main effect of Mecamylamine in an ANOVA. (C) Effects of spontaneous nicotine/saline withdrawal on ICSS thresholds in mGluR5-/- and mGluR5+/+ mice. Asterisks (*p < 0.05 and **p < 0.01) indicate significant differences between nicotine- and saline-treated mGluR5+/+ mice at specific time-points with pre-planned comparisons after an ANOVA indicate a significant main effect of Nicotine/Saline Exposure (##p < 0.01). Nicotine-withdrawing mGluR5+/+ mice exhibited elevated thresholds compared with saline-treated mGluR5+/+ mice. Although no statistically significant difference was found between nicotine-withdrawing mGluR5-/- mice and mGluR5+/+ mice, thresholds in mGluR5-/- mice did not differ significantly from thresholds in their saline-treated counterparts. Inset to C: Area-under-the-curve calculated as the sum of threshold values exhibited 3-100 h post-pump removal. Asterisks indicate a significant elevation of the area-under-the-curve in nicotine-withdrawing mGluR5+/+ mice compared with saline-treated controls (*p < 0.05) with pre-planned analyses after an ANOVA revealed a significant main effect of Nicotine/ Saline Exposure.
Mecamylamine-precipitated nicotine withdrawal
Mecamylamine administration induced similar ICSS threshold elevations in nicotine-treated mGluR5+/+ and mGluR5-/- mice compared with their saline-treated counterparts (Fig. 2B), reflected by significant main effects of Mecamylamine Dose (F3,156 = 6.093, p < 0.01) and Nicotine/Saline Exposure (F1,52 = 26.625, p < 0.001), and the absence of a significant main effect of Genotype and no Nicotine/Saline Exposure × Genotype × Mecamylamine Dose interaction, although no Mecamylamine Dose × Nicotine/Saline Exposure interaction was observed. Pre-planned comparisons did not reveal significant differences in ICSS thresholds among experimental groups after administration of any mecamylamine dose. The latency to turn the wheel manipulandum remained unaffected during mecamylamine-precipitated nicotine withdrawal (data not shown).
Spontaneous nicotine withdrawal
ICSS thresholds were elevated in mGluR5+/+ and mGluR5-/- mice, reflected by a significant main effect of Nicotine/Saline Exposure (F1,52 = 9.862, p < 0.01; Fig. 2C). The ANOVA did not reveal any significant interaction or main effects of Genotype or Withdrawal Day. Pre-planned comparisons, however, demonstrated that ICSS thresholds were significantly elevated in nicotine-withdrawing mGluR5+/+ mice on days 1, 3, and 4 post-pump removal (p < 0.05) compared with the thresholds of saline-withdrawing mGluR5+/+ mice, while the thresholds of nicotine-withdrawing mGluR5-/- mice were not significantly different from thresholds of their saline-withdrawing counterparts at any time-point. Nicotine withdrawal was also attenuated in mGluR5-/- mice when the area-under-the-curve data were analyzed (Fig. 2C inset), as revealed by a significant main effect of Nicotine/Saline Exposure (F1,52 = 9.862, p < 0.01), with no significant main effect of Genotype and no interaction. Pre-planned analyses indicated that only nicotine-withdrawing mGluR5+/+, and not mGluR5-/-, mice had significantly elevated thresholds compared with thresholds of saline-treated controls (p < 0.05). The latency to turn the wheel manipulandum remained unaffected during spontaneous nicotine withdrawal (data not shown).
Increases in somatic signs seen in nicotine-withdrawing mGluR5+/+ mice were absent in mGluR5-/- mice (Fig. 3), reflected by significant main effects of Genotype (F1,52 = 6.686, p < 0.01) and Nicotine/Saline Exposure (F1,52 = 4.049, p < 0.05), although no significant Genotype × Nicotine/Saline Exposure interaction was found. Pre-planned comparisons revealed that nicotine-treated mGluR5+/+ mice had a significantly higher number of somatic signs than saline-treated mGluR5+/+ mice (p < 0.05) and nicotine-treated mGluR5-/- mice (p < 0.01).
Figure 3.
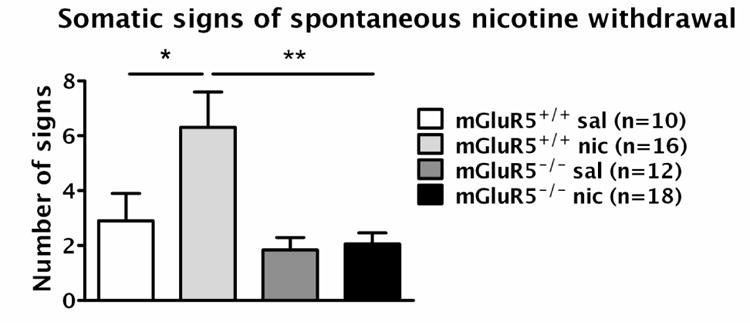
Somatic signs at 24 h of nicotine/saline withdrawal in mGluR5-/- and mGluR5+/+ mice. The data are represented as mean ± SEM. Asterisks indicate a significant difference between saline- and nicotine-treated mGluR5+/+ mice (*p < 0.05) and between nicotine-treated mGluR5-/- and mGluR5+/+ mice (**p < 0.01) with pre-planned comparisons after significant main effects of Genotype (p < 0.01) and Nicotine/Saline Exposure (p < 0.05) in an ANOVA.
Discussion
During withdrawal from chronic cocaine administration, ICSS thresholds of mGluR5+/+, but not mGluR5-/-, mice were significantly elevated compared with thresholds of their respective saline-treated controls. Moreover, thresholds in cocaine-withdrawing mGluR5-/- mice were significantly lower than thresholds of cocaine-withdrawing mGluR5+/+ mice. This pattern of results indicates an attenuation of the anhedonic aspects of cocaine withdrawal in mice null for the mGlu5 receptor. Furthermore, the cessation of chronic nicotine administration resulted in significant elevations of ICSS thresholds in mGluR5+/+ mice, whereas thresholds of mGluR5-/- mice were not significantly elevated compared with thresholds of their saline-treated counterparts. The difference in ICSS thresholds between mGluR5+/+ and mGluR5-/- mice was markedly more pronounced during cocaine, than nicotine, withdrawal. During nicotine withdrawal, ICSS thresholds of mGluR5+/+ mice were only moderately elevated, possibly not allowing for detection of differences in thresholds between nicotine-withdrawing mGluR5+/+ and mGluR5-/- mice. In addition, the somatic signs of nicotine withdrawal were absent in mGluR5-/- mice, while increases in somatic signs characterized nicotine withdrawal in mGluR5+/+ mice.
Elevations in brain reward thresholds have previously been reported in C57BL/6J mice during withdrawal from chronic nicotine (Johnson et al. 2008; Stoker et al. 2008) and cocaine (Stoker and Markou 2011) administration and are well-established in rats for cocaine (Baldo et al. 1999; Kokkinidis and McCarter 1990; Markou et al. 1992; Markou and Koob 1991; 1992) and nicotine (Cryan et al. 2003; Epping-Jordan et al. 1998; Kenny et al. 2003; Skjei and Markou 2003) withdrawal. The present study demonstrated an attenuation of ICSS threshold elevations during drug withdrawal in mice null for the mGlu5 receptor compared with wildtype mice.
The attenuation of the aversive aspects of withdrawal in mGluR5-/- mice was further supported by the lack of increases in somatic signs that characterized nicotine withdrawal in mGluR5+/+ mice. In addition to expression of the mGlu5 receptor in central brain sites (see Introduction), the localization of this receptor has also been shown in the periphery (Young et al. 2007). The somatic signs observed during nicotine withdrawal are primarily peripherally mediated (Watkins et al. 2000; but see Malin et al. 1997). Thus, the lack of mGlu5 receptors in the periphery may be responsible for the absence of somatic signs of nicotine withdrawal in mGluR5-/- mice.
At first glance, the present finding may appear contradictory to previous results demonstrating that pharmacological antagonism of mGlu5 receptors in rats exacerbates somewhat brain reward thresholds during psychostimulant withdrawal (Harrison et al. 2002; Kenny et al. 2005; Liechti and Markou 2007) and increases the somatic signs of nicotine withdrawal (Liechti and Markou 2007). However, the present findings in mGlu5 receptor knockout mice are entirely consistent with these previous pharmacological findings in outbred rats, as both sets of findings suggest an involvement of these receptors in the development of psychostimulant withdrawal. That is, the lack of the receptor in the knockout mice prevented the development of psychostimulant dependence reflected in attenuated anhedonic and somatic aspects of drug withdrawal, while the presence of the receptors during drug exposure in the rats rendered these receptors more sensitive to blockade in the outbred rats.
Excessive activation of the circuits involved in the positive reinforcing effects of drugs of abuse induces neuroadaptations to prevent these systems from overactivation. These neuroadaptations induced by drug administration are hypothesized to lead to the negative affect induced by the absence of the drug (Koob and Le Moal 2008; Markou et al. 1998). The lack of neuroadaptations in mGlu5 receptors in brain reward circuits in mGluR5-/- mice during chronic drug administration may therefore have resulted in less development of drug dependence, and consequently in the attenuation of the negative state of drug withdrawal compared with wildtype mice. These hypothesized effects are reflected in the attenuated threshold elevations in mGluR5-/- mice compared with mGluR5+/+ mice during cocaine and nicotine withdrawal and the attenuation of the somatic signs of nicotine withdrawal. In addition, the upregulation of other receptors may have occurred in mGluR5-/- mice during development to compensate for the lack of the permissive role of mGlu5 receptor activation in reward processes in these mice. For example, mGlu1 and mGlu5 receptors belong to the same group of metabotropic glutamate receptors based on similarities in their molecular structure and physiological functions. To exemplify, pharmacological antagonism of mGlu1 and mGlu5 receptors similarly decreased reinstatement of nicotine seeking in rats (Bespalov et al. 2005; Dravolina et al. 2007). Therefore, adaptations in mGlu1 receptor function may potentially compensate for the absence of mGlu5 receptor activation in reward processes in mGluR5-/- mice. Alternatively or in addition, neuroadaptations in N-methyl-D-aspartate (NMDA) receptor function could compensate for the lack of mGlu5 receptors. Although a hypofunctional state of NMDA receptors was previously suggested for mGluR5-/- mice (Chen et al. 2010), nicotine or cocaine administration may have induced neuroadaptations that resulted in NMDA receptor upregulation in mGlu5 receptor knockout mice. NMDA receptors are positively coupled to mGlu5 receptors in rats (Awad et al. 2000; Pisani et al. 2001), and studies have demonstrated increased NMDA receptor subunit expression during nicotine withdrawal in rats (Kenny et al. 2009; Wang et al. 2008). Increased NMDA receptor function, therefore, may have been induced by the administration of nicotine and cocaine in constitutively mGlu5 receptor knockout mice to compensate for the lack of the excitatory control exerted by mGlu5 receptor activation.
Previous studies demonstrating that blockade of mGlu5 receptors in healthy drug-naive subjects negatively regulates reward function and results in a mild anhedonic mood state (Harrison et al. 2002; Kenny et al. 2003; Liechti and Markou 2007), suggest a role for mGlu5 receptor function in the regulation of mood, which may have resulted in compensatory adaptations in mood-regulatory systems in mGluR5-/- mice because of the chronic absence of mGlu5 receptors during the development of these mice. Consistent with this hypothesized role of mGlu5 receptors in affective regulation and the present findings, an antidepressant behavioral profile was seen in mGluR5-/- mice and wildtype mice treated with of the mGlu5 receptor antagonists MPEP and MTEP in the tail suspension and forced swim tests (Belozertseva et al. 2007; Li et al. 2006; Palucha et al. 2005).
In summary, the present study demonstrated an attenuation of the anhedonic aspects of the cocaine withdrawal syndrome in mGlu5 receptor null mice compared with their wildtype counterparts. Further, mGlu5 receptor null mice demonstrated a tendency towards attenuation of the anhedonic aspects of the nicotine withdrawal syndrome. Increases in the somatic signs of nicotine withdrawal were also attenuated in mGlu5 receptor knockout mice. The attenuation of the aversive aspects of drug withdrawal in mGluR5-/- mice may have resulted from the lack of adaptations in mGlu5 receptor function induced by the administration by cocaine and nicotine that may have occurred in mGluR5+/+ mice. This lack of drug-induced adaptations in mGlu5 receptor function may have consequently resulted in an attenuation of the anhedonic aspects of the cocaine and nicotine withdrawal syndromes and in an attenuation of the somatic signs of nicotine withdrawal. Thus, these results suggest the critical involvement of mGlu5 receptors in the development of nicotine and cocaine dependence and mediation of the anhedonic and somatic signs of drug withdrawal.
Interestingly, the chromosomal region 11q14, on which the GRM5 gene is located, has been linked to habitual smoking behavior (Bierut et al. 2004). The findings of the present study may suggest that individuals with genetic variations in the mGlu5 receptor, resulting in decreased mGlu5 receptor-mediated neurotransmission, may be less sensitive to the reinforcing effects of nicotine and cocaine and the depressive-like symptoms of psychostimulant drug withdrawal.
Acknowledgments
This work was supported by NIH grant R01DA023209 to AM. The authors would like to thank Novartis for providing the mGlu5 knockout breeding pairs, Mr. Edwin Obaña and Mrs. Kimberly Edwards for their assistance with mouse colony maintenance and genotyping of the mGlu5 receptor knockout line, Dr. Manoranjan D'Souza for input during the preparation of this manuscript, and Mr. Michael Arends for editorial assistance.
Funding: This work was supported by NIH grant R01DA023209 to AM.
AM has received contract research support from Intracellular Therapeutics, Inc., Bristol-Myers-Squibb Co., F. Hoffman-La Roche Inc., Pfizer, and Astra-Zeneca and honorarium/consulting fees from Abbott GmbH and Company, AstraZeneca, and Pfizer during the past 3 years. AM has a patent application on metabotropic glutamate receptors and drug dependence.
Footnotes
Disclosure/Conflict of interest: AKS and BO have no disclosures and no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th, text rev. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–9. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–8. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–86. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Koob GF, Markou A. Role of adenosine A2 receptors in brain stimulation reward under baseline conditions and during cocaine withdrawal in rats. J Neurosci. 1999;19:11017–26. doi: 10.1523/JNEUROSCI.19-24-11017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–48. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007;17:172–9. doi: 10.1016/j.euroneuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–78. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Goate A, Hinrichs AL, Saccone NL, Foroud T, Edenberg HJ, Cloninger CR, Begleiter H, Conneally PM, Crowe RR, Hesselbrock V, Li TK, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. A genomic scan for habitual smoking in families of alcoholics: common and specific genetic factors in substance dependence. Am J Med Genet A. 2004;124A:19–27. doi: 10.1002/ajmg.a.20329. [DOI] [PubMed] [Google Scholar]
- Chen HH, Stoker AK, Markou A. The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology (Berl) 2010;209:343–50. doi: 10.1007/s00213-010-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–34. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–9. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–8. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–84. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology (Berl) 1984;83:82–7. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–96. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–43. [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav. 2008;90:409–15. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–88. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–54. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–81. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–72. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–8. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, McCarter BD. Postcocaine depression and sensitization of brain-stimulation reward: analysis of reinforcement and performance effects. Pharmacol Biochem Behav. 1990;36:463–71. doi: 10.1016/0091-3057(90)90242-a. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–44. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Need AB, Baez M, Witkin JM. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther. 2006;319:254–9. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–74. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence : implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–24. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of glutamate receptor subunit/subtype messenger RNAS for NMDAR1, GLuR1, GLuR2 and mGLuR5 by accumbal projection neurons. Brain Res Mol Brain Res. 1999;63:287–96. doi: 10.1016/s0169-328x(98)00288-5. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Schopen CK, Kirk JW, Sailer EE, Lawless BA, Upchurch TP, Shenoi M, Rajan N. Nicotine abstinence syndrome precipitated by central but not peripheral hexamethonium. Pharmacology, biochemistry, and behavior. 1997;58:695–9. doi: 10.1016/s0091-3057(97)90006-x. [DOI] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology (Berl) 1992;109:305–14. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology. 1992;7:213–24. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–74. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–90. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Janak PH, Olive MF. Effect of the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) on the acute locomotor stimulant properties of cocaine, D-amphetamine, and the dopamine reuptake inhibitor GBR12909 in mice. Psychopharmacology (Berl) 2004;174:266–73. doi: 10.1007/s00213-003-1733-2. [DOI] [PubMed] [Google Scholar]
- McKee BL, Meshul CK. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience. 2005;133:605–13. doi: 10.1016/j.neuroscience.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav. 2005;81:901–6. doi: 10.1016/j.pbb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–61. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–64. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Mendez-Franco J, Salceda R, Aguirre JA, Fuxe K. Neurochemical effects of nicotine on glutamate and GABA mechanisms in the rat brain. Acta Physiol Scand. 1991;141:241–50. doi: 10.1111/j.1748-1716.1991.tb09074.x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–87. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35:129–36. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Saellstroem Baum S, Huebner A, Krimphove M, Morgenstern R, Badawy AA, Spies CD. Nicotine stimulation on extracellular glutamate levels in the nucleus accumbens of ethanol-withdrawn rats in vivo. Alcohol Clin Exp Res. 2006;30:1414–21. doi: 10.1111/j.1530-0277.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–9. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG, Svensson TH. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–83. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–70. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Bespalov A, Markou A. Decreased prepulse inhibition during nicotine withdrawal in DBA/2J mice is reversed by nicotine self-administration. Eur J Pharmacol. 2003a;472:99–110. doi: 10.1016/s0014-2999(03)01904-6. [DOI] [PubMed] [Google Scholar]
- Shiffman SM. The tobacco withdrawal syndrome. NIDA Res Monogr. 1979:158–84. [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–7. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 2003;168:280–92. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–9. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A. Withdrawal from chronic cocaine administration induces brain reward threshold elevations in C57BL/6J mice. Behav Brain Res. 2011;223:176–81. doi: 10.1016/j.bbr.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–32. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–33. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–18. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, Spooren W, Vanderschuren LJ. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM. Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J Neurochem. 2008;106:943–56. doi: 10.1111/j.1471-4159.2008.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–64. [PubMed] [Google Scholar]
- Young RL, Page AJ, O'Donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol. 2007;292:G501–11. doi: 10.1152/ajpgi.00353.2006. [DOI] [PubMed] [Google Scholar]


